-
PDF
- Split View
-
Views
-
Cite
Cite
Michael J Law, Kerri Ciccaglione, Fine-Tuning of Histone H3 Lys4 Methylation During Pseudohyphal Differentiation by the CDK Submodule of RNA Polymerase II, Genetics, Volume 199, Issue 2, 1 February 2015, Pages 435–453, https://doi.org/10.1534/genetics.114.172841
Close - Share Icon Share
Abstract
Transcriptional regulation is dependent upon the interactions between the RNA pol II holoenzyme complex and chromatin. RNA pol II is part of a highly conserved multiprotein complex that includes the core mediator and CDK8 subcomplex. In Saccharomyces cerevisiae, the CDK8 subcomplex, composed of Ssn2p, Ssn3p, Ssn8p, and Srb8p, is thought to play important roles in mediating transcriptional control of stress-responsive genes. Also central to transcriptional control are histone post-translational modifications. Lysine methylation, dynamically balanced by lysine methyltransferases and demethylases, has been intensively studied, uncovering significant functions in transcriptional control. A key question remains in understanding how these enzymes are targeted during stress response. To determine the relationship between lysine methylation, the CDK8 complex, and transcriptional control, we performed phenotype analyses of yeast lacking known lysine methyltransferases or demethylases in isolation or in tandem with SSN8 deletions. We show that the RNA pol II CDK8 submodule components SSN8/SSN3 and the histone demethylase JHD2 are required to inhibit pseudohyphal growth—a differentiation pathway induced during nutrient limitation—under rich conditions. Yeast lacking both SSN8 and JHD2 constitutively express FLO11, a major regulator of pseudohyphal growth. Interestingly, deleting known FLO11 activators including FLO8, MSS11, MFG1, TEC1, SNF1, KSS1, and GCN4 results in a range of phenotypic suppression. Using chromatin immunoprecipitation, we found that SSN8 inhibits H3 Lys4 trimethylation independently of JHD2 at the FLO11 locus, suggesting that H3 Lys4 hypermethylation is locking FLO11 into a transcriptionally active state. These studies implicate the CDK8 subcomplex in fine-tuning H3 Lys4 methylation levels during pseudohyphal differentiation.
A robust and dynamic transcriptional response requires cells to integrate extracellular signaling cues into an appropriate output. Inappropriate responses to external cues can lead to developmental defects, programmed cell death, and cancer. Both transcriptional induction and repression require the coordinated activity of transcription factors, histone-modifying enzymes, chromatin-remodeling proteins, and histone chaperone proteins (reviewed in Rando and Winston 2012). The budding yeast Saccharomyces cerevisiae has proven to be a powerful model in the understanding of how extracellular environmental signals elicit transcriptional responses.
Post-translational histone modifications play a central role in a signaling network that regulates transcriptional activation, attenuation, or repression (Rea et al. 2000; Strahl and Allis 2000; Jenuwein and Allis 2001; Berger 2007; Smith and Shilatifard 2010; Rando and Winston 2012). Histone proteins that are responsible for packaging DNA in the nucleus can be post-translationally modified via acetylation, ubiquitination, sumolation, phosphorylation, and methylation (Strahl and Allis 2000). These modifications are dynamic and are controlled by opposing classes of enzymes, termed “writers” and “erasers” (Ruthenburg et al. 2007a,b). These enzymes, as well as the protein domains that interpret the modifications termed “readers,” are well conserved throughout eukaryotes (reviewed in Rando and Winston 2012). Therefore, the coordinated regulation of histone writing, erasing, and reading is of central importance to transcriptional responses and phenotypic outcomes. Recent studies focused on histone methylation have been important in forwarding our understanding of histone modifications in transcription.
In budding yeast, lysine methylation targets include histone H3 Lys4, Lys36, and Lys79 and histone H4 Lys5, Lys12, and Lys18. Methylation of these residues is catalyzed by the enzymes Set1p, Set2p, Dot1p, and Set5p, respectively (Krogan et al. 2002; Strahl et al. 2002; van Leeuwen et al. 2002; Edwards et al. 2011; Green et al. 2012). Lysine can be modified by one, two, or three methyl groups, and each level of methylation results in different functional consequences (Fingerman et al. 2005). Conversely, lysine demethylases have been identified for H3 Lys4 and H3 Lys36; Jhd2p demethylates H3 Lys4 (Liang et al. 2007; Seward et al. 2007), while Jhd1p, and the paralogs Rph1p and Gis1p target H3 Lys36 for demethylation (Tu et al. 2007). Yeast harboring deletions of these enzymatic regulators of methylation have a myriad of phenotypes including loss of telomeric silencing and ribosomal DNA (rDNA), sensitivity to cellular stressors, and misregulation of apoptosis and meiosis (Singer et al. 1998; San-Segundo and Roeder 2000; Deutschbauer et al. 2002; Krogan et al. 2002; Santos-Rosa et al. 2002; Boa et al. 2003; Schaft et al. 2003; Sollier et al. 2004; Carrozza et al. 2005; Fingerman et al. 2005; Morohashi et al. 2005; Trelles-Sticken et al. 2005; Merker et al. 2008; Walter et al. 2014).
The best-characterized histone methyl mark occurs on histone H3 Lys4. While histone H3 Lys4 trimethylation at the promoters of genes has been associated with active transcription, the methyltransferase SET1 is also required for transcriptional silencing at rDNA and telomeres (Nislow et al. 1997; Bernstein et al. 2002; Bryk et al. 2002; Krogan et al. 2002; Nagy and Denison 2002; Santos-Rosa et al. 2002; Boa et al. 2003). In yeast, both SET1 and JHD2 are required for efficient meiotic differentiation. SET1 yeast mutants have major defects in meiosis due to delayed meiotic S-phase, defects in centromere and telomere structure, and inefficient double-strand break formation (Sollier et al. 2004; Trelles-Sticken et al. 2005; Borde et al. 2009). The JHD2 demethylase has a critical function in completing meiosis and supporting gamete fitness (Xu et al. 2012). These functions are controlled in part by regulating meiotic noncoding RNA (ncRNA), rRNA, and protein-coding gene expression during spore morphogenesis (Xu et al. 2012). H3 Lys4 methylation is also important for pseudohyphal differentiation. For example, deleting the SWD3 component of COMPASS, the Set1p-containing enzymatic complex, results in enhancement of flocculation, one of the hallmarks of psuedohyphal growth (Dietvorst and Brandt 2008). Together, these data highlight the importance of tight control of H3 Lys4 methylation levels during yeast cell fate determination.
Lysine methylation can be regulated by a diverse array of molecular interactions, including those with sequence-specific transcription factors, crosstalk with other histone modifications, and interactions with the RNA pol II holoenzyme. For example, histone H3 Lys4 and Lys79 methylation are dependent upon H2B ubiquitylation, which is regulated in part by the polymerase-associated factor (PAF1) complex subunit Rtf1p (Krogan et al. 2003a; Ng et al. 2003a; Wood et al. 2003a). In addition, phosphorylation of the RNA pol II large subunit C-terminal domain (RNA pol II CTD) stimulates interactions with the H3 Lys36 methyltransferase Set2p during transcriptional elongation (Krogan et al. 2003b; Xiao et al. 2003). Both H3 Lys36 trimethylation and phosphorylated Rpo21p combine to recruit the histone deacetylase complex Rpd3S into coding regions, leading to deacetylation in coding regions of actively transcribed genes (Carrozza et al. 2005; Li et al. 2007; Govind et al. 2010). These studies highlight the intimate communication between lysine methylation, the PAF complex, and phosphorylated RNA pol II CTD.
Gene-specific transcriptional activation depends upon a modular, multisubunit RNA pol II holoenzyme complex, which is composed of RNA pol II and mediator. The CDK8 submodule associates with mediator, but is genetically and biochemically distinct from the core mediator (Liao et al. 1995; Carlson 1997; Cooper and Strich 1999; Borggrefe et al. 2002). This submodule, containing Ssn2p (Med13p), Ssn3p (Cdk8p), Ssn8p (Cyclin Cp), and Srb8p (Med12p), is a highly conserved protein complex that can both positively and negatively regulate transcription in a locus-specific fashion (Strich et al. 1989; Hirst et al. 1994; Myers et al. 1998; Chi et al. 2001; Vincent et al. 2001; van de Peppel et al. 2005). CDK8 submodule function requires the Ssn3p/Ssn8p kinase complex and acts in part via the RNA pol II CTD. In vivo work showed that it can suppress growth defects due to RNA pol II CTD truncations, while in vitro studies suggested that it may function by phosphorylating the CTD (Liao et al. 1995; Hengartner et al. 1998). Ssn8p also has a nontranscriptional role in stress response as it transits to the mitochondria inducing mitochondrial fission during reactive oxygen stress (Cooper et al. 2014). Further support of its central importance in nutritional and stress responses is evident as it is of critical importance to meiosis (Cooper and Strich 2002), apoptosis (Cooper et al. 2014), diauxic shift (Holstege et al. 1998), and pseudohyphal growth (Nelson et al. 2003). Due to the genetic evidence indicating that SSN8 and SSN3 impinge upon RNA pol II CTD and that the CTD can influence histone methylation, we hypothesized that SSN8/SSN3 and histone methylation are interdependent.
In this study, we examined the genetic relationship between the histone lysine methylase and demethylase enzymes and the SSN8/SSN3 complex. Surprisingly, we observed that SSN8 and the histone demethylase JHD2 are required to repress pseudohyphal growth in rich media. Pseudohyphal growth occurs when yeast are deprived of nitrogen, fermentable carbon, or amino acids and is characterized by enhanced cell–cell adhesion, changes in cell polarity, increases in cell length, adherence to plastic surfaces, and invasive growth into substrates (Gimeno and Fink 1992; Gimeno et al. 1992; Ljungdahl et al. 1992; Cullen and Sprague 2000). Similarly, diploid yeast can enter meiotic differentiation when deprived of both fermentable carbon and nitrogen. Historically, studies focused on pseudohyphal growth have been performed in Σ1278B yeast strains, which execute meiotic differentiation at low efficiencies. More recently, the meiotic strain SK1 was shown to undergo pseudohyphal growth (Strudwick et al. 2010), making it well suited for understanding the transitions from mitosis to either pseudohyphal growth or meiosis.
The morphological changes of pseudohyphal yeast are highly correlated with expression of the mannoprotein-encoding FLO gene family (Dranginis et al. 2007). Work focused on understanding transcriptional regulation of FLO genes has centered around FLO11, the only nonsubtelomeric family member and thus not subject to transcriptional silencing (Guo et al. 2000; Verstrepen et al. 2004; Chen and Thorner 2007). Studies of FLO11 have indicated that it is necessary for pseudohyphal growth, but other investigations have suggested that FLO1 and FLO10 compensate in strains lacking FLO11 (Guo et al. 2000; Smukalla et al. 2008; Bester et al. 2012). Previous work from many laboratories has shown that FLO11 is regulated by the integration of multiple cell-signaling pathways, including RAS/PKA, MAPK, SNF, TOR, and mitochondrial retrograde transport (Halme et al. 2004; reviewed in Bruckner and Mosch 2012; Cullen and Sprague 2012). These signaling pathways converge to either downregulate transcriptional repressors or stimulate transcriptional activators leading to FLO11 transcription. Additionally, FLO11 transcription is subject to multiple types of “epigenetic control” including a ncRNA toggle, histone deacetylation, and chromatin-remodeling proteins (Bumgarner et al. 2009, 2012; Barrales et al. 2012).
In this study, we identify an unexpected genetic relationship between SSN8 and JHD2. We find that mutant yeast lacking both SSN8 and JHD2 constitutively activate FLO11 transcription and that this activation requires known FLO11 transcriptional activators at varying degrees. We further characterized a role for SSN8 in repressing H3 Lys4 trimethylation independently of JHD2. This study uncovers an important relationship between the CDK8 subcomplex and locus-specific control of H3 Lys4 methylation, raising the possibility that it can guide cell-fate decisions by regulating the dynamic balance of H3 Lys4 methylation levels.
Materials and Methods
Yeast strains, microbiological techniques, and growth conditions
Yeast used in this study are listed in Table 1. All strains are in the SK1 genetic background unless otherwise noted. Yeast deletions were generated using homologous recombination, and gene deletions were shuttled from the Research Genetics strain collection (KanMX) or using hygromycin B resistance cassettes (Goldstein and Mccusker 1999). Construction of diploid homozygous double-null yeast strains utilized either homologous recombination or crosses between each single-deletion mutant. Following generation of heterozygous diploids, cells were sporulated and dissected. The resulting haploid double-null yeast strains were then transformed with Ycp50-ho, giving rise to diploid homozygous mutants. Yeast were cultured in rich media (YEPD) or Synthetic Low Ammonia Dextrose (SLAD; 0.17% yeast nitrogen base, 50 μM ammonium sulfate, 2% dextrose) liquid or plates (2% washed agar) (Ryan 1950; Gimeno et al. 1992) supplemented with amino acids for auxotrophies. For SLAD time-course experiments, yeast were grown to mid-logarithmic phase in YEPD containing 0.5% peptone, harvested, washed with water, and split into either SLAD or YEPD media. Initial experiments analyzed FLO11 messenger RNA (mRNA) expression in wild-type yeast 0, 1, 2, and 4 hr postshift. These experiments determined that the optimal time to assay FLO11 expression was at t = 0 and t = 4 hr.
Yeast strains used in this study
| Strain . | Genotypea . | Source . |
|---|---|---|
| RSY883 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ | Strich et al. (2004) |
| MLY2 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX | This study |
| MLY3 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ jhd2::KanMx | This study |
| MLY4 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 | This study |
| MLY10 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::KanMx::hisG-URA3-hisG jhd2::KanMX | This study |
| MLY11 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::KanMX | This study |
| MLY25 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX ime1::KanMX | This study |
| MLY37 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX flo8::KanMX | This study |
| MLY43 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX mss11::KanMX | This study |
| MLY66 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 rph1::KanMX | This study |
| MLY67 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set2::KanMX | This study |
| MLY68 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX kss1::KanMX | This study |
| MLY71 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd1::KanMX | This study |
| MLY72 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 gis1::KanMX | This study |
| MLY74 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ jhd1::KanMX | This study |
| MLY80 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 dot1::KanMX | This study |
| MLY83 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ dot1::KanMX | This study |
| MLY84 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set1::KanMX | This study |
| MLY85 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX tec1::KanMX | This study |
| MLY86 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ set1::KanMX | This study |
| MLY108 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ gcn4::HphMX | This study |
| MLY109 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ rtg3::HphMX | This study |
| MLY110 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ snf1::HphMX | This study |
| MLY121 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX rtg3::HphMX | This study |
| MLY124 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ set5::KanMX | This study |
| MLY125 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set5::KanMX | This study |
| MLY128 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX gcn4::HphMX | This study |
| MLY136 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX snf1::HphMX | This study |
| MLY140 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::Kanmx::hisG-URA3-hisG set1::KanMX | This study |
| MLY141 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ set2::KanMx | This study |
| MLY142 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ gis1::KanMx | This study |
| MLY143 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ rph1::KanMx | This study |
| MLY 147 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ mss11::KanMX | This study |
| MLY148 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ flo8::KanMX | This study |
| MLY201 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ mfg1::HphMX | This study |
| MLY203 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX mfg1::HphMX | This study |
| MLY209 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ flo11::GFP-KanMX | This study |
| MLY210 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::hisG flo11::GFP-KanMX | This study |
| MLY218 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ime1::KanMX | This study |
| MLY224 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ tec1::KanMX | This study |
| MLY225 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ kss1::KanMX | This study |
| Strain . | Genotypea . | Source . |
|---|---|---|
| RSY883 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ | Strich et al. (2004) |
| MLY2 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX | This study |
| MLY3 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ jhd2::KanMx | This study |
| MLY4 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 | This study |
| MLY10 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::KanMx::hisG-URA3-hisG jhd2::KanMX | This study |
| MLY11 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::KanMX | This study |
| MLY25 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX ime1::KanMX | This study |
| MLY37 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX flo8::KanMX | This study |
| MLY43 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX mss11::KanMX | This study |
| MLY66 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 rph1::KanMX | This study |
| MLY67 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set2::KanMX | This study |
| MLY68 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX kss1::KanMX | This study |
| MLY71 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd1::KanMX | This study |
| MLY72 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 gis1::KanMX | This study |
| MLY74 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ jhd1::KanMX | This study |
| MLY80 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 dot1::KanMX | This study |
| MLY83 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ dot1::KanMX | This study |
| MLY84 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set1::KanMX | This study |
| MLY85 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX tec1::KanMX | This study |
| MLY86 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ set1::KanMX | This study |
| MLY108 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ gcn4::HphMX | This study |
| MLY109 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ rtg3::HphMX | This study |
| MLY110 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ snf1::HphMX | This study |
| MLY121 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX rtg3::HphMX | This study |
| MLY124 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ set5::KanMX | This study |
| MLY125 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set5::KanMX | This study |
| MLY128 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX gcn4::HphMX | This study |
| MLY136 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX snf1::HphMX | This study |
| MLY140 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::Kanmx::hisG-URA3-hisG set1::KanMX | This study |
| MLY141 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ set2::KanMx | This study |
| MLY142 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ gis1::KanMx | This study |
| MLY143 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ rph1::KanMx | This study |
| MLY 147 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ mss11::KanMX | This study |
| MLY148 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ flo8::KanMX | This study |
| MLY201 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ mfg1::HphMX | This study |
| MLY203 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX mfg1::HphMX | This study |
| MLY209 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ flo11::GFP-KanMX | This study |
| MLY210 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::hisG flo11::GFP-KanMX | This study |
| MLY218 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ime1::KanMX | This study |
| MLY224 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ tec1::KanMX | This study |
| MLY225 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ kss1::KanMX | This study |
All strains are isogenic to RSY883 except as noted. All genotypes are homozygous except as noted.
| Strain . | Genotypea . | Source . |
|---|---|---|
| RSY883 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ | Strich et al. (2004) |
| MLY2 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX | This study |
| MLY3 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ jhd2::KanMx | This study |
| MLY4 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 | This study |
| MLY10 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::KanMx::hisG-URA3-hisG jhd2::KanMX | This study |
| MLY11 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::KanMX | This study |
| MLY25 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX ime1::KanMX | This study |
| MLY37 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX flo8::KanMX | This study |
| MLY43 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX mss11::KanMX | This study |
| MLY66 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 rph1::KanMX | This study |
| MLY67 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set2::KanMX | This study |
| MLY68 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX kss1::KanMX | This study |
| MLY71 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd1::KanMX | This study |
| MLY72 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 gis1::KanMX | This study |
| MLY74 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ jhd1::KanMX | This study |
| MLY80 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 dot1::KanMX | This study |
| MLY83 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ dot1::KanMX | This study |
| MLY84 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set1::KanMX | This study |
| MLY85 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX tec1::KanMX | This study |
| MLY86 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ set1::KanMX | This study |
| MLY108 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ gcn4::HphMX | This study |
| MLY109 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ rtg3::HphMX | This study |
| MLY110 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ snf1::HphMX | This study |
| MLY121 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX rtg3::HphMX | This study |
| MLY124 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ set5::KanMX | This study |
| MLY125 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set5::KanMX | This study |
| MLY128 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX gcn4::HphMX | This study |
| MLY136 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX snf1::HphMX | This study |
| MLY140 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::Kanmx::hisG-URA3-hisG set1::KanMX | This study |
| MLY141 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ set2::KanMx | This study |
| MLY142 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ gis1::KanMx | This study |
| MLY143 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ rph1::KanMx | This study |
| MLY 147 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ mss11::KanMX | This study |
| MLY148 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ flo8::KanMX | This study |
| MLY201 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ mfg1::HphMX | This study |
| MLY203 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX mfg1::HphMX | This study |
| MLY209 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ flo11::GFP-KanMX | This study |
| MLY210 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::hisG flo11::GFP-KanMX | This study |
| MLY218 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ime1::KanMX | This study |
| MLY224 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ tec1::KanMX | This study |
| MLY225 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ kss1::KanMX | This study |
| Strain . | Genotypea . | Source . |
|---|---|---|
| RSY883 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ | Strich et al. (2004) |
| MLY2 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX | This study |
| MLY3 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ jhd2::KanMx | This study |
| MLY4 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 | This study |
| MLY10 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::KanMx::hisG-URA3-hisG jhd2::KanMX | This study |
| MLY11 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::KanMX | This study |
| MLY25 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX ime1::KanMX | This study |
| MLY37 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX flo8::KanMX | This study |
| MLY43 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX mss11::KanMX | This study |
| MLY66 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 rph1::KanMX | This study |
| MLY67 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set2::KanMX | This study |
| MLY68 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX kss1::KanMX | This study |
| MLY71 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd1::KanMX | This study |
| MLY72 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 gis1::KanMX | This study |
| MLY74 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ jhd1::KanMX | This study |
| MLY80 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 dot1::KanMX | This study |
| MLY83 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ dot1::KanMX | This study |
| MLY84 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set1::KanMX | This study |
| MLY85 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX tec1::KanMX | This study |
| MLY86 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ set1::KanMX | This study |
| MLY108 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ gcn4::HphMX | This study |
| MLY109 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ rtg3::HphMX | This study |
| MLY110 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ snf1::HphMX | This study |
| MLY121 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX rtg3::HphMX | This study |
| MLY124 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ set5::KanMX | This study |
| MLY125 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 set5::KanMX | This study |
| MLY128 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX gcn4::HphMX | This study |
| MLY136 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX snf1::HphMX | This study |
| MLY140 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn3::Kanmx::hisG-URA3-hisG set1::KanMX | This study |
| MLY141 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ set2::KanMx | This study |
| MLY142 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ gis1::KanMx | This study |
| MLY143 | MATa/MATαlys2 TRP1 ura3 LYS2::ho∆ rph1::KanMx | This study |
| MLY 147 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ mss11::KanMX | This study |
| MLY148 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ flo8::KanMX | This study |
| MLY201 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ mfg1::HphMX | This study |
| MLY203 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::KanMX mfg1::HphMX | This study |
| MLY209 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ flo11::GFP-KanMX | This study |
| MLY210 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ssn8::TRP1 jhd2::hisG flo11::GFP-KanMX | This study |
| MLY218 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ ime1::KanMX | This study |
| MLY224 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ tec1::KanMX | This study |
| MLY225 | MATa/MATαlys2 trp1::hisG ura3 LYS2::ho∆ kss1::KanMX | This study |
All strains are isogenic to RSY883 except as noted. All genotypes are homozygous except as noted.
Microscopy, cytometry, and phenotype characterization
Bright-field and fluorescence microscopy images were acquired with a Nikon Eclipse 90i microscope equipped with a Retiga Exi CCD camera and NIS software for data analysis.
Flocculation rates were determined for overnight cultures grown in YEPD media using a Klett–Summerson photoelectric colorimeter. Absorbances were measured at the indicated time points, and the reading at t = 0 was set equal to 100%.
Invasive growth assays were performed essentially as described (Roberts and Fink 1994). Briefly, individual colonies of yeast were spread onto YEPD containing 2% agar and grown for 3 days at 30°. Yeast were then washed away from the plate using a gentle stream of water with light scrubbing.
Budding patterns were determined using calcofluor staining essentially as previously described (Pringle et al. 1989). Briefly, mid-logarithmic YEPD cultures were fixed with 3.7% formaldehyde for 30 min at room temperature. Fixed cells were then washed with ddH20 and resuspended in 100 μg/ml Calcofluor white (Sigma, catalog no. 18909) and incubated in the dark at 4° for <48 hr. After light sonication to remove flocs, bud scars were analyzed using the DAPI filter. Budding patterns were scored as follows: cells containing proximal and distal bud scars were bipolar, those with only distal bud scars were unipolar, and cells with equatorial bud scars were random. At least 200 cells per genotype were assayed.
Length-to-width measurements were performed on mid-logarithmic cultures in rich media (Gimeno et al. 1992). Photographs were taken on the Zeiss axioscope and measurements were performed using Axiovision 4.3. At least 100 cells were measured per genotype. Statistically significant differences were determined using the Mann–Whitney U-test with a correction for multiple comparisons.
Cell cytometry was performed on an Accuri C6 instrument. Cells containing flo11-GFP were grown in the indicated media to mid-logarithmic phase. Following light sonication to disrupt flocs, 30,000 cells were analyzed for their GFP expression using the FL1 channel. Once collected, data were analyzed using Flowjo software (Tree Star, Ashland, OR). All samples were gated equally, and median GFP expression was reported for cells that are in the positive population.
RT-qPCR
Total nucleic acids were prepared from 20-ml mid-logarithmic cultures. Approximately 500 ng of total nucleic acid preparations were then treated with DNase I (New England Biolabs), followed by reverse transcription using Mu-MLV reverse transcriptase (New England Biolabs) in oligo(dT)-primed reactions to allow reverse transcription of poly(A) mRNA. Subsequent qPCR reactions were prepared using the Power SYBR Master mix (Applied Biosystems) containing primers listed in Table 2. All CT values were normalized first to NUP85 and then to wild-type values (ΔΔCT). Values reported are the average of three or more independent biological replicates; error bars represent the standard error of the mean.
qPCR primers used in this study
| Primer name . | Primer sequence (5′ → 3′) . |
|---|---|
| NUP85-coding forward | TTCGCGAAGGAGCATAATGC |
| NUP85-coding reverse | ACACTTCCAATTCATTCAGAATCG |
| −733 FLO11 promoter forward | CAACAATACGGGCACAACTCA |
| −733 FLO11 promoter reverse | TCACACCACCGATAGGCAATAG |
| −1325 FLO11 promoter forward | GAACGCCGGTAGGCAAATT |
| −1325 FLO11 promoter reverse | TGGGCGACATTCTTGTCAAG |
| FLO11-coding forward | GTTCAACCAGTCCAAGCGAAA |
| FLO11-coding reverse | GTAGTTACAGGTGGGTAGGTGAAGTG |
| IME1-coding forward | TCCCCTAGAAGTTGGCATTTTG |
| IME1-coding reverse | CCAAGTTCTGCAGCTGAGATGA |
| −250 SUC2 promoter forward | GGTACGCCCGATGTTTGC |
| −250 SUC2 promoter reverse | AGTCGTTTAAGCATTCCTCGAAA |
| 18S rDNA forward | AATAAGGGTTCGATTCCGGAG |
| 18S rDNA reverse | TGGATGTGGTAGCCGTTTCTC |
| Primer name . | Primer sequence (5′ → 3′) . |
|---|---|
| NUP85-coding forward | TTCGCGAAGGAGCATAATGC |
| NUP85-coding reverse | ACACTTCCAATTCATTCAGAATCG |
| −733 FLO11 promoter forward | CAACAATACGGGCACAACTCA |
| −733 FLO11 promoter reverse | TCACACCACCGATAGGCAATAG |
| −1325 FLO11 promoter forward | GAACGCCGGTAGGCAAATT |
| −1325 FLO11 promoter reverse | TGGGCGACATTCTTGTCAAG |
| FLO11-coding forward | GTTCAACCAGTCCAAGCGAAA |
| FLO11-coding reverse | GTAGTTACAGGTGGGTAGGTGAAGTG |
| IME1-coding forward | TCCCCTAGAAGTTGGCATTTTG |
| IME1-coding reverse | CCAAGTTCTGCAGCTGAGATGA |
| −250 SUC2 promoter forward | GGTACGCCCGATGTTTGC |
| −250 SUC2 promoter reverse | AGTCGTTTAAGCATTCCTCGAAA |
| 18S rDNA forward | AATAAGGGTTCGATTCCGGAG |
| 18S rDNA reverse | TGGATGTGGTAGCCGTTTCTC |
| Primer name . | Primer sequence (5′ → 3′) . |
|---|---|
| NUP85-coding forward | TTCGCGAAGGAGCATAATGC |
| NUP85-coding reverse | ACACTTCCAATTCATTCAGAATCG |
| −733 FLO11 promoter forward | CAACAATACGGGCACAACTCA |
| −733 FLO11 promoter reverse | TCACACCACCGATAGGCAATAG |
| −1325 FLO11 promoter forward | GAACGCCGGTAGGCAAATT |
| −1325 FLO11 promoter reverse | TGGGCGACATTCTTGTCAAG |
| FLO11-coding forward | GTTCAACCAGTCCAAGCGAAA |
| FLO11-coding reverse | GTAGTTACAGGTGGGTAGGTGAAGTG |
| IME1-coding forward | TCCCCTAGAAGTTGGCATTTTG |
| IME1-coding reverse | CCAAGTTCTGCAGCTGAGATGA |
| −250 SUC2 promoter forward | GGTACGCCCGATGTTTGC |
| −250 SUC2 promoter reverse | AGTCGTTTAAGCATTCCTCGAAA |
| 18S rDNA forward | AATAAGGGTTCGATTCCGGAG |
| 18S rDNA reverse | TGGATGTGGTAGCCGTTTCTC |
| Primer name . | Primer sequence (5′ → 3′) . |
|---|---|
| NUP85-coding forward | TTCGCGAAGGAGCATAATGC |
| NUP85-coding reverse | ACACTTCCAATTCATTCAGAATCG |
| −733 FLO11 promoter forward | CAACAATACGGGCACAACTCA |
| −733 FLO11 promoter reverse | TCACACCACCGATAGGCAATAG |
| −1325 FLO11 promoter forward | GAACGCCGGTAGGCAAATT |
| −1325 FLO11 promoter reverse | TGGGCGACATTCTTGTCAAG |
| FLO11-coding forward | GTTCAACCAGTCCAAGCGAAA |
| FLO11-coding reverse | GTAGTTACAGGTGGGTAGGTGAAGTG |
| IME1-coding forward | TCCCCTAGAAGTTGGCATTTTG |
| IME1-coding reverse | CCAAGTTCTGCAGCTGAGATGA |
| −250 SUC2 promoter forward | GGTACGCCCGATGTTTGC |
| −250 SUC2 promoter reverse | AGTCGTTTAAGCATTCCTCGAAA |
| 18S rDNA forward | AATAAGGGTTCGATTCCGGAG |
| 18S rDNA reverse | TGGATGTGGTAGCCGTTTCTC |
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed essentially as described previously (Meluh and Koshland 1997) with the following modifications. Fifty milliliters of mid-log dextrose cultures were cross-linked with 1% formaldehyde (15 min at room temperature) followed by quenching of cross-linked protein/DNA complexes with 140 mM glycine for 5 min. Cross-linked cells were then spheroplasted, washed extensively, and sonicated using a Bioruptor UCD-200 (Diagenode) to generate fragments ∼300–750 nt in length. IPs were performed on 50 μg of chromatin solution using antibodies directed toward trimethylated H3 Lys4 (Abcam, ab8580) or histone H3 CTD (Abcam, ab1791). Immune complexes were collected, washed sequentially with TSE-150 and -500, LiCl/Det, and TE and then eluted prior to reversing cross-links. DNA was precipitated, treated with proteinase K, and subjected to qPCR. The percentage of input of each IP was calculated using a standard curve for each genomic locus assayed. Each ChIP experiment was performed on three or more independent biological repeats.
Results
SSN8 and JHD2 are required to inhibit pseudohyphal growth under rich conditions
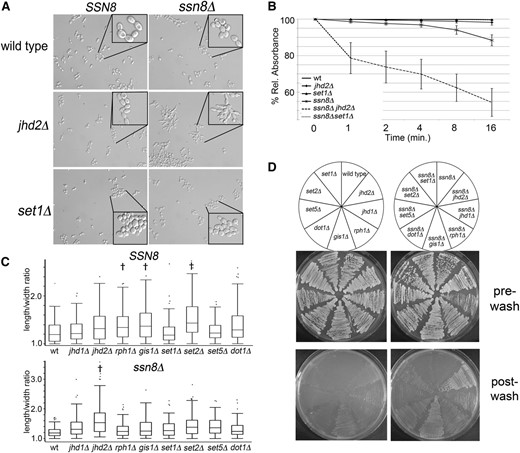
We wanted to determine whether SSN8 and histone methylation are interdependent in regulating transcription. To do this, strains lacking a known lysine methyltransferase or demethylase, with and without SSN8, were generated (Table 3). Since both lysine methylation and SSN8 play major roles in transcription, one might expect that removal of these genes would cause pleiotropic affects, resulting in reduced doubling times under rich conditions. To test this possibility, growth rates of the mutant yeast strains cultured in rich media were measured, but this did not identify any statistically significant differences (Supporting Information, Table S1). While conducting these measurements, we noted that the strain lacking both SSN8 and JHD2 displayed a pseudohyphal budding pattern (Figure 1A). This phenotype was not observed in any of the other ssn8Δ yeast mutants analyzed in this study (Figure 1A and Figure S1A). These data indicate that both SSN8 and JHD2 are required to inhibit pseudohyphal differentiation under rich conditions.
Phenotype analysis of histone methylation and SSN8 mutant yeast
| Histone target . | Genotype . | SSN8 . | Unipolar buds . | Elongated buds . | Invasive growth . | Flocculation . |
|---|---|---|---|---|---|---|
| Wild type | + | − | − | − | − | |
| Δ | + | − | + | + | ||
| +, ssn3Δ | + | + | + | + | ||
| H3 Lys4 | set1Δ | + | + | − | − | − |
| Δ | ++ | − | − | − | ||
| +, ssn3Δ | − | − | + | − | ||
| jhd2Δ | + | + | + | + | − | |
| Δ | ++ | ++ | ++ | ++ | ||
| +, ssn3Δ | ++ | ++ | ++ | ++ | ||
| H3 Lys36 | set2Δ | + | + | ++ | − | + |
| Δ | − | + | + | + | ||
| jhd1Δ | + | − | − | + | − | |
| Δ | − | + | + | + | ||
| gis1Δ | + | + | + | + | − | |
| Δ | ++ | − | ++ | + | ||
| rph1Δ | + | + | + | − | − | |
| Δ | + | − | + | + | ||
| H3 Lys79 | dot1Δ | + | + | + | − | − |
| Δ | + | − | + | + | ||
| H4 Lys5, -8, -12 | set5Δ | + | − | − | − | + |
| Δ | + | + | − | ++ |
| Histone target . | Genotype . | SSN8 . | Unipolar buds . | Elongated buds . | Invasive growth . | Flocculation . |
|---|---|---|---|---|---|---|
| Wild type | + | − | − | − | − | |
| Δ | + | − | + | + | ||
| +, ssn3Δ | + | + | + | + | ||
| H3 Lys4 | set1Δ | + | + | − | − | − |
| Δ | ++ | − | − | − | ||
| +, ssn3Δ | − | − | + | − | ||
| jhd2Δ | + | + | + | + | − | |
| Δ | ++ | ++ | ++ | ++ | ||
| +, ssn3Δ | ++ | ++ | ++ | ++ | ||
| H3 Lys36 | set2Δ | + | + | ++ | − | + |
| Δ | − | + | + | + | ||
| jhd1Δ | + | − | − | + | − | |
| Δ | − | + | + | + | ||
| gis1Δ | + | + | + | + | − | |
| Δ | ++ | − | ++ | + | ||
| rph1Δ | + | + | + | − | − | |
| Δ | + | − | + | + | ||
| H3 Lys79 | dot1Δ | + | + | + | − | − |
| Δ | + | − | + | + | ||
| H4 Lys5, -8, -12 | set5Δ | + | − | − | − | + |
| Δ | + | + | − | ++ |
Phenotypes were scored from yeast harboring the indicated deletions cultured in rich media to mid-logarithmic phase. Yeast were scored as follows: unipolar buds: “−” < 30%; “+” = 30–80%; “++” ≥ 80%; elongated buds, median length-to-width values <1.3 = “−”, ≥ 1.3–1.4 = “+”, ≥ 1.4 = “++” (Table S1). Invasive growth and flocculation scores are based on data in Figure 1, C and D
| Histone target . | Genotype . | SSN8 . | Unipolar buds . | Elongated buds . | Invasive growth . | Flocculation . |
|---|---|---|---|---|---|---|
| Wild type | + | − | − | − | − | |
| Δ | + | − | + | + | ||
| +, ssn3Δ | + | + | + | + | ||
| H3 Lys4 | set1Δ | + | + | − | − | − |
| Δ | ++ | − | − | − | ||
| +, ssn3Δ | − | − | + | − | ||
| jhd2Δ | + | + | + | + | − | |
| Δ | ++ | ++ | ++ | ++ | ||
| +, ssn3Δ | ++ | ++ | ++ | ++ | ||
| H3 Lys36 | set2Δ | + | + | ++ | − | + |
| Δ | − | + | + | + | ||
| jhd1Δ | + | − | − | + | − | |
| Δ | − | + | + | + | ||
| gis1Δ | + | + | + | + | − | |
| Δ | ++ | − | ++ | + | ||
| rph1Δ | + | + | + | − | − | |
| Δ | + | − | + | + | ||
| H3 Lys79 | dot1Δ | + | + | + | − | − |
| Δ | + | − | + | + | ||
| H4 Lys5, -8, -12 | set5Δ | + | − | − | − | + |
| Δ | + | + | − | ++ |
| Histone target . | Genotype . | SSN8 . | Unipolar buds . | Elongated buds . | Invasive growth . | Flocculation . |
|---|---|---|---|---|---|---|
| Wild type | + | − | − | − | − | |
| Δ | + | − | + | + | ||
| +, ssn3Δ | + | + | + | + | ||
| H3 Lys4 | set1Δ | + | + | − | − | − |
| Δ | ++ | − | − | − | ||
| +, ssn3Δ | − | − | + | − | ||
| jhd2Δ | + | + | + | + | − | |
| Δ | ++ | ++ | ++ | ++ | ||
| +, ssn3Δ | ++ | ++ | ++ | ++ | ||
| H3 Lys36 | set2Δ | + | + | ++ | − | + |
| Δ | − | + | + | + | ||
| jhd1Δ | + | − | − | + | − | |
| Δ | − | + | + | + | ||
| gis1Δ | + | + | + | + | − | |
| Δ | ++ | − | ++ | + | ||
| rph1Δ | + | + | + | − | − | |
| Δ | + | − | + | + | ||
| H3 Lys79 | dot1Δ | + | + | + | − | − |
| Δ | + | − | + | + | ||
| H4 Lys5, -8, -12 | set5Δ | + | − | − | − | + |
| Δ | + | + | − | ++ |
Phenotypes were scored from yeast harboring the indicated deletions cultured in rich media to mid-logarithmic phase. Yeast were scored as follows: unipolar buds: “−” < 30%; “+” = 30–80%; “++” ≥ 80%; elongated buds, median length-to-width values <1.3 = “−”, ≥ 1.3–1.4 = “+”, ≥ 1.4 = “++” (Table S1). Invasive growth and flocculation scores are based on data in Figure 1, C and D
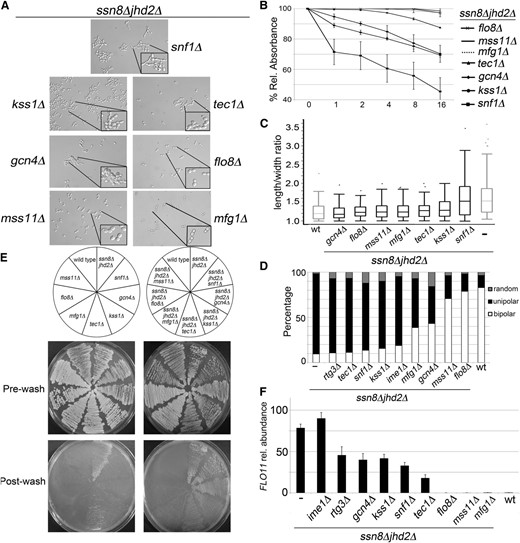
Phenotype analyses of SSN8 and histone methylation mutants. (A) Bright-field microscopy images of yeast with the indicated genotypes grown to mid-logarithmic phase in rich media. Note the pseudohyphal budding pattern of yeast lacking both SSN8 and JHD2. Images of remaining genotypes can be seen in Figure S1A. (B) Flocculation time-course experiment. Yeast with the indicated genotypes were grown overnight in rich media, and flocculation rates were measured using a Klett colorimeter. Absorbances were measured at t = 0, 1, 2, 4, 8, and 16 min with absorbance at t = 0 set equal to 100%. Error bars represent SEM for three independent biological replicates; time courses for the remaining genotypes can be seen in Figure S1B. (C) Box-whisker plots of length-to-width ratios for yeast with the indicated genotypes cultured to mid-logarithmic phase in rich media. At least 100 cells per genotype were measured; the dots above whiskers show the outliers, and the “†” symbol indicates statistically significant differences from wild type (Mann–Whitney U-test with correction for multiple comparisons, P < 10−4). (D) Invasive growth assays of yeast with the indicated genotypes grown on YEPD plates for 3 days. Invasiveness was determined by washing with water while scrubbing the plate.
Pseudohyphal yeast display enhanced cell-to-cell adhesion, a unipolar budding pattern, elongated buds, and invasive growth into solid substrates (reviewed in Cullen and Sprague 2012). To assess the contribution of SSN8 and histone methylation regulators to pseudohyphal growth, these individual phenotypes were determined in each genetic background (Table 3 and Table S1). First, cell-to-cell adhesion was assayed using a quantitative flocculation assay. Overnight cultures were grown in rich media and assayed for their flocculation rates using measurements from a Klett colorimeter. Initial Klett readings were set equal to 100%, and decreases in absorbance were monitored over a 16-min time-course experiment (Figure 1B and Figure S1B). None of the strains containing SSN8 displayed significant enhancement of flocculation over the duration of the time course (Figure 1B and Figure S1B). However, deleting SSN8 resulted in increased flocculation rates, which is consistent with earlier reports documenting this phenotype (Nelson et al. 2003; Raithatha et al. 2012). Surprisingly, the histone H3 Lys4 methylation regulators JHD2 and SET1 influenced flocculation in the SSN8 mutant. Removal of the JHD2 demethylase increased the flocculation rate of the ssn8Δ yeast strain, while deleting SET1 completely abrogated flocculation (Figure 1B). The enhanced flocculation observed in the ssn8Δjhd2Δ strain is most pronounced at the earliest time points; after only 1 min an ∼20% decrease in absorbance was observed (Figure 1B), indicating that these mutant yeast contain a high percentage of flocs in rich media. SET1 was the only lysine methylation regulator that was required for ssn8Δ flocculation phenotypes (Figure 1B, Figure S1B, and Table 3), suggesting that the dynamics of H3 Lys4 methylation play a central role in cell-to-cell adhesion.
To determine budding patterns, wild-type and mutant yeast were grown to mid-logarithmic phase in rich media and stained with calcofluor white, and the position of bud scars was quantified (for details see Materials and Methods). Notably, yeast lacking SET1, JHD2, SET2, GIS1, RPH1, or DOT1 all displayed moderate increases in the percentage of cells with unipolar budding patterns. (Table 3 and Table S1). Consistent with these observations, abnormal budding patterns have been noted for set1Δ mutants, cellular morphologies are altered in gis1Δ mutants, and a chitin deposition phenotype has been observed in dot1Δ mutants (Nislow et al. 1997; Sopko et al. 2006; Frederiks et al. 2009). These minor increases in unipolar buds have not been previously reported for jhd2Δ, set2Δ, or rph1Δ mutants. The impact of SSN8 on bud-site selection was next determined in double-mutant yeast strains. Removal of SSN8 alone resulted in modest enhancement of unipolar budding. Deleting GIS1, SET1, or JHD2 in the ssn8Δ mutant background resulted in dramatic increases in unipolar budding (>80% cells unipolar; Table 3 and Table S1). While ssn8Δgis1Δ mutants displayed increased unipolar budding, deleting the GIS1 paralog RPH1 in the ssn8Δ mutant background did not provide a phenocopy. This indicates that, while both enzymes catalyze H3Lys36 demethylation, they regulate nonoverlapping genes or gene products, which is also consistent with earlier reports (see below; Sopko et al. 2006). Interestingly, both H3Lys4 methylation regulators SET1 and JHD2 displayed genetic interactions with SSN8 for bud-site selection. Together, these data indicate that an intermediary level of H3Lys4 methylation is important for bud-site determination. Additionally, they highlight the complexity with which bud-site selection is controlled by histone methylation regulators.
Next, elongated budding was measured by calculating length-to-width ratios. Yeast were cultured to mid-logarithmic phase in rich media, and at least 100 cells were measured per genotype. The ratio of cell length to width was measured and is displayed as box-whisker plots (Figure 1C, Table 3, and Table S1). Statistical analyses of these data show that most of the yeast mutants assayed do not have elongated buds relative to wild type. Statistically significant increases in median length-to-width ratio in cells lacking RPH1, GIS1, and SET2 were identified. Since all of these genes regulate H3 Lys36 methylation, these results suggest that this methyl mark may play important roles in the G2/M transition. The most pronounced increase in length-to-width ratio was observed in the strain lacking both SSN8 and JHD2 (Figure 1C, Table 3, and Table S1). Yeast harboring these mutations displayed a statistically significant increase in cell length relative to wild type, consistent with the observed pseudohyphal growth patterns (P < 0.0001; Mann–Whitney U-test).
Finally, invasive growth was determined using a plate wash assay. Cells were grown for 3 days on rich plates and washed with ddH2O while scrubbing the plate to determine which cells were embedded in the agar. Yeast lacking JHD1 or DOT1 display an increase in invasive growth relative to wild type, but these increases are not enhanced upon removal of SSN8 (Figure 1D and Table 3). Yeast lacking SSN8 and either JHD2 or GIS1 all displayed elevated agar invasion. This may be due to upregulation of multiple genes that are involved in this phenotypic switch, as systematic overexpression studies have shown that invasive growth is a complex phenotype, controlled by diverse gene products including transcription factors and chromatin-modifying enzymes (Shively et al. 2013). In agreement with our flocculation assays and length-to-width measurements, the enhanced invasion phenotype of the ssn8Δ strain was SET1-dependent. Interestingly, the invasive growth phenotypes of yeast lacking GIS1 or RPH1 in the ssn8Δ mutant background were not identical. Invasive growth in these strains mirrored the unipolar budding phenotypes described above, providing further support for the separable functions of these two demethylase genes. Importantly, the only yeast mutant strain assayed that displayed all of the hallmarks of pseudohyphal growth in rich media (herein referred to as “synthetic pseudohyphal growth”) was the strain lacking both SSN8 and JHD2 (Table 3). Consistent with the idea that H3 Lys4 methylation dynamics play a central role in the pseudohyphal transition, all filamentation-related phenotypes except for abnormal budding patterns of ssn8Δ yeast strains are SET1-dependent (Table 3).
Synthetic pseudohyphal growth relies on the transcriptional function of SSN8
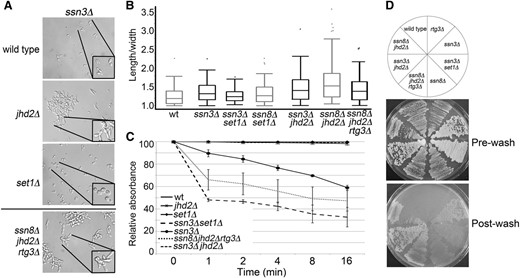
SSN8 encodes a cyclin (cyclin C, CNC1) whose gene product physically interacts with the cyclin-dependent kinase Ssn3p (Cdk8p) to regulate the expression of stress response genes (Carlson et al. 1984; Kuchin et al. 1995; Cooper et al. 1997; Liu et al. 2001; Cohen et al. 2008). In addition to its function as a transcriptional regulator, Ssn8p also plays a regulatory role in inducing mitochondrial fission upon H2O2 stress that is independent of Ssn3p (Cooper et al. 2014). Given the requirement of mitochondrial function for pseudohyphae formation (Starovoytova et al. 2013), we wanted to address if pseudohyphal growth in rich media observed in yeast lacking SSN8 and JHD2 is primarily attributed to the transcriptional or mitochondrial role of SSN8. Two independent approaches were used to test this. First, yeast mutants lacking SSN3/CDK8 in tandem with JHD2 or SET1 were constructed and their phenotypes were scored. Similar to our results observed in the SSN8/CNC1 yeast mutants, these yeast mutants displayed unipolar budding, increased cell-to-cell adhesion, elongated buds, and enhanced invasive growth (Figure 2, A–D, and Table 3). Interestingly, ssn3Δ mutant pseudohyphal phenotypes were dependent upon SET1, further supporting a genetic relationship between the CDK8 complex, H3 Lys4 methylation regulators, and pseudohyphal growth.
Phenotype analyses of yeast mutant discriminating between transcriptional and mitochondrial roles in synthetic pseudohyphal growth. (A) Bright-field microscopy. (B) Box-whisker plots showing length-to-width measurements. (C) Flocculation assays. (D) Invasive growth assays of yeast harboring the indicated mutations performed as described in Figure 1.
To determine if the synthetic pseudohyphal growth observed in ssn8Δjhd2Δ yeast mutants was dependent upon mitochondrial retrograde transport, yeast mutants lacking SSN8, JHD2, and RTG3 were constructed. RTG3 is required for expression of filamentation reporter genes and invasive growth in respiration-competent yeast (Chavel et al. 2010). Additionally, respiration competency is a prerequisite for filamentous growth via mechanisms that support a major role for retrograde transport genes (Lorenz et al. 2000; Kang and Jiang 2005; Jin et al. 2008; Chavel et al. 2010; Starovoytova et al. 2013). Removal of RTG3 in the ssn8Δjhd2Δ mutant background did not suppress any of the hallmarks of filamentous growth (Figure 2, A–D). This indicates that synthetic pseudohyphal growth occurs independently of mitochondrial retrograde transport, supporting a model in which the transcriptional function of SSN8/SSN3 is primarily responsible for inducing this dimorphic switch.
SSN8 and JHD2 are required for FLO11 transcriptional repression in rich media
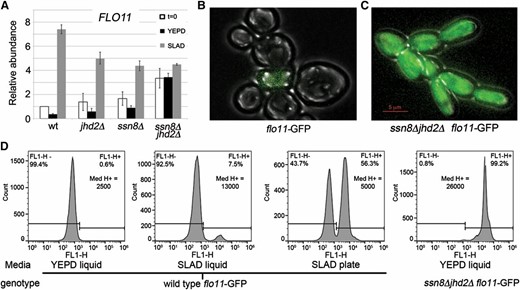
The experiments described above suggest that the pseudohyphal phenotype observed in ssn8Δjhd2Δ mutant yeast is due primarily to transcriptional defects. Previous work from multiple labs has identified FLO11 as a central hub that is necessary but not sufficient for the yeast morphological switch to pseudohyphal growth (Pan and Heitman 1999; Conlan and Tzamarias 2001; Reynolds and Fink 2001; Halme et al. 2004; Voordeckers et al. 2012). To determine if FLO11 mRNA is elevated in ssn8Δjhd2Δ mutant yeast, we performed RT-qPCR analysis in a time-course experiment. First, FLO11 transcriptional induction kinetics were determined in wild-type yeast. Yeast cultures were grown to mid-logarithmic phase in YEPD containing 0.5% peptone (t = 0) and then shifted to either rich (YEPD; 2% peptone) or liquid low-ammonia (SLAD) media to induce FLO11 transcription. Time points were taken at t = 0.5, 1, 2, and 4 hr postshift. These initial studies showed that maximal FLO11 induction in SLAD and repression in YEPD occurred at the 4-hr time point (Figure S2). Using this information, time-course experiments were performed comparing FLO11 mRNA expression in wild-type, jhd2Δ, ssn8Δ, and ssn8Δjhd2Δ mutant yeast. Wild-type and both single mutants displayed similar levels of FLO11 transcript at t = 0. Shifting these cultures to rich media resulted in FLO11 repression, while shifting to SLAD resulted in FLO11 induction (Figure 3A). Interestingly, yeast lacking both SSN8 and JHD2 display elevated FLO11 expression under all three growth conditions assayed (Figure 3A). This indicates that both SSN8 and JHD2 are required to repress FLO11 transcription in rich media.
FLO11 transcriptional regulation in wild-type and ssn8Δjhd2Δ yeast mutants. (A) SLAD time course measuring FLO11 mRNA levels using RT-qPCR. Wild-type and single or double SSN8 and JHD2 mutant yeast strains were grown as described in Materials and Methods. Average FLO11 expression normalized to NUP85 and wild-type levels at t = 0 for three independent biological replicates is reported; error bars represent SEM. (B and C) Representative flo11-GFP reporter expression for (B) wild-type yeast cultured in SLAD media for 5 hr or (C) ssn8Δjhd2Δ mutants cultured to mid-logarithmic phase in YEPD. (D) Flow cytometry data quantifying flo11-GFP reporter expression of wild-type or ssn8Δjhd2Δ mutants cultured in the indicated media. The percentages and median levels for GFP-positive signal were determined from 30,000 cells.
Since FLO11 transcription is variegated in a population of pseudohyphal yeast (Halme et al. 2004), we wanted to determine if ssn8Δjhd2Δ mutant yeast express FLO11 mRNA in every cell or in a subset of cells. Two independent models could be used to explain the observed increases in FLO11 mRNA levels. One model is that all yeast in the culture are expressing FLO11 mRNA at similar elevated levels, while an alternative to this is that FLO11 mRNA is increased dramatically in some cells in the population while absent in others. To differentiate between these possibilities, the FLO11 ORF was replaced with GFP in wild-type or ssn8Δjhd2Δ mutant yeast (Figure 3, B and C). A SLAD time-course experiment in wild-type strains was performed to examine flo11-GFP induction. Cell cytometry showed that 0.6% of wild-type cells express flo11-GFP in YEPD media. Shifting wild-type flo11-GFP cultures to SLAD media resulted in an ∼12-fold increase in the percentage of cells expressing the GFP reporter, consistent with our RT-qPCR data (Figure 3, A and C). While culturing yeast in SLAD liquid media can induce FLO11 expression, it fails to result in pseudohyphal growth. For this to occur, wild-type yeast must be grown on SLAD plates. To determine the percentage of cells expressing flo11-GFP during pseudohyphal induction, wild-type yeast containing the reporter were grown on SLAD plates for 4 days, and cytometry was performed. These assays showed that ∼56% of cells were now expressing the reporter gene. Both the percentage of GFP-expressing cells (∼99%) and the levels of GFP expressed in each cell were dramatically increased in the ssn8Δjhd2Δ mutant (GFP positive median signal = 1300 wild type SLAD liquid, = 5000 wild type SLAD plate, = 26,000 ssn8Δjhd2Δ YEPD liquid; Figure 3, B–D). This supports the idea that FLO11 transcription is locked into the “on” state, even while grown under repressive conditions. Interestingly, replacing the FLO11 ORF with GFP in the ssn8Δjhd2Δ yeast mutant did not inhibit synthetic pseudohyphal growth (Figure 3C). This suggests that other members of the FLO gene family may be compensating for the absence of FLO11, indicating that SSN8 and JHD2 may be responsible for repressing other genes related to pseudohyphal growth. Additionally, these data show that both SSN8 and JHD2 are required for the FLO11 repression in the total population of cells cultured in rich media.
SSN8 and JDH2 bypass the requirement for IME1 in pseudohyphal growth
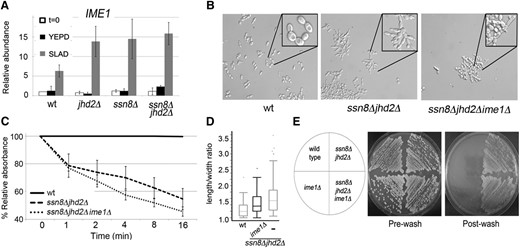
Recent reports using genome-wide approaches to investigate pseudohyphal differentiation have uncovered an important role for meiotic genes (Shively et al. 2013). In the SK1 yeast background, IME1, the master regulator of meiosis, is required for pseudohyphal growth on nonfermentable carbon sources (Kassir et al. 1988; Strudwick et al. 2010). Similar to FLO11 transcription, IME1 transcription is responsive to mating type, nitrogen, and carbon sources (Kassir et al. 1988, 2003). Due to these regulatory overlaps, we hypothesized that IME1 mRNA is upregulated in the ssn8Δjhd2Δ mutant yeast and that this upregulation stimulates FLO11 transcription. This hypothesis was tested in two ways. First, IME1 mRNA levels were determined using RT-qPCR in a SLAD time-course experiment. Unlike FLO11 transcriptional upregulation, IME1 expression profiles in wild type were similar to those measured in mutant yeast during the time course (Figure 4A). This indicates that SSN8 and JHD2 are not required to repress IME1 mRNA expression in rich media, supporting a model in which both SSN8 and JHD2 contribute to locus-specific transcriptional repression.
IME1 transcriptional regulation and ssn8Δjhd2Δime1Δ yeast mutant phenotypes. (A) SLAD time course of wild-type and single or double SSN8 and JHD2 yeast mutants measuring IME1 mRNA levels using RT-qPCR. Average IME1 expression is normalized to NUP85 and wild-type levels at t = 0 for three independent biological replicates. Error bars represent SEM. (B) Bright-field microscopy. (C) Flocculation time courses. (D) Length-to-width ratios. (E) Plate wash assays for the indicated mutants performed as in Figure 1.
Next the requirement for IME1 in synthetic pseudohyphal growth was tested by generating triple-mutant yeast that were deleted for SSN8, JHD2, and IME1. While this strain did exhibit decreased cell length-to-width ratios (Figure 4, B and D), both flocculation and invasive growth were unaffected (Figure 4, C and E). These data indicate that IME1 is not essential for all of the phenotypes associated with synthetic pseudohyphal induction and support negative control of FLO gene transcription by SSN8 and JHD2.
Psuedohyphal growth in ssn8Δjhd2Δ mutants requires a subset of the known FLO11 activators
Our data have shown that FLO11 transcriptional repression in rich media requires both SSN8 and JHD2. FLO11 transcriptional induction and pseudohyphal differentiation are dependent upon the integration of multiple signaling pathways including SNF, MAPK, cAMP/PKA, and TOR that converge upon the FLO11 promoter region to regulate transcription factor activity (reviewed in (Bruckner and Mosch 2012; Cullen and Sprague 2012). Together, these pathways combine to signal when yeast should enter the pseudohyphal differentiation pathway. To address which of these pathways are required for synthetic pseudohyphal cell divisions, we performed phenotypic analysis of yeast lacking components of each pathway in the ssn8Δjhd2Δ mutant yeast background.
First, a pathway that responds to low glucose and is required for invasive growth, adhesion to plastic, and pseudohyphal growth was examined by generating triple-null SNF1 yeast mutants (Cullen and Sprague 2000; Reynolds and Fink 2001). Since SSN8 was initially characterized as a suppressor of the SNF1 kinase, it is likely that deleting SSN8 may result in its hyperactivation (Carlson et al. 1984). Surprisingly, deleting SNF1 in the ssn8Δjhd2Δ mutant background did not affect flocculation rates, cell length-to-width ratio, unipolar budding, or invasive growth (Figure 5, A–E). It should be noted that the SNF1 triple-mutant yeast cells appear sick, which is also supported by increased doubling times (data not shown). Together, these data suggest that SNF1 is not essential for the synthetic pseudohyphal phenotype, indicating that SSN8 is acting independently of its previously described role as a suppressor of SNF.
Phenotype and FLO11 transcriptional analyses of synthetic pseudohyphal yeast mutants lacking transcriptional activators of pseudohyphal growth. (A–E) Phenotype analyses of synthetic pseudohyphal yeast mutants with each indicated triple deletion were analyzed as described in Figure 1. (A) Bright-field microscopy. (B) Flocculation time courses. (C) Length-to-width ratios. (D) Budding patterns. (E) Invasive growth assays. (F) Steady-state FLO11 mRNA measured by RT-qPCR of yeast harboring the indicated mutations. Expression levels were normalized to NUP85 and wild type; data show the average of three or four independent biological replicates; error bars depict SEM.
Next, the relationship between the MAPK-signaling pathway and the ssn8Δjhd2Δ mutant phenotypes was investigated. When yeast are cultured in media containing low nitrogen, the MAPK-signaling cascade is initiated, leading to activation of the filamentous growth MAPK, Kss1p (Cullen and Sprague 2012). Kss1p stimulates filamentous growth by phosphorylating and inactivating the inhibitors of FLO11 expression, Dig1p and Dig2p (Roberts and Fink 1994; Mosch et al. 1996; Cook et al. 1997). Dig1p and Dig2p inhibit FLO11 expression by interacting with and repressing the transcriptional activation complex Tec1p/Ste12p; both of these genes are required for pseudohyphal growth (Gavrias et al. 1996; Madhani and Fink 1997; Madhani et al. 1997; Bardwell et al. 1998; Kohler et al. 2002; Zeitlinger et al. 2003; Borneman et al. 2006; Chou et al. 2006; Borneman et al. 2007a,b). Additionally, previous work has shown that the Ssn8p/Ssn3p complex can inhibit the Tec1p/Ste12p heterodimer by phosphorylating Ste12p and triggering its degradation (Nelson et al. 2003). This work suggests that SSN8 mutant yeast may enhance pseudohyphal growth by acting through the Kss1p MAPK and/or Ste12p/Tec1p heterodimer. To test this possibility, triple-null yeast lacking SSN8, JHD2, and KSS1 or TEC1 were generated. Deleting KSS1 in ssn8Δjhd2Δ mutants resulted in partial suppression of pseudohyphal growth in rich media; yeast harboring this deletion had reduced, but not eliminated, flocs and partial suppression of increased cell length, but still displayed elevated unipolar budding patterns and invasive growth (Figure 5, A–E). These data show that KSS1 is required for some, but not all, of the ssn8Δjhd2Δ mutant pseudohyphal phenotype. This may be due to incomplete inactivation of the Dig1p and Dig2p transcriptional repressors in the KSS1 mutants.
We next assayed the impact of removing TEC1 from the synthetic pseudohyphal yeast strain. Strains harboring TEC1 deletions showed more exaggerated phenotypes than the KSS1 deletions with an almost complete suppression of elongated buds and flocculation (Figure 5, A–C). However, the ssn8Δjhd2Δtec1Δ yeast mutant still displayed increased unipolar budding and agar invasiveness (Figure 5, D and E). The maintenance of unipolar budding in the ssn8Δjhd2Δtec1Δ mutants supports previous work indicating that STE20 and not STE12 plays a critical role in bipolar budding of diploid cells (Sheu et al. 2000; Cullen and Sprague 2002). Importantly, the failure of TEC1 mutants to completely suppress synthetic pseudohyphal growth indicates that the ssn8Δjhd2Δ mutants are not acting solely through the upregulation of Ste12p.
Next the role of the nitrogen limitation response was assayed by examining a component of the TOR pathway, which helps to coordinate cellular response to limited nitrogen. TOR pathway activation signals to the transcription factor Gcn4p to induce filamentation and FLO11 transcription (Gimeno et al. 1992; Crespo and Hall 2002). In addition to its role in general nitrogen response, previous work has shown that Gcn4p can be activated during amino acid limitation even while in the presence of glucose and ammonium (Braus et al. 2003; Hinnebusch 2005; Kleinschmidt et al. 2005). Therefore, GCN4 plays important roles in FLO11 transcriptional induction by sensing both amino acid stress and nitrogen limitation. Analyzing ssn8Δjhd2Δgcn4Δ mutants revealed partial suppression of all of the hallmarks of pseudohyphal growth. While GCN4 was required for elongated, unipolar buds, it was only partially responsible for enhanced flocculation and invasive growth (Figure 5, A–E). This indicates that, unlike KSS1 and TEC1, which are essential for some, but not all, of the hallmarks of pseudohyphal growth, GCN4 is responsible for the maximal induction of all pseudohyphal phenotypes. Together, these different classes of partial suppressors reflect the complex genetic regulation of the pseudohyphal phenotype.
Finally, the role of the cAMP/PKA pathway in the ssn8Δjhd2Δ mutant phenotype was investigated by deleting the transcription factors FLO8, MSS11, and MFG1. The cAMP/PKA pathway is induced by low glucose and may also be regulated by low ammonia levels. The ammonia response requires the ammonium permease Mep2p, which is also required for filamentation (Lorenz and Heitman 1998; Van Nuland et al. 2006). Inhibition of the Bcy1p subunit by cAMP leads to activation of the protein kinase Tpk2p. Tpk2p can induce FLO11 expression in two ways; it positively regulates the transcriptional activator Flo8p and negatively regulates the transcriptional repressor Sfl1p (Robertson and Fink 1998; Pan and Heitman 2002). The Flo8p, Mss11p, and Mfg1p proteins form a complex that stimulates FLO11 transcription and pseudohyphal growth (Gagiano et al. 1999; Van Dyk et al. 2005; Ryan et al. 2012; Mayhew and Mitra 2014). The requirement of this complex for synthetic pseudohyphal growth was assayed by deleting each component individually in ssn8Δjhd2Δ mutant yeast. Deleting FLO8 and MSS11 in ssn8Δjhd2Δ mutants resulted in complete suppression of all pseudohyphal phenotypes; deleting MFG1 suppressed all phenotypes except for unipolar buds, which were reduced, but not eliminated, in ssn8Δjhd2Δmfg1Δ mutants (Figure 5, A–E). Given our previous results showing FLO11 independence for synthetic pseudohyphal growth (Figure 3C), these results also indicate that the other genes required for synthetic pseudohyphal growth are regulated by FLO8, MSS11, and MFG1, perhaps acting as a protein complex.
Transcription factor requirements for constitutive FLO11 expression
Our previous experimentation showed that ssn8Δjhd2Δ mutants bypass a subset of the transcriptional activators of FLO11 for some of the pseudohyphal phenotypes. In addition, we observed constitutive upregulation of FLO11 in ssn8Δjhd2Δ mutants independent of culture conditions. To determine if the suppression phenotypes result from repression of constitutive FLO11 expression, we assayed FLO11 mRNA levels in the triple-null yeast mutants discussed above. Yeast harboring triple deletions were cultured to mid-logarithmic phase in YEPD media. FLO11 expression was monitored in these cultures using RT-qPCR. IME1 was expendable for constitutive FLO11 activation, supporting our previous observations indicating that control of synthetic pseudohyphal growth by IME1 is bypassed (Figure 5F). These experiments showed an approximately twofold reduction in FLO11 mRNA levels in triple-null yeast mutants lacking RTG3, GCN4, KSS1, and SNF1, and an approximately threefold reduction in yeast lacking TEC1 (Figure 5F). These results are intriguing, given the results of our suppression of the pseudohyphal phenotypes in these yeast mutants. For example, SNF1 and RTG3 appear to be partially required for constitutive FLO11 transcription, but are mostly bypassed for the synthetic pseudohyphal phenotypes. The partial suppressors GCN4, KSS1, and TEC1 are also important for maintaining maximal FLO11 expression observed in ssn8Δjhd2Δ mutants. These results are in agreement with the partial suppression of pseudohyphal phenotypes observed in these triple-mutant yeast. Finally, FLO11 expression was completely abrogated in yeast lacking FLO8, MSS11, or MFG1, which again agrees well with the phenotypic data (Figure 5F). Together, these data show that, while FLO11 is not essential for the synthetic pseudohyphal phenotypes, its transcriptional regulators are important for both pseudohyphal induction and FLO11 constitutive expression. This is in support of previous work characterizing the complex genetic networks underpinning the pseudohyphal dimorphic switch.
Histone H3 Lys4 trimethylation is repressed by SSN8 independently of JHD2 at the FLO11 promoter
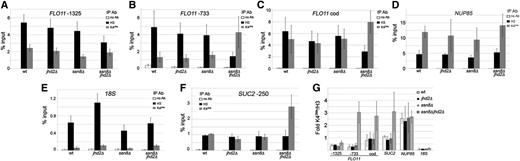
Since FLO11 transcription is constitutively activated in rich media in yeast mutants that lack both SSN8 and the histone H3 Lys4 demethylase JHD2, we wanted to understand if this transcriptional misregulation is the result of changes in the H3 Lys4 methylation levels at the FLO11 promoter. To test this possibility, ChIP was performed in wild-type or mutant mid-logarithmic yeast cultures lacking SSN8, JHD2, or both, using antibodies directed toward trimethylated histone H3 Lys4 or an antibody that recognizes the C-terminal domain of histone H3. Histone H3 Lys4 trimethylation was determined at four independent genomic loci, FLO11, SUC2, 18S, and NUP85. First, qPCR reactions were directed toward three regions of FLO11: two primer pairs were in the promoter, while one was in the coding region. Removal of either SSN8 or JHD2 did not significantly impact the levels of H3 Lys4 methylation at this locus relative to wild type (Figure 6, A–C and G). Interestingly, the levels of H3 Lys4 trimethylation were increased in yeast lacking both SSN8 and JHD2. This enrichment was present at the promoter sequence most proximal to the ATG (FLO11-733), but not observed in qPCR reactions directed toward a region located only ∼600 nucleotides upstream that encompasses the Flo8p binding site (Figure 6, A, B, and G). The enhanced methylation levels in the FLO11 promoter region observed in ssn8Δjhd2Δ mutants was extended into the FLO11-coding region, consistent with constitutive FLO11 expression in these mutants (Figure 6C). These increases were specific to the double-null yeast mutants, as each single mutation did not alter H3 Lys4 trimethylation.
Chromatin immunoprecipitations for H3 Lys4 trimethylation levels in SSN8 and JHD2 yeast mutants. (A–F) ChIP assays were performed on yeast harboring the indicated mutations grown to mid-logarithmic phase in rich media using antibodies directed toward the C-terminal domain of histone H3 or toward trimethylated histone H3 Lys4. No antibody (IgG alone) was used as a negative control. qPCR reactions were directed toward (A and B) FLO11 promoter (numbers relative to ATG), (C) the FLO11-coding region, (D) the NUP85-coding region, (E) the 18S rDNA locus, or (F) the SUC2 promoter. Enrichment values were calculated as the percentage input using a standard curve and represent the average for three independent biological repeats. Error bars show the standard deviation. (G) Ratio of trimethylated histone H3 Lys4 relative to histone H3 CTD. Error bars show the standard deviation.
Two independent models may explain the increased H3 Lys4 trimethylation levels at the FLO11 locus in ssn8Δjhd2Δ . First, these genes may be regulating histone methylation on a genome-wide basis. Alternatively, SSN8 and JHD2 may fine-tune locus-specific H3 Lys4 trimethylation levels. To discriminate between these two models, qPCR reactions were directed toward two control loci: the coding region of NUP85, a constitutively expressed gene that should contain high levels of H3 Lys4 trimethylation, or 18S rDNA, a genomic locus previously identified to contain low H3 Lys4 methylation levels (Bernstein et al. 2002). Unlike FLO11, H3 Lys4 trimethylation levels were similarly elevated at the NUP85 locus in wild-type and SSN8JHD2 single and double mutants (Figure 6, D and G). Contrary to this, H3Lys4 trimethylation was nearly absent at the 18S rDNA locus in all genotypes examined (Figure 6, E and G). These data suggest that SSN8 and JHD2 are not globally repressing histone H3 Lys4 trimethylation.
Finally, H3 Lys4 trimethylation levels were examined at the SUC2 promoter. SUC2 encodes a sucrose invertase that is not involved in pseudohyphal growth but is negatively regulated by SSN8 (Carlson et al. 1984). ChIP experiments using qPCR primers directed toward the SUC2 promoter revealed that H3 Lys4 trimethylation levels display a similar pattern to the FLO11 promoter (Figure 6, F and G). While deleting either SSN8 or JHD2 resulted in minor impacts on H3 Lys4 trimethylation, deleting both genes resulted in elevated H3 Lys4 trimethylation. These results, combined with the direct regulatory role for SSN8 at the SUC2 locus, are consistent with a model in which SSN8 represses locus-specific H3 Lys4 trimethylation independently of JHD2, raising the possibility that the CDK8 subcomplex may direct transcription by influencing chromatin modifications.
SSN8 and JHD2 repress H3 Lys4 trimethylation independently of FLO8
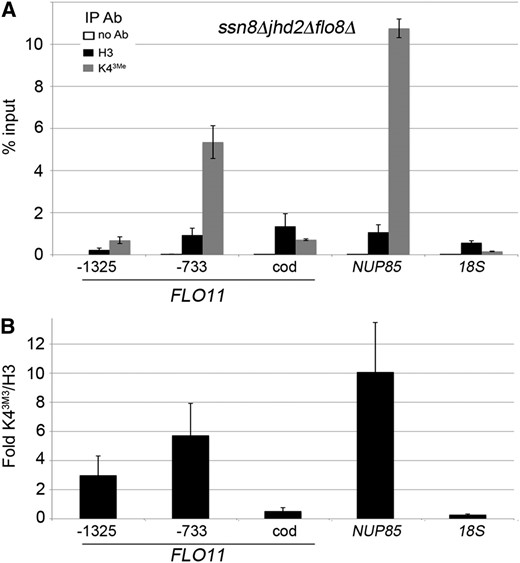
Our genetic analyses have revealed that ssn8Δjhd2Δ yeast mutants enter pseudohyphal growth in rich media and constitutively express FLO11 in a FLO8-, MSS11-, and MFG1-dependent manner. ChIP data show that yeast lacking both SSN8 and JHD2 display increased H3 Lys4 trimethylation levels at the FLO11 promoter relative to either single mutant or wild type. These results suggest that the Flo8p/Mss11p/Mfg1p complex may be recruiting COMPASS to FLO11, resulting in increases in H3 Lys4 trimethylation. To test this possibility, ChIP experiments were performed in ssn8Δjhd2Δflo8Δ yeast mutants using immunoprecipitations specific for histone H3 or H3 Lys4 trimethylation. These experiments revealed that SSN8 and JHD2 repress H3 Lys4 trimethylation at the FLO11 promoter independently of FLO8 (Figure 7A). Comparing the ChIP data from ssn8Δjhd2Δ and ssn8Δjhd2Δflo8Δ mutants revealed similarities and differences between the H3 Lys4 trimethylation patterns at the FLO11 locus. For example, primer pairs directed toward the upstream promoter region detected elevated H3 Lys4 trimethylation in ssn8Δjhd2Δflo8Δ yeast mutants, similar to those observed in ssn8Δjhd2Δ mutants (compare Figure 7, A and B, to Figure 6, B and G; FLO11-733 primers). Contrary to this, the elevated methylation observed in the FLO11-coding region in ssn8Δjhd2Δ mutants is dependent upon the presence of FLO8 (compare Figure 7, A and B, to Figure 6, C and G). These results are consistent with the requirement of FLO8 for the constitutive activation of FLO11; since H3 Lys4 trimethylation correlates with active transcription, loss of this transcription would result in losing elevated methylation. Interestingly, the opposite result is observed in qPCR primers that encompass the Flo8p-binding site (FLO11-1325). While ssn8Δjhd2Δ mutants display no change in methylation relative to wild type at this site, ssn8Δjhd2Δflo8Δ mutants show minor changes in the ratios of H3 Lys4 trimethylation relative to histone H3 (compare Figure 7B and Figure 6G), although the total enrichments are decreased. Importantly, the control qPCR reactions directed toward NUP85 and 18S rDNA display the same trends for H3 Lys4 trimethylation as all other genotypes (Figure 7, A and B). These data indicate that SSN8 and JHD2 repress H3 Lys4 trimethylation at the FLO11 promoter independently of FLO8 and support a direct regulation of histone methylation by SSN8. Additionally, they also show that FLO8 acts downstream of histone H3 Lys4 trimethylation in stimulating FLO11 transcriptional activation and pseudohyphal induction, similar to previous observations reporting this relationship between Flo8p and the Rpd3L HDAC complex (Bumgarner et al. 2009).
ChIP assays in ssn8Δjhd2Δflo8Δ yeast mutants (A) % inputs for ChIPs performed on ssn8Δjhd2Δflo8Δ yeast mutants grown to mid-logarithmic phase in rich media. qPCR reactions were directed at the indicated regions as described in Figure 6. Values represent the average for three independent biological replicates and error bars are standard deviations. (B) Fold H3 Lys4 trimethylation relative to total H3 from assays reported in (A). Error bars show the standard deviations.
Discussion
The results described here uncover a mechanism in which the CDK8 subcomplex of mediator may direct transcription by fine-tuning the H3 Lys4 trimethylation levels at specific loci. Our data indicate that H3 Lys4 methylation and the CDK8 subcomplex play important roles in this decision-making process. We have shown that both SSN8/SSN3 and JHD2 are required to inhibit filamentous growth under rich conditions. Epistasis analyses determined that constitutive FLO11 expression in yeast lacking both SSN8 and JHD2 requires FLO8, MSS11, and MFG1 and that other transcriptional activators for FLO11 including TEC1, SNF1, KSS1, GCN4, and RTG3 play a partial role in this process. Using ChIP, we found that SSN8 represses H3 Lys4 trimethylation independently of JHD2 at both the FLO11 and SUC2 loci, but not at the NUP85 and 18S rDNA control loci. These studies have uncovered a previously undescribed function for the CDK8 subcomplex in regulating locus-specific transcription through modulating histone H3 Lys4 methylation levels.
Since its identification, the Ssn8p/Ssn3p (Cnc1p/Cdk8p) protein complex has been characterized as both an activator and a repressor of gene transcription, with many molecular targets identified including pol II CTD, other mediator subunits, and transcription factors (Hengartner et al. 1995; Chi et al. 2001; Nelson et al. 2003; Raithatha et al. 2012; Nemet et al. 2014). Microarray studies of yeast lacking the SSN3 kinase showed that ∼40% of the 173 genes that were upregulated were also increased during diauxic shift (Holstege et al. 1998). Two of the top hits for gene upregulation in this study were the pseudohyphal regulatory genes FLO1 and FLO11. Our data show that ssn8Δjhd2Δ mutants can induce pseudohyphal growth independently of FLO11, but this induction requires the FLO8, MSS11, and MFG1 activators. Both FLO8 and MSS11 are also required for FLO1 transcription (Fichtner et al. 2007), making it likely that ssn8Δjhd2Δ mutants are upregulating this FLO gene family member as well. It is also possible that deleting SSN8 and thereby eliminating Ssn3p kinase activity has broad impacts on many pseudohyphal regulators. For example, phosphorylation of Ste12p, Gcn4p, Msn2p, and Phd1p stimulates their proteasome-dependent degradation (Chi et al. 2001; Nelson et al. 2003; Raithatha et al. 2012). All of these targets are known to stimulate FLO11 transcription, providing a mechanism in which SSN8 may negatively coregulate multiple transcription factors that all function in the same biological process. Interestingly, our qPCR experiments measuring steady-state FLO11 transcript levels show that deleting TEC1 (the binding partner for Ste12p) and GCN4 results in a partial reduction in FLO11 transcript levels. These results suggest that, while protein upregulation for these transcriptional activators is important for maximal FLO11 induction, they are not essential.
Our data support a model in which the Ssn8p/Ssn3p kinase complex regulates locus-specific H3 Lys4 methylation levels. A major question remains in how the Ssn8p/Ssn3p complex might be specifically targeting H3 Lys4 at FLO11 and SUC2, but not other loci. Previous work identified a physical interaction between the transcriptional repressor Sfl1p (Suppressor gene for flocculation) and Ssn8p (Song and Carlson 1998). Sfl1p and Flo8p antagonize each other to regulate FLO11 transcription (Halme et al. 2004; Bumgarner et al. 2009, 2012). Since FLO8 is also required for synthetic pseudohyphal growth (Figure 5), these data support a model in which Ssn8p may be recruited to the FLO11 promoter by physically interacting with Sfl1p. In support of this hypothesis, Sfl1p and Ssn8p physically interact at the SUC2 locus to inhibit its expression. Our ChIP experiments show that histone H3 Lys4 trimethylation at the SUC2 promoter is also repressed by SSN8 (Figure 6). This interaction would help to suppress aberrant FLO11 (or SUC2) transcription by inhibiting H3 Lys4 methylation. Deleting SSN8 would allow H3 Lys4 methylation, but in the presence of the demethylase Jhd2p these methylation marks would be quickly erased. In the absence of JHD2, enhanced methylation would be inhibited by the presence of Ssn8p. Therefore, we would observe increased H3 Lys4 methylation only in the absence of both SSN8 and JHD2, supporting a dual regulatory mechanism for H3 Lys4 methylation targeting.
Our data showing elevated H3 Lys4 trimethylation only in yeast lacking both SSN8 and JHD2 are intriguing (Figure 6). The observation that the CDK8 submodule may regulate transcription by fine-tuning H3 Lys4 methylation levels provides another example for the intricacies of transcriptional regulation. Given the aforementioned diverse targets already identified for Ssn3p, we provide multiple models to explain how SSN8 represses H3 Lys4 methylation. The Ssn8p/Ssn3p complex may impact Set1p function directly via phosphorylation. As has been shown for other examples, Set1p phosphorylation would be followed by protein ubiquitination and degradation. Therefore, locus-specific Ssn8p/Ssn3p presence would prevent H3 Lys4 methylation by stimulating Set1p turnover. An alternative model involves histone crosstalk mechanisms. H3 Lys4 trimethylation requires H2B Lys123 ubiquitination, leading to COMPASS complex recruitment and H3 Lys4 methylation (Wood et al. 2003b). Given the extensive crosstalk between histone amino acids, it remains possible that Ssn8p/Ssn3p kinase phosphorylates a histone amino acid, which in turn prevents H2B Lys123 ubiquitination and/or H3 Lys4 methylation. Removal of this kinase activity would then result in enhancement of H3 Lys4 methylation. Alternatively, H2B Lys123 ubiquitination may be inhibited by Ssn8p/Ssn3p phosphorylating the E3 ligase Bre1p. Another potential model involves the PAF1 complex, which interconnects Bre1p, COMPASS, and RNA pol II CTD (Ng et al. 2003b). In this model, protein phosphorylation would inhibit complex formation or activity, resulting in low H3 Lys4 methylation levels independent of JHD2. It will be interesting to uncover the mechanism by which SSN8 can inhibit H3 Lys4 trimethylation.
The evolutionary conservation of SET1, JHD2, SSN8 (CNC1), and SSN3 (CDK8) raises the possibility that their molecular function and regulation may also be conserved. For example, recent work in Candida albicans has shown that both SSN8 and SSN3 are required for biofilm formation in the presence of the biofilm inhibitor PYO (Lindsay et al. 2014). Additionally, CDK8 knockout mice are embryonic lethal at embryonic day 2.5, which is prior to compaction and implantation (Westerling et al. 2007). This suggests that mammalian CDK8 is critical for cell-fate determination during early embryogenesis. Further evidence for the crucial role played by CNC1 and CDK8 in cell-fate decisions comes from studies on cancer, where genetic evidence has shown that both genes are misregulated. Somewhat paradoxically, CDK8 is found upregulated in colon cancers and melanoma, but is also reduced, mutated, or deleted in diverse cancer types, including esophageal squamous cell carcinoma and bladder cancers (Mitra et al. 2006; Greenman et al. 2007; Firestein et al. 2008; Chattopadhyay et al. 2010; Kapoor et al. 2010). Similarly, CNC1 is upregulated in many cancers including colorectal cancer, lymphoblastic leukemia, and adenocarcinoma, but is frequently deleted in osteosarcomas and gastric cancers (reviewed in Xu and Ji 2011). These studies have suggested that both CDK8 and CNC1 play critical roles during oncogenesis, but the in vivo role for these genes remains elusive. How could amplifications and deletions of the same target genes lead to similar cell-fate decisions and cancer development? Our data indicate that these genes are important for repressing H3 Lys4 trimethylation at specific loci. Therefore, crippling CNC1 or CDK8 may lead to increased H3 Lys4 methylation levels at targeted loci, while overexpressing CNC1 or CDK8 may lead to decreased methylation at other loci. In support of this, locus-specific regulation of H3 Lys4 methylation is critical for cell-fate decisions. For example, misregulating the writing, erasing, or reading of H3 Lys4 methylation has been linked to multiple types of cancers including myeloid and lymphoid leukemias (reviewed in Chi et al. 2010). Therefore, future studies investigating the evolutionary conservation of CNC1 and CDK8 in regulating histone H3 Lys4 methylation will be of critical importance.
Acknowledgments
The authors thank Eric G. Moss and Michael F. Henry for careful reading of the manuscript; Randy Strich and Katrina F. Cooper for reagents; Randy Strich for stimulating conversations; and Sarah Hatton, Joseph P. Sheehan, and Stephen K. Kim for providing technical assistance. Funding for this project was provided by New Jersey Health Foundation grant #PC 79-15.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.172841/-/DC1.
Communicating editor: M. Freitag
Literature Cited
Morohashi, N., A. P. Mitchell, and M. Shimizu, 2005 Effect of histone methyltransferase gene mutations on sporulation in S. cerevisiae. Nucleic Acids Symp. Ser. (Oxf.) 49: 325–326.