-
PDF
- Split View
-
Views
-
Cite
Cite
Olga Puchta, Michal Lubas, Kamil A Lipinski, Jakub Piatkowski, Michal Malecki, Pawel Golik, DMR1 (CCM1/YGR150C) of Saccharomyces cerevisiae Encodes an RNA-Binding Protein from the Pentatricopeptide Repeat Family Required for the Maintenance of the Mitochondrial 15S Ribosomal RNA, Genetics, Volume 184, Issue 4, 1 April 2010, Pages 959–973, https://doi.org/10.1534/genetics.110.113969
Close - Share Icon Share
Abstract
Pentatricopeptide repeat (PPR) proteins form the largest known RNA-binding protein family and are found in all eukaryotes, being particularly abundant in higher plants. PPR proteins localize mostly in mitochondria and chloroplasts, where they modulate organellar genome expression on the post-transcriptional level. The Saccharomyces cerevisiae DMR1 (CCM1, YGR150C) encodes a PPR protein that localizes to mitochondria. Deletion of DMR1 results in a complete and irreversible loss of respiratory capacity and loss of wild-type mtDNA by conversion to ρ−/ρ0 petites, regardless of the presence of introns in mtDNA. The phenotype of the dmr1Δ mitochondria is characterized by fragmentation of the small subunit mitochondrial rRNA (15S rRNA), that can be reversed by wild-type Dmr1p. Other mitochondrial transcripts, including the large subunit mitochondrial rRNA (21S rRNA), are not affected by the lack of Dmr1p. The purified Dmr1 protein specifically binds to different regions of 15S rRNA in vitro, consistent with the deletion phenotype. Dmr1p is therefore the first yeast PPR protein, which has an rRNA target and is probably involved in the biogenesis of mitochondrial ribosomes and translation.
PROGRESS in plant genomics, particularly sequencing of the Arabidopsis thaliana genome, led to the discovery of a large protein family characterized by tandem repeats of a degenerate 35-amino-acid motif and thus named the pentatricopeptide repeat (PPR) family (Small and Peeters 2000; Lurin et al. 2004). This family underwent a striking expansion in terrestrial plants, with typical genomes encoding several hundred PPR proteins; their presence is, however, a common feature of all eukaryotic lineages, including protists, metazoa, and fungi. The number of confirmed PPR proteins encoded by nonplant genomes ranges from ∼30 in protists to 7 in humans and 3 in Saccharomyces cerevisiae (Lightowlers and Chrzanowska-Lightowlers 2008; Schmitz-Linneweber and Sluyter 2008; Davies et al. 2009); it should be noted, however, that due to the degenerate nature of the pentatricopeptide motif, these numbers may not be exact.
Two common features of the PPR family are the organellar localization and RNA binding. About 50% of the A. thaliana PPR proteins are predicted to be targeted to mitochondria, and 25% to chloroplasts using in silico methods (Lurin et al. 2004). All the seven human PPR proteins are predicted to be mitochondrial, and functional analysis confirms their role in the expression of the mitochondrial genome (Lightowlers and Chrzanowska-Lightowlers 2008; Davies et al. 2009). Three yeast proteins that can be unambiguously assigned to the PPR family: Pet309p (Manthey and McEwen 1995; Perez-Martinez et al. 2003; Tavares-Carreon et al. 2008), Aep3p (Ellis et al. 2004), and Dmr1p also have mitochondrial localization and function. Involvement in mitochondrial genome expression was also confirmed for PPR proteins in Trypanosoma (Pusnik et al. 2007). In contrast, cytoplasmic (Mancebo et al. 2001) or nuclear (Ding et al. 2006) localization of PPR proteins was found only in a few cases. The vast majority of PPR proteins are therefore targeted to mitochondria and/or chloroplasts.
Structural predictions as well as available genetic and biochemical data suggest that the PPR proteins bind RNA and participate in various post-transcriptional steps of organellar gene expression (Andres et al. 2007; Delannoy et al. 2007; Pusnik et al. 2007; Lightowlers and Chrzanowska-Lightowlers 2008; Schmitz-Linneweber and Sluyter 2008). The PPR motif is structurally (and presumably also phylogenetically) related to the tetratricopeptide repeat (TPR) motif, which mediates protein–protein interactions (Blatch and Lassle 1999). A single PPR motif consists of a pair of antiparallel α-helices (A and B) forming in tandem arrays a superhelix that encloses a central groove—a putative ligand-binding site. While in TPR motifs the residues projecting into the groove vary considerably (Das et al. 1998), the side chains lining the central groove in PPR motifs are clearly hydrophilic and form a positively charged surface (Small and Peeters 2000). Thus, in contrast to the TPR motifs, which are responsible for protein–protein interactions, it was proposed that the PPR motifs could constitute a novel RNA-binding domain (Small and Peeters 2000; Lurin et al. 2004).
All PPR proteins are presumed to bind RNA, but with the possible exception of certain P-subfamily and combinatorial and modular protein (PCMP) subfamily members (found only in plants) containing the DYW domain (Nakamura and Sugita 2008), they do not exhibit any enzymatic activities. They are therefore described as “adaptor proteins” or mediators of protein-RNA interactions at various stages of organellar gene expression (Lurin et al. 2004; Schmitz-Linneweber and Sluyter 2008). Both mRNAs and noncoding transcripts, such as ribosomal RNAs were found among their targets, and they were implicated in processes such as RNA editing, cleavage, degradation, stability, translation, splicing, and transcription (Andres et al. 2007; Delannoy et al. 2007; Pusnik et al. 2007; Lightowlers and Chrzanowska-Lightowlers 2008; Schmitz-Linneweber and Sluyter 2008). Little is known, however, about the mechanistic aspects of substrate binding and activity of PPR proteins. In spite of a rapid progress in understanding the functions of PPR proteins at the molecular level, the basis for RNA recognition by these proteins remains obscure and no common features in sequence motifs or secondary structures have been identified (Schmitz-Linneweber and Sluyter 2008).
Of the three bona fide PPR proteins of yeast S. cerevisiae detected using available bioinformatic tools (Karpenahalli et al. 2007), two are involved in the expression of specific mRNAs. The Pet309 protein (Manthey and McEwen 1995; Perez-Martinez et al. 2003; Tavares-Carreon et al. 2008) is involved in the processing, stability, and translation of COX1 mRNA, while Aep3p (Ellis et al. 2004) is involved in maintaining the stability of the ATP6/8 bicistronic mRNA. The third yeast PPR protein, encoded by the YGR150C ORF, which is the subject of this study, is also found in the proteome of mitochondria (Reinders et al. 2006), and a mitochondrial localization (Huh et al. 2003) and respiratory deficient phenotype were reported in high-throughput studies (Steinmetz et al. 2002; Merz and Westermann 2009) and mentioned by other investigators (Ellis et al. 2004; Moreno et al. 2009). The exact nature of its involvement in the mitochondrial function, particularly its RNA target, however, was unknown until now.
MATERIALS AND METHODS
Strains, media, and yeast techniques:
The S. cerevisiae strains used in this study are listed in Table 1. Standard yeast media and basic genetic methods were as described previously (Dujardin et al. 1980; Burke et al. 2000). Yeasts were transformed using either the rapid or high-efficiency LiAc/SS-DNA/PEG protocol (Gietz and Woods 2002).
S. cerevisiae strains used in this study
. | Genotype . | . | |
|---|---|---|---|
| Name . | Nuclear . | Mitochondrial . | Origin . |
| CW04 | MATα, ade2; trp1; ura3; leu2; his3 | ρ+, 13 intronsa | Chiron et al. (2005) |
| CW252 | MATα, ade2; trp1; ura3; leu2; his3 | ρ+, intronlessb | Saint-Georges et al. (2002) |
| KL14-4A/60 | MATa, his1; trp2 | ρ0 | Groudinsky et al. (1981) |
| Y14780 | MATα, his3; leu2; lys2; ura3; dmr1∷kanMX4 | ρ−/ρ0 | EUROSCARF |
| DPPR1 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ−/ρ0 | This work |
| DPPR2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ−/ρ0 | This work |
| JC8/55 | MATa, Kar1-1; leu1 | ρ0 | Conde and Fink (1976) |
| W303/1B/60 | MATα, ade2; trp1; ura3; leu2; his3 | ρ0 | Bonnefoy and Fox (2002) |
| CK15S | MATa, Kar1-1; leu1 | ρ− [15S rRNA] | This work |
| CW15S | MATα, ade2; trp1; ura3; leu2; his3 | ρ− [15S rRNA] | This work |
| DPPR2/15S-3 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA] | This work |
| DPPR2/15S-7 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA]c | This work |
| DPPR2/15S-24 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA] | This work |
| DPPR2/21S-1 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [21S rRNA] | This work |
| DPPR2/21S-2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [21S rRNA] | This work |
| DPPR2/21S-COX2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [COX2] | This work |
| Y11793 | MATα, his3; leu2; lys2; ura3; pet127∷kanMX4 | ρ+ | EUROSCARF |
| DPET1 | MATα, his3; leu2; lys2; ura3; pet127∷hphMX4 | ρ+ | This work |
| DPET2 | MATα, ade2; trp1; ura3; leu2; his3; pet127∷hphMX4 | ρ+, intronlessb | This work |
| DPETCW15S | MATα, ade2; trp1; ura3; leu2; his3; pet127∷hphMX4 | ρ− [15S rRNA]c | This work |
| DDP/15S-7 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4; pet127∷hphMX4 | ρ− [15S rRNA]c | This work |
| YSC1178-7500684 | MATa, his3; leu2; met15; ura3; DMR1-TAP | ρ+ | Open Biosystems, Ghaemmaghami (2003) |
| BY4742 | MATα, his3; leu2; lys2; ura3; pet127∷hphMX4 | ρ+ | Brachmann (1998) |
. | Genotype . | . | |
|---|---|---|---|
| Name . | Nuclear . | Mitochondrial . | Origin . |
| CW04 | MATα, ade2; trp1; ura3; leu2; his3 | ρ+, 13 intronsa | Chiron et al. (2005) |
| CW252 | MATα, ade2; trp1; ura3; leu2; his3 | ρ+, intronlessb | Saint-Georges et al. (2002) |
| KL14-4A/60 | MATa, his1; trp2 | ρ0 | Groudinsky et al. (1981) |
| Y14780 | MATα, his3; leu2; lys2; ura3; dmr1∷kanMX4 | ρ−/ρ0 | EUROSCARF |
| DPPR1 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ−/ρ0 | This work |
| DPPR2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ−/ρ0 | This work |
| JC8/55 | MATa, Kar1-1; leu1 | ρ0 | Conde and Fink (1976) |
| W303/1B/60 | MATα, ade2; trp1; ura3; leu2; his3 | ρ0 | Bonnefoy and Fox (2002) |
| CK15S | MATa, Kar1-1; leu1 | ρ− [15S rRNA] | This work |
| CW15S | MATα, ade2; trp1; ura3; leu2; his3 | ρ− [15S rRNA] | This work |
| DPPR2/15S-3 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA] | This work |
| DPPR2/15S-7 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA]c | This work |
| DPPR2/15S-24 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA] | This work |
| DPPR2/21S-1 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [21S rRNA] | This work |
| DPPR2/21S-2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [21S rRNA] | This work |
| DPPR2/21S-COX2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [COX2] | This work |
| Y11793 | MATα, his3; leu2; lys2; ura3; pet127∷kanMX4 | ρ+ | EUROSCARF |
| DPET1 | MATα, his3; leu2; lys2; ura3; pet127∷hphMX4 | ρ+ | This work |
| DPET2 | MATα, ade2; trp1; ura3; leu2; his3; pet127∷hphMX4 | ρ+, intronlessb | This work |
| DPETCW15S | MATα, ade2; trp1; ura3; leu2; his3; pet127∷hphMX4 | ρ− [15S rRNA]c | This work |
| DDP/15S-7 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4; pet127∷hphMX4 | ρ− [15S rRNA]c | This work |
| YSC1178-7500684 | MATa, his3; leu2; met15; ura3; DMR1-TAP | ρ+ | Open Biosystems, Ghaemmaghami (2003) |
| BY4742 | MATα, his3; leu2; lys2; ura3; pet127∷hphMX4 | ρ+ | Brachmann (1998) |
EUROSCARF, EUROpean Saccharomyces Cerevisiae ARchive for Functional Analysis.
Isomitochondrial to the 777-3A strain.
Has the wild-type “corrected” 252G allele of COB, unlike the original intronless mtDNA (Seraphin et al. 1987), which carries a hypomorphic 252D allele.
Isomitochondrial ρ− clones.
S. cerevisiae strains used in this study
. | Genotype . | . | |
|---|---|---|---|
| Name . | Nuclear . | Mitochondrial . | Origin . |
| CW04 | MATα, ade2; trp1; ura3; leu2; his3 | ρ+, 13 intronsa | Chiron et al. (2005) |
| CW252 | MATα, ade2; trp1; ura3; leu2; his3 | ρ+, intronlessb | Saint-Georges et al. (2002) |
| KL14-4A/60 | MATa, his1; trp2 | ρ0 | Groudinsky et al. (1981) |
| Y14780 | MATα, his3; leu2; lys2; ura3; dmr1∷kanMX4 | ρ−/ρ0 | EUROSCARF |
| DPPR1 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ−/ρ0 | This work |
| DPPR2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ−/ρ0 | This work |
| JC8/55 | MATa, Kar1-1; leu1 | ρ0 | Conde and Fink (1976) |
| W303/1B/60 | MATα, ade2; trp1; ura3; leu2; his3 | ρ0 | Bonnefoy and Fox (2002) |
| CK15S | MATa, Kar1-1; leu1 | ρ− [15S rRNA] | This work |
| CW15S | MATα, ade2; trp1; ura3; leu2; his3 | ρ− [15S rRNA] | This work |
| DPPR2/15S-3 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA] | This work |
| DPPR2/15S-7 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA]c | This work |
| DPPR2/15S-24 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA] | This work |
| DPPR2/21S-1 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [21S rRNA] | This work |
| DPPR2/21S-2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [21S rRNA] | This work |
| DPPR2/21S-COX2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [COX2] | This work |
| Y11793 | MATα, his3; leu2; lys2; ura3; pet127∷kanMX4 | ρ+ | EUROSCARF |
| DPET1 | MATα, his3; leu2; lys2; ura3; pet127∷hphMX4 | ρ+ | This work |
| DPET2 | MATα, ade2; trp1; ura3; leu2; his3; pet127∷hphMX4 | ρ+, intronlessb | This work |
| DPETCW15S | MATα, ade2; trp1; ura3; leu2; his3; pet127∷hphMX4 | ρ− [15S rRNA]c | This work |
| DDP/15S-7 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4; pet127∷hphMX4 | ρ− [15S rRNA]c | This work |
| YSC1178-7500684 | MATa, his3; leu2; met15; ura3; DMR1-TAP | ρ+ | Open Biosystems, Ghaemmaghami (2003) |
| BY4742 | MATα, his3; leu2; lys2; ura3; pet127∷hphMX4 | ρ+ | Brachmann (1998) |
. | Genotype . | . | |
|---|---|---|---|
| Name . | Nuclear . | Mitochondrial . | Origin . |
| CW04 | MATα, ade2; trp1; ura3; leu2; his3 | ρ+, 13 intronsa | Chiron et al. (2005) |
| CW252 | MATα, ade2; trp1; ura3; leu2; his3 | ρ+, intronlessb | Saint-Georges et al. (2002) |
| KL14-4A/60 | MATa, his1; trp2 | ρ0 | Groudinsky et al. (1981) |
| Y14780 | MATα, his3; leu2; lys2; ura3; dmr1∷kanMX4 | ρ−/ρ0 | EUROSCARF |
| DPPR1 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ−/ρ0 | This work |
| DPPR2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ−/ρ0 | This work |
| JC8/55 | MATa, Kar1-1; leu1 | ρ0 | Conde and Fink (1976) |
| W303/1B/60 | MATα, ade2; trp1; ura3; leu2; his3 | ρ0 | Bonnefoy and Fox (2002) |
| CK15S | MATa, Kar1-1; leu1 | ρ− [15S rRNA] | This work |
| CW15S | MATα, ade2; trp1; ura3; leu2; his3 | ρ− [15S rRNA] | This work |
| DPPR2/15S-3 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA] | This work |
| DPPR2/15S-7 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA]c | This work |
| DPPR2/15S-24 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [15S rRNA] | This work |
| DPPR2/21S-1 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [21S rRNA] | This work |
| DPPR2/21S-2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [21S rRNA] | This work |
| DPPR2/21S-COX2 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4 | ρ− [COX2] | This work |
| Y11793 | MATα, his3; leu2; lys2; ura3; pet127∷kanMX4 | ρ+ | EUROSCARF |
| DPET1 | MATα, his3; leu2; lys2; ura3; pet127∷hphMX4 | ρ+ | This work |
| DPET2 | MATα, ade2; trp1; ura3; leu2; his3; pet127∷hphMX4 | ρ+, intronlessb | This work |
| DPETCW15S | MATα, ade2; trp1; ura3; leu2; his3; pet127∷hphMX4 | ρ− [15S rRNA]c | This work |
| DDP/15S-7 | MATα, ade2; trp1; ura3; leu2; his3; dmr1∷kanMX4; pet127∷hphMX4 | ρ− [15S rRNA]c | This work |
| YSC1178-7500684 | MATa, his3; leu2; met15; ura3; DMR1-TAP | ρ+ | Open Biosystems, Ghaemmaghami (2003) |
| BY4742 | MATα, his3; leu2; lys2; ura3; pet127∷hphMX4 | ρ+ | Brachmann (1998) |
EUROSCARF, EUROpean Saccharomyces Cerevisiae ARchive for Functional Analysis.
Isomitochondrial to the 777-3A strain.
Has the wild-type “corrected” 252G allele of COB, unlike the original intronless mtDNA (Seraphin et al. 1987), which carries a hypomorphic 252D allele.
Isomitochondrial ρ− clones.
The dmr1Δ strains were constructed by replacing the entire YGR150C ORF with the KanMX4 selection module. We used DNA from the strain Y14780 originating from the Saccharomyces Genome Deletion Project (Winzeler et al. 1999; Giaever et al. 2002), which harbors the YGR150C deletion, as a template for PCR using the YGR150C_A (see Table 2 for all oligonucleotide sequences) and YGR150C_D primers. This gave a product containing the KanMX4 module with yeast genomic flanks upstream from the ATG and downstream from the termination codon of YGR150C. This deletion cassette was then used to transform the CW04 and CW252 strains, which provide isogenic haploid W303 nuclear genotype, with the “long” mtDNA containing 13 introns, and intronless mtDNA, respectively. The resulting dmr1Δ strains were named DPPR1 and DPPR2, respectively.
Oligonucleotides used in this study
Name . | Sequence (5′ to 3′) . |
|---|---|
| YGR150C_A | CTTCCTTGTCGCACATTATCTTACT |
| YGR150C_D | TACCTACTATATCGACCACTACGGG |
| PET127_L | |
| PET127_R | |
| DMR1_LBam | ATGGATCCTTCCTTGTCGCACATTATCTTACT |
| DMR1_RPSt | ATCTGCAGTACCTACTATATCGACCACTACGGG |
| 15S_3ter | TATAAGCCCACCGCAGGTTCCCCTACGGTAACTGTA |
| RT | CATGCTCCACTGCTTAAGTC |
| L1 | CGCACTAATCACTCATCAC |
| L2 | AACTGTTTCGCACTAATCAC |
| R2 | GCTAACGTACTCTTCAGGTG |
| yDmr1_N1 | GAGAACCTGTACTTCCAGGGTCTAATAAATAAGCGTTTCAAGT |
| yDMR1_C | GGGGACCACTTTGTACAAGAAAGCTGGGTTATTACATGTTAAGTTCTTGTTCCT |
| N2 | GGGGACAAGTTTGTACAAAAAAGCAGGCTCGGAGAACCTGTACTTCCAG |
| 15S1L | TAATACGACTCACTATAGGAATTTATAAGAATATGATGTTGGTTCAG |
| 15S1R | AAACCATTATGATTAACGCTC |
| 15S2L | TAATACGACTCACTATAGGGAGACGGTAACACAAAGAGGG |
| 15S2R | GAACTAAAGACAACAATGTAACG |
| 15S3L | TAATACGACTCACTATAGGGTTGTCTTTAGTTCGTGCTG |
| 15S3R | TGTAAGAATATTTAAGATATTTATAAGCCCAC |
| 21S1L | GATGCATAATACGACTCACTATAGGTAAAAAGTAGAATAATAGATTTG |
| 21S1R | TTGCTGACCCATTATAC |
| 21S2L | TAATACGACTCACTATAGGAGTAACTGTGCGATAATTGTAAC |
| 21S2R | TTATCAACTTAGCTTATCTACTATG |
Name . | Sequence (5′ to 3′) . |
|---|---|
| YGR150C_A | CTTCCTTGTCGCACATTATCTTACT |
| YGR150C_D | TACCTACTATATCGACCACTACGGG |
| PET127_L | |
| PET127_R | |
| DMR1_LBam | ATGGATCCTTCCTTGTCGCACATTATCTTACT |
| DMR1_RPSt | ATCTGCAGTACCTACTATATCGACCACTACGGG |
| 15S_3ter | TATAAGCCCACCGCAGGTTCCCCTACGGTAACTGTA |
| RT | CATGCTCCACTGCTTAAGTC |
| L1 | CGCACTAATCACTCATCAC |
| L2 | AACTGTTTCGCACTAATCAC |
| R2 | GCTAACGTACTCTTCAGGTG |
| yDmr1_N1 | GAGAACCTGTACTTCCAGGGTCTAATAAATAAGCGTTTCAAGT |
| yDMR1_C | GGGGACCACTTTGTACAAGAAAGCTGGGTTATTACATGTTAAGTTCTTGTTCCT |
| N2 | GGGGACAAGTTTGTACAAAAAAGCAGGCTCGGAGAACCTGTACTTCCAG |
| 15S1L | TAATACGACTCACTATAGGAATTTATAAGAATATGATGTTGGTTCAG |
| 15S1R | AAACCATTATGATTAACGCTC |
| 15S2L | TAATACGACTCACTATAGGGAGACGGTAACACAAAGAGGG |
| 15S2R | GAACTAAAGACAACAATGTAACG |
| 15S3L | TAATACGACTCACTATAGGGTTGTCTTTAGTTCGTGCTG |
| 15S3R | TGTAAGAATATTTAAGATATTTATAAGCCCAC |
| 21S1L | GATGCATAATACGACTCACTATAGGTAAAAAGTAGAATAATAGATTTG |
| 21S1R | TTGCTGACCCATTATAC |
| 21S2L | TAATACGACTCACTATAGGAGTAACTGTGCGATAATTGTAAC |
| 21S2R | TTATCAACTTAGCTTATCTACTATG |
Oligonucleotides used in this study
Name . | Sequence (5′ to 3′) . |
|---|---|
| YGR150C_A | CTTCCTTGTCGCACATTATCTTACT |
| YGR150C_D | TACCTACTATATCGACCACTACGGG |
| PET127_L | |
| PET127_R | |
| DMR1_LBam | ATGGATCCTTCCTTGTCGCACATTATCTTACT |
| DMR1_RPSt | ATCTGCAGTACCTACTATATCGACCACTACGGG |
| 15S_3ter | TATAAGCCCACCGCAGGTTCCCCTACGGTAACTGTA |
| RT | CATGCTCCACTGCTTAAGTC |
| L1 | CGCACTAATCACTCATCAC |
| L2 | AACTGTTTCGCACTAATCAC |
| R2 | GCTAACGTACTCTTCAGGTG |
| yDmr1_N1 | GAGAACCTGTACTTCCAGGGTCTAATAAATAAGCGTTTCAAGT |
| yDMR1_C | GGGGACCACTTTGTACAAGAAAGCTGGGTTATTACATGTTAAGTTCTTGTTCCT |
| N2 | GGGGACAAGTTTGTACAAAAAAGCAGGCTCGGAGAACCTGTACTTCCAG |
| 15S1L | TAATACGACTCACTATAGGAATTTATAAGAATATGATGTTGGTTCAG |
| 15S1R | AAACCATTATGATTAACGCTC |
| 15S2L | TAATACGACTCACTATAGGGAGACGGTAACACAAAGAGGG |
| 15S2R | GAACTAAAGACAACAATGTAACG |
| 15S3L | TAATACGACTCACTATAGGGTTGTCTTTAGTTCGTGCTG |
| 15S3R | TGTAAGAATATTTAAGATATTTATAAGCCCAC |
| 21S1L | GATGCATAATACGACTCACTATAGGTAAAAAGTAGAATAATAGATTTG |
| 21S1R | TTGCTGACCCATTATAC |
| 21S2L | TAATACGACTCACTATAGGAGTAACTGTGCGATAATTGTAAC |
| 21S2R | TTATCAACTTAGCTTATCTACTATG |
Name . | Sequence (5′ to 3′) . |
|---|---|
| YGR150C_A | CTTCCTTGTCGCACATTATCTTACT |
| YGR150C_D | TACCTACTATATCGACCACTACGGG |
| PET127_L | |
| PET127_R | |
| DMR1_LBam | ATGGATCCTTCCTTGTCGCACATTATCTTACT |
| DMR1_RPSt | ATCTGCAGTACCTACTATATCGACCACTACGGG |
| 15S_3ter | TATAAGCCCACCGCAGGTTCCCCTACGGTAACTGTA |
| RT | CATGCTCCACTGCTTAAGTC |
| L1 | CGCACTAATCACTCATCAC |
| L2 | AACTGTTTCGCACTAATCAC |
| R2 | GCTAACGTACTCTTCAGGTG |
| yDmr1_N1 | GAGAACCTGTACTTCCAGGGTCTAATAAATAAGCGTTTCAAGT |
| yDMR1_C | GGGGACCACTTTGTACAAGAAAGCTGGGTTATTACATGTTAAGTTCTTGTTCCT |
| N2 | GGGGACAAGTTTGTACAAAAAAGCAGGCTCGGAGAACCTGTACTTCCAG |
| 15S1L | TAATACGACTCACTATAGGAATTTATAAGAATATGATGTTGGTTCAG |
| 15S1R | AAACCATTATGATTAACGCTC |
| 15S2L | TAATACGACTCACTATAGGGAGACGGTAACACAAAGAGGG |
| 15S2R | GAACTAAAGACAACAATGTAACG |
| 15S3L | TAATACGACTCACTATAGGGTTGTCTTTAGTTCGTGCTG |
| 15S3R | TGTAAGAATATTTAAGATATTTATAAGCCCAC |
| 21S1L | GATGCATAATACGACTCACTATAGGTAAAAAGTAGAATAATAGATTTG |
| 21S1R | TTGCTGACCCATTATAC |
| 21S2L | TAATACGACTCACTATAGGAGTAACTGTGCGATAATTGTAAC |
| 21S2R | TTATCAACTTAGCTTATCTACTATG |
The presence of functional ρ+ mtDNA was tested by crossing to the ρ0 tester strain KL14-4A/60, selection of diploids on minimal media, and testing the obtained heterozygous diploids on respiratory medium (YPG).
As the phenotype of severe respiratory deficiency coupled with the loss of any functional ρ+ mtDNA was identical in both deletant strains, DPPR2 was used as the dmr1Δ strain in all the subsequent experiments.
The CW15S control strain was constructed in a series of cytoductions, first from the DPPR2/15S-7 ρ− into JC8/55, giving CK15S, and then from CK15S into W303/1B/60, giving CW15S, which carries the ρ− mtDNA encoding the 15S rRNA in a wild-type W303 nuclear background. The ρ− cytoductants containing the 15S rRNA gene were distinguished from the ρ0 parent by PCR using primers 15S2L and 15S2R (see below).
The pet127Δ strain DPET1 in BY4742 background was constructed by substituting the KanMX4 selection marker in the Y11793 strain (Saccharomyces Genome Deletion Project) with the HphMX4 marker (conferring hygromycin B resistance) according to Goldstein and McCusker (1999). DNA from the DPET1 strain was then used as a template for PCR using the PET127_L and PET127_R primers to give a product containing the HphMX4 module with yeast genomic flanks upstream from the ATG and downstream from the termination codon of the PET127 ORF. This deletion cassette was then used to transform the CW252, CW15S, and DPPR2/15S-7 strains to give DPET2, DPETCW15S, and DDP1/15S-7, respectively.
Hoechst staining:
Formaldehyde was added to fresh YPD log-phase cultures to a final concentration of 3.7% (w/v). Cells were then incubated for 30 min at room temperature, washed twice in phosphate buffered saline (PBS), and resuspended in PBS with 1 μg/ml Hoechst 3324 (Invitrogen). After a 5-min incubation at room temperature, cells were washed twice with PBS, dropped on the slides, covered, and sealed with nail polish. Fluorescence was visualized using an Olympus 1X81 inverted fluorescence microscope with a 100×/1.4 oil immersion objective (UPLSAPO 100×/1.4) and an MT20 illumination system with a mercury-xenon lamp. Images were taken with an XM10 monochromatic CCD camera. Visible light microscopy was used to examine cell morphology on the same slides.
DNA preparations and Southern blotting:
Genomic DNA for Southerns and PCR was prepared according to the rapid protocol (Hoffman and Winston 1987). For the selection of ρ− clones carrying particular sequences in the residual mitochondrial genome, clones derived from single colonies of the dmr1Δ strain (DPPR2) were subcloned several times and then grown in YPD in 96-well plates. Genomic DNA was isolated and dot-blot hybridizations performed as described previously (Szczepanek and Lazowska 1996), using the same probes that were subsequently used for Northern blot experiments (described below).
Plasmid construction:
A 3280-bp genomic fragment, encompassing the entire sequence of the YGR150C ORF (2595 bp) was amplified from the genome of the CW04 strain (W303 background) using primers DMR1_LBam and DMR1_RPSt, with BamHI and PstI sites, respectively, added as 5′ overhangs, using the Phusion High-Fidelity DNA polymerase (Finnzymes). The PCR product was digested with BamHI and PstI and cloned into the respective sites of YEplac195 (2μ, URA3) vector (Gietz and Sugino 1988) to yield pDMR1-2μ. The insert was then subcloned, using the same enzyme pair, into the YCplac33 (ARS-CEN, URA3) vector (Gietz and Sugino 1988), giving pDMR1-CEN.
RNA methods:
Extraction, electrophoresis, and Northern blotting of mitochondrial or total RNA from log-phase liquid cultures were performed as described previously (Malecki et al. 2008). Whenever mitochondrial RNA was needed, it was prepared from mitochondria purified by differential centrifugation as described previously (Malecki et al. 2008). Oligonucleotide 15S_3ter was used as a probe for the 15S rRNA, recognizing the 3′ terminus of the transcript. Plasmids pSCM51 (Jacquier and Dujon 1983) and pJM2 (Mulero and Fox 1993) were used to detect 21S rRNA and COX2 mRNA, respectively. The probes were labeled with 32P using T4 PNK (New England Biolabs) in the case of oligonucleotides, and the nick-translation kit (Invitrogen) in the case of plasmids, according to the manufacturers' instructions.
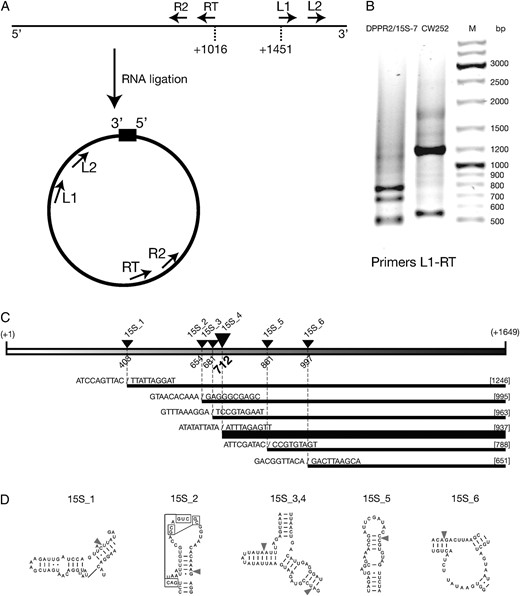
Circularization RT–PCR mapping of 15S rRNA ends was essentially performed as described (Kuhn and Binder 2002). Two micrograms of mitochondrial RNA was self-ligated in a 15-μl reaction using T4 RNA ligase (Fermentas) according to the manufacturer's instructions. After ligation, RNA was extracted with phenol:chloroform:isoamyl (pH 5.2), precipitated with ethanol and resuspended in 10 μl of DEPC-treated water. Reverse transcription was performed using 10 pmol of primer RT and 2 μg of self-ligated RNA using AMV reverse transcriptase (New England Biolabs). The first round of PCR amplification was performed using primers RT and L1. Nested amplification was performed using primers L2 and R2 and 1 μl of 100× diluted product of the first amplification as template. Products of the second round of amplification were separated by agarose gel electrophoresis, bands corresponding to different products were excised from the gel, isolated using the Gel-Out kit (A&A Biotechnology), cloned into the pGEM-T Easy vector (Promega), according to the manufacturers' instructions, and sequenced in both directions using universal primers.
Mitochondrial fractionation and immunoblotting:
Yeast strain YSC1178-7500684, expressing a Dmr1p–TAP fusion from the genomic locus in a S288C background is a part of the Yeast TAP-Fusion Library (scTAP) (Ghaemmaghami et al. 2003) and was purchased from Open Biosystems through BioCat GmbH (Heidelberg, Germany). For all the fractionation experiments strains were grown on YP-glycerol media. For fractionation of yeast cells into the mitochondrial and postmitochondrial (supernatant) fractions spheroplasts were lysed and subjected to successive differential centrifugation according to Chacinska et al. (2004). Briefly, cellular debris was pelleted at 1500 × g and the cleared lysate was subsequently centrifuged at 13,000 × g to provide the mitochondria-containing pellet and the postmitochondrial supernatant. Forty micrograms of proteins from the pellet and supernatant fractions were analyzed by SDS–PAGE (12%), followed by immunoblotting. Submitochondrial fractionation was performed according to Chacinska et al. (2004). Briefly, 50 μg of isolated mitochondria, resuspended in ice cold buffer containing 250 mm sucrose, 1 mm EDTA, and 10 mm MOPS, pH 7.2, were converted to mitoplasts by hypotonic swelling and were optionally treated with 20 μg/ml of proteinase K. Proteins were resolved on SDS–PAGE (15%) followed by immunoblotting (Protran nitrocellulose transfer membrane, Whatman). Rabbit peroxidase anti-peroxidase soluble complex antibody (Sigma), mouse monoclonal antibodies against yeast Cox2p (MitoSciences), and polyclonal rabbit antibodies against yeast Pgk1p, Tom70p, Fis1p, Cyc3p, Tim23p, and Tim13p were used for detection of relevant proteins. Goat anti-rabbit-HRP and anti-mouse-HRP secondary antibodies were purchased from Calbiochem (through Merck KGaA, Darmstadt, Germany).
Protein expression and purification:
Construction of His6MBP fusion protein vectors for Dmr1p was performed using Gateway recombinational cloning (Invitrogen) according to the procedure described previously (Malecki et al. 2007, 2008; Tropea et al. 2007). DNA encoding the Dmr1 protein without the 26 amino acid N-terminal sequence was amplified using primers yDmr1_N1 and yDMR1_C, and subsequently reamplified using primers N2 and yDMR1_C. Phusion high-fidelity DNA polymerase (Finnzymes) was used according to the manufacturer's instructions. The PCR product was cloned into pDONR201 (Invitrogen) by recombinational cloning to generate the entry plasmid pDONR–yDMR1. Finally, recombination of the encoding fragment from entry plasmid to pDEST–His6MBP (Tropea et al. 2007) produced destination vector pHis6MBP–yDMR1. Correct sequence was confirmed by sequencing.
Recombinant Dmr1p was purified from bacteria transformed with pHis6MBP–yDMR1 according to the protocol described previously (Malecki et al. 2008).
RNA binding assays:
The single-stranded RNA substrates were generated by in vitro transcription using the T7 transcription kit (Fermentas), according to the manufacturer's instructions. α-32P-UTP was added to the reaction to produce radiolabeled substrate. The templates were PCR products corresponding to bases 7–680, 642–1119, or 1107–1649 of mature 15S rRNA, obtained using primers 15S1L and 15S1R, 15S2L and 15S2R, or 15S3L and 15S3R, respectively, and to bases 1–485 or 2600–3070 of mature 21S rRNA, obtained using primers 21S1L and 21S1R or 21S2L and 21S2R, respectively. In each pair the left primer contained the promoter sequence for the T7 polymerase in a 5′ overhang of 20 nt.
The RNA transcripts were then digested with RNase-free DNase I (Roche) and purified by electrophoresis in 5% denaturing acrylamide gels and isolation of the appropriate bands as described previously (Malecki et al. 2008).
Binding reactions were preformed in 15 μl of binding buffer (100 mm KCl; 2 mm DTT; 20 mm Tris pH 8; 10% glycerol). Spermidine (1 mm final concentration) and rNTP mix (8 mm) was added to the buffer to improve the resolution of the assay (Brule et al. 2002). A total of 0.2 μg of unlabeled bacterial tRNA (Roche) was added to each sample as a nonspecific competitor. Recombinant Dmr1 protein was added in a series of twofold dilutions, with 0.6 μg per reaction being the starting point (1×). BSA (Sigma) was used as the negative control. In reactions containing diluted Dmr1p, BSA was also added to bring the total concentration of protein in each sample to the same value (0.6 μg per reaction). Approximately 200 cps of appropriate labeled RNA was added to each reaction sample. Samples were incubated on ice for 10 min. After incubation, 2 μl of loading buffer (30% glycerol; 0.25% xylene cyanol; 0.25% bromophenol blue) were added to each reaction. Samples were then resolved on a native (1× TBE) 5% acrylamide gel for 6 hr at 15 mA at 6°. The gel was subsequently dried and overnight autoradiography was performed on a phosphor screen (Fuji).
RESULTS
Deletion of DMR1 results in a complete loss of respiratory capacity with concomitant generation of ρ−/ρ0 cytoplasmic petites:
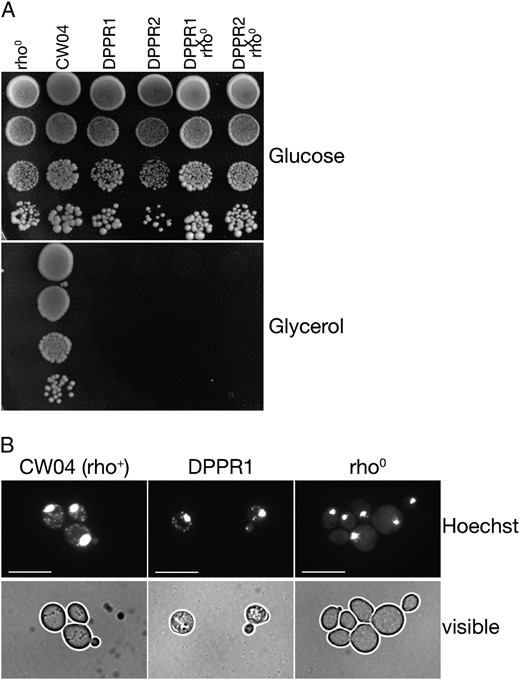
Respiratory deficiency of strains carrying the null allele of DMR1 was reported in high-throughput studies (Dimmer et al. 2002; Steinmetz et al. 2002; Merz and Westermann 2009) and observed previously by other authors (Ellis et al. 2004; Moreno et al. 2009). We constructed strains bearing the deletion of DMR1 in the background of the strain W303, which has been extensively used in the study of nucleo-mitochondrial interactions. To test the possible involvement of Dmr1p in the splicing of mitochondrial introns, we constructed dmr1Δ strains bearing either wild-type long mtDNA (13 introns), or the intronless variant (Seraphin et al. 1987; Saint-Georges et al. 2002). The phenotype of tight respiratory deficiency (Figure 1A) was identical for both strains, which rules out the involvement in the splicing of mitochondrial introns as the primary defect in dmr1Δ mutants. Moreover, crossing to the ρ0 tester strain KL14-4A/60, performed with 200 single-colony clones from both dmr1Δ strains, indicated that the defect was irreversible, and no ρ+ colonies, carrying intact mitochondrial genomes, could be identified among the deletants.
Phenotype of the DMR1 deletants. (A) Deletant strains are respiratory deficient and fail to grow on glycerol media, regardless of the presence (strain DPPR1) or absence (strain DPPR2) of mitochondrial introns. Diploids constructed from these dmr1Δ strains with a wild-type ρ0 tester (KL14-4A/60) are respiratory deficient as well, suggesting that functional mtDNA is irreversibly lost. Four 10-fold serial dilutions, beginning with the 10−1 dilution of the saturated liquid YPD preculture are arranged vertically in each panel. Plates were grown for 3 days. (B) Residual mtDNA is still present in some dmr1Δ cells. Hoechst staining shows extranuclear signal in DPPR1 (dmr1Δ) cells, like in the ρ+ control CW04, but unlike in the ρ0 negative control (KL14-4A/60), leading to the conclusion that the dmr1Δ cells are ρ−. Bar, 10 μm.
Mutations in nuclear genes causing such loss of mtDNA stability fall into two broad categories. Defects in genes encoding proteins involved directly in the replication, recombination, repair, maintenance, and transmission of the mitochondrial genome usually lead to a complete loss of mitochondrial DNA—termed ρ0, while disruption of various aspects of mitochondrial gene expression often leads to the generation of ρ− petites, that undergo large random deletions and retain only a short fragment of the original mitochondrial genome in a concatamer form (reviewed by Contamine and Picard 2000). To verify whether the cytoplasmic petites generated in dmr1Δ strains were of the ρ0 or ρ− type, we performed Hoechst staining to detect nuclear and extranuclear DNA. The results (Figure 1B) indicate that some, but not all, dmr1Δ cells still retain DNA in their mitochondria. This allowed us to conclude that deletion of DMR1 leads to a complete loss of functional (ρ+) mtDNA, but at least some of the resulting petite cells are ρ− and possibly ρ0 clones. Similar analysis performed on 20 independent single-colony clones allowed the detection of extranuclear DNA signal in at least 13 of them. Of the clones isolated as mtDNA-containing ρ− petites for further characterization (see subsequent sections) some were stable, while some lost mtDNA upon repeated passaging, which is common for this class of mutants. While the total absence of any residual mtDNA in clones giving no detectable extranuclear Hoechst staining was not unequivocally confirmed, it is very likely that at least some are true ρ0 clones, and thus the phenotype of the dmr1Δ cells can be described as the conversion to ρ−/ρ0 petites. Most ρ− strains display limited mtDNA stability and evolve to ρ0 as the culture ages (Contamine and Picard 2000); presence of some ρ0 cells is thus to be expected.
Among different dysfunctions leading to the generation of ρ−/ρ0 petites, defects in mitochondrial translation play a major role (Myers et al. 1985; Contamine and Picard 2000; Duvezin-Caubet et al. 2006; Merz and Westermann 2009). Therefore the phenotype of the deletion of the DMR1 gene strongly suggests that its product is essential for the expression of the mitochondrial genome, regardless of the presence or absence of introns, and may be involved in translation.
Lack of the DMR1 gene product leads to the degradation of the small subunit mitochondrial rRNA (15S rRNA):
Mitochondrial protein synthesis is completely abolished in ρ− cells, but at least some of the RNA transcripts are still produced (Morimoto et al. 1979). We therefore decided to analyze the transcripts in mitochondria of ρ− clones derived from the dmr1Δ strain, paying particular attention to the RNA elements of the translation machinery. Synthesis of complex, polycistronic transcripts in ρ− cells is severely perturbed due to multiple processing defects, related, among others, to the lack of functional RNase P, but several simpler mitochondrial transcripts, including both ribosomal RNAs (Osinga et al. 1981; Tabak et al. 1981) are produced correctly in petite cells and thus amenable to analysis. We used ρ− clones obtained in the dmr1Δ strain derived from the strain that harbors the intronless mtDNA (DPPR2), to avoid the pleiotropic splicing defects, invariably associated with the ρ− phenotype.
Petite dmr1Δ clones expressing the small subunit ribosomal RNA (15S rRNA), large subunit ribosomal RNA (21S rRNA), and the COX2 mRNA were selected by dot-blot screening using probes recognizing the appropriate gene sequences, and the production of full-length transcripts was confirmed by Northern blotting of total RNA preparations using the same probes. Mitochondrial transcripts in the selected ρ− clones were further analyzed by Northern blotting using mitochondrial RNA preparations. Three independent clones, expressing 15S rRNA, named DPPR2/15S-3, DPPR2/15S-7, and DPPR2/15S-24, were chosen for further analysis.
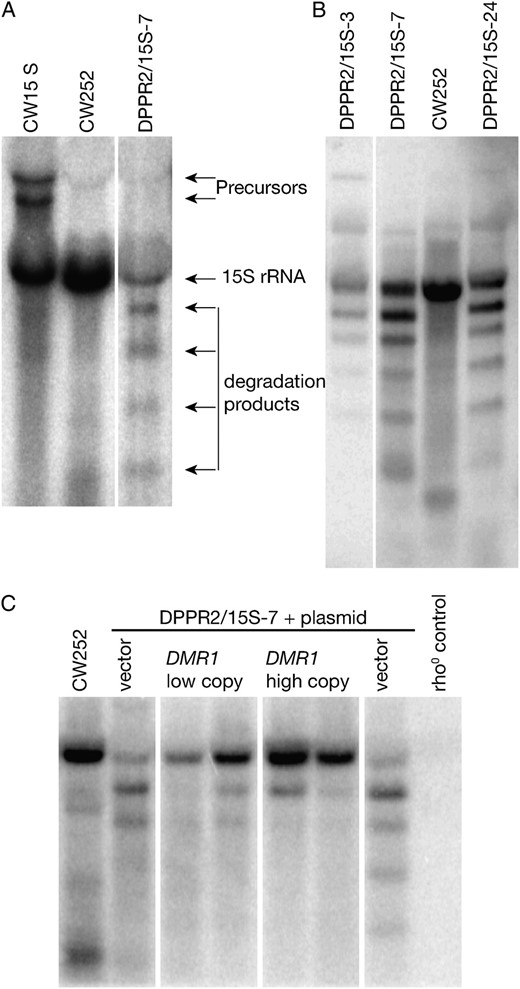
A striking phenotype was observed in dmr1Δ clones expressing the small subunit ribosomal RNA (15S rRNA, Figure 2A). Very little of the full-length transcript is present, compared to the wild-type control, and several bands of lower molecular weight are apparent. As the oligonucleotide probe used in this hybridization recognizes the 3′ terminal sequence of the 15S rRNA, these bands correspond to the products of degradation, rather than abortive transcription. The full-length transcript in ρ− mitochondria is slightly larger than in wild-type cells, as it contains an additional 5′ extension of ∼80 nt, which is removed only in the context of ρ+ mtDNA (Osinga et al. 1981). To confirm that the observed phenotype of 15S rRNA degradation is related to the deletion of DMR1, rather than to the general ρ− phenotype, an additional control strain (CW15S) was constructed by cytoduction of the ρ− mitochondria expressing the 15S rRNA into the wild-type nuclear context. While this strain differs from the wild-type ρ+ in overaccumulation of high molecular weight precursors containing the downstream tRNA-Trp (probably due to the lack of RNase P activity in ρ− mitochondria), no degradation products are observed. The phenotype of 15S rRNA degradation is therefore related to the absence of Dmr1p, not to the defects in RNA metabolism due to the ρ− mtDNA background. The same degradation pattern is observed when total RNA preparations were used instead of mitochondrial RNA (supporting information, Figure S2), demonstrating that it is not related to the mtRNA preparation protocol.
Specific degradation of the 15S rRNA transcript in dmr1Δ mitochondria. (A) Hybridization with an oligonucleotide probe recognizing the 3′ fragment of the 15S rRNA shows that in ρ− dmr1Δ cells (DPPR2/15S-7) expressing this gene the transcript is degraded into a series of defined fragments. Neither wild-type ρ+ strain (CW252), nor a strain with the same ρ− genome introduced into wild-type nuclear background (CW15S), shows any signs of this degradation. (B) Independent dmr1Δ ρ− clones (DPPR2/15S-2, DPPR2/15S-7, DPPR2/15S-24) expressing the 15S rRNA show the same degradation pattern. (C) Low-copy (pDMR1-CEN) or high-copy (pDMR1-2μ) plasmid vectors expressing the wild-type DMR1 gene, but not empty vector controls, rescue the phenotype of 15S rRNA degradation in dmr1Δ ρ− mitochondria of the DPPR2/15S-7 strain. RNA prepared from mitochondria purified by differential centrifugation was used in all the hybridizations shown.
Analysis of the three independent ρ− clones expressing the 15S rRNA transcript (Figure 2B) showed a very similar pattern of degradation products in all of them. Analysis of the mitochondrial DNA by restriction mapping, followed by Southern hybridization (Figure S1) proved that these three clones had different maps (and thus different fragments of the original genome), but all retained the ∼2-kb HapII fragment, which is sufficient to encode the full-length 15S rRNA transcript (Osinga et al. 1981). Therefore, the phenomenon of 15S rRNA degradation in dmr1Δ mitochondria is not related to any particular ρ− clone.
To further confirm that the phenotype of 15S rRNA degradation is in fact related to the lack of the product of the DMR1 gene, we transformed the ρ− dmr1Δ strain DPPR2/15S-7, expressing this transcript, with plasmids carrying the wild-type DMR1, and analyzed the 15S rRNA transcript by Northern hybridization (Figure 2C). Both centromeric (pDMR1-CEN) and multicopy (pDMR1-2μ) constructs reversed the 15S rRNA degradation phenotype, even though very weak bands corresponding to degradation products could be observed in some transformants, particularly those carrying the centromeric construct. This further confirms that the phenotype of 15S rRNA degradation is indeed the result of the lack of the DMR1 gene product. The presence of residual degradation products in some transformants carrying the centromeric DMR1 vector hints at a possibility of gene-dosage effect (low-level haploinsufficiency).
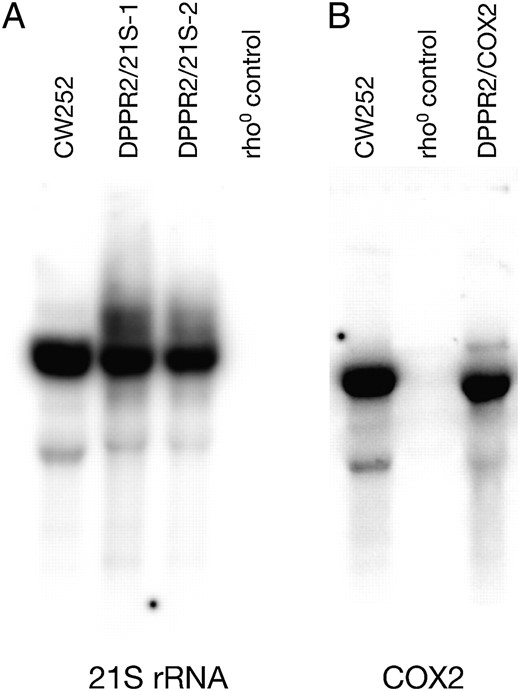
On the other hand, in ρ− dmr1Δ strains expressing the large subunit 21S rRNA (DPPR2/21S-1 and DPPR2/21S-2; Figure 3A) or the COX2 mRNA (DPPR2/COX2; Figure 3B) no alterations in the respective transcripts can be detected by Northern hybridization. Transcripts other than 15S rRNA, 21S rRNA (intronless allele), and COX2 mRNA are not amenable to analysis in a ρ− background due to the processing defects related to the absence of mitochondrial gene expression (including the RNase P function). These results suggest that the phenotype of the DMR1 gene inactivation is specific to the small subunit 15S rRNA and does not directly affect other transcripts. Obviously, perturbations in the expression of the 15S rRNA would affect ribosome biogenesis and translation and thus could explain the phenotype of irreversible respiratory deficiency with the production of ρ− petites, described above.
Other mitochondrial transcripts are not affected by the deletion of DMR1. Mitochondrial RNA preparations from dmr1Δ ρ− clones expressing the 21S rRNA (DPPR2/21S-1 and DPPR2/21S-2) (A), or the COX2 mRNA (DPPR2/COX2) (B), together with positive (CW252, wild-type ρ+) and negative (KL14-4A/60, ρ0) controls were hybridized with appropriate plasmid probes (described in materials and methods).
To further characterize the degradation of 15S rRNA in dmr1Δ mitochondria, we mapped the 5′ ends of the degradation products by circularization of mitochondrial RNA, followed by RT–PCR using primers RT and L1, designed to amplify the three largest degradation products (Figure 4A). The pattern of RT–PCR products (Figure 4B) is consistent with the apparent molecular weights of the bands observed on Northern blots, although no amplicon corresponding to the full-length transcript can be detected in reactions from the mutant strain. The RT–PCR products were then reamplified with a nested pair of primers R2 and L2 and the reamplification products were cloned into the pGEM-T Easy plasmid vector and sequenced. All the sequenced clones contained the intact 3′ terminal sequence of 15S rRNA and a variety of 5′ sequences (Figure 4C), clustering in three regions, corresponding to products of ∼1250 nt, ∼960 nt, and ∼720 nt, which is consistent with the apparent molecular weights of the three largest degradation products observed on Northern blots. With the exception of the largest band, however, all these degradation products seem to actually consist of heterogeneous populations of fragments that cannot be resolved by Northern analysis. The most common is the 937 nt product (corresponding to a break at position 712 of the 15S rRNA sequence), indeed appearing as the strongest band on Northerns. Mapping the 5′ ends of the degradation products onto the putative secondary structure of 15S rRNA obtained by comparative modeling from the Comparative RNA Project (Cannone et al. 2002) failed to reveal any clear sequence or structure pattern. All the breakpoints localize in double-stranded regions in the neighborhood of single-stranded loops, but such features are very common in ribosomal RNAs. The phenomenon of the degradation of 15S rRNA in the absence of the functional DMR1 gene is therefore not sequence specific.
Mapping the ends of the degradation products. RNA from ρ− dmr1Δ cells expressing the 15S rRNA of the DPPR2/15S-7 strain and the CW252 wild-type control was circularized by ligation and amplified in two successive rounds of RT–PCR and PCR. (A) General design of the experiment, showing the location of primers. (B) Results of the first round of amplification (PCR using primers L1 and RT) of the DPPR2/15S-7 strain and the CW252 wild-type control RNA preparations. M is the size marker (GeneRuler DNA ladder mix; Fermentas). (C) Locations of the 5′ ends of the degradation products in the mature 15S rRNA sequence. Fragment lengths shown in brackets, breakpoint positions in the sequence indicated by slashes. Line width is proportional to the number of times the particular fragment was identified in the sequenced pool. (D) Regions containing the breakpoints in the putative secondary structure model of 15S rRNA from the Comparative RNA Project (http://www.rna.ccbb.utexas.edu/).
Dmr1p is an integral mitochondrial protein:
To verify the mitochondrial localization of the Dmr1 protein we used the strain YSC1178-7500684, expressing a Dmr1p–TAP fusion from the genomic locus in a S288C background and obtained from the Yeast TAP-Fusion Library (scTAP) (Ghaemmaghami et al. 2003). The TAP epitope is fused to the C terminus of the protein, and the fusion is expressed from the native genomic promoter. The Dmr1p–TAP fusion strain grows on nonfermentable carbon sources as well as the wild-type BY4742 strain derived from the same S288C background. Northern blot analysis of the RNA from the Dmr1p–TAP fusion strain compared to the wild-type BY4742 strain (Figure 5A) with the oligonucleotide probe 15S_3ter recognizing the 3′ terminal sequence of the 15S rRNA, showed no discernible differences in the transcript. This result indicates that the Dmr1p–TAP fusion is functionally equivalent to the wild-type Dmr1 protein, and can therefore be used for subcellular localization experiments.
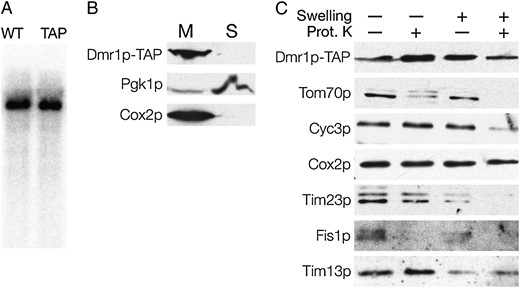
Dmr1p is an integral mitochondrial protein. Mitochondria were purified from a strain expressing the Dmr1p–TAP fusion. (A) The TAP tag fusion does not affect the Dmr1p function. Total RNA from the isogenic wild-type strain (WT) and the Dmr1p–TAP strain was hybridized with an oligonucleotide probe recognizing the 3′ fragment of the 15S rRNA. (B) The Dmr1p–TAP fusion is detected in the mitochondrial fraction. Spheroplasts from the Dmr1–TAP strain were lysed and debris was removed by centrifugation at 1500 × g. Mitochondria (M) were then pelleted by centrifugation at 13,000 × g, and the postmitochondrial supernatant (S) corresponded mostly to the soluble cytoplasmic fraction. Proteins were analyzed by SDS–PAGE (12%) and immunoblotting with antibodies recognizing the TAP tag (PAP), the cytosolic protein Pgk1p, and the integral inner mitochondrial membrane protein Cox2p. (C) Purified mitochondria from the Dmr1p–TAP strain were converted to mitoplasts by hypotonic swelling. Intact mitochondria and mitoplasts were optionally treated with 20 μg/ml of proteinase K. Proteins from each fraction were then resolved on SDS–PAGE (15%) followed by Western blot analysis with antibodies recognizing the TAP tag (PAP), and the markers for the mitochondrial outer membrane (Tim70p and Fis1p), the intermembrane space (Cyc3p, Tim23p, and Tim13p), and the integral mitochondrial membrane protein Cox2p.
To confirm the mitochondrial localization of Dmr1p, we fractionated the spheroplasts from the Dmr1p–TAP strain by differential centrifugation (Chacinska et al. 2004). Proteins from the mitochondrial pellet and the postmitochondrial supernatant were separated by SDS–PAGE and immunoblotted using antibodies recognizing the TAP tag, a mitochondrial protein marker Cox2p and a cytoplasmic protein marker Pgk1p. The results, shown in Figure 5B, clearly show that the Dmr1p signal is detected exclusively in the mitochondrial fraction (like the Cox2p marker), thus indicating that Dmr1p is indeed a mitochondrial protein.
To test whether the Dmr1 protein resides inside the mitochondrial compartment, purified mitochondria were converted to mitoplasts by hypotonic swelling. Both intact mitochondria and mitoplasts were also treated with proteinase K to remove proteins that are located on the external face of the membranes. The resulting protein preparations were separated by SDS–PAGE and immunoblotted with antibodies recognizing the TAP tag as well as a series of marker proteins located in the outer membrane (Tim70p and Fis1p), the intermembrane space (Cyc3p, Tim23p, and Tim13p), or inside the inner membrane (Cox2p).
The results, presented in Figure 5C, show that the Dmr1p signal is resistant to proteinase K treatment, both in the intact mitochondria and in mitoplast preparations, similar to the Cox2 protein. This indicates that Dmr1p is located either in the mitochondrial matrix or in the inner membrane (not protruding into the intermembrane space). Such localization is consistent with the proposed role of Dmr1p in the mitochondrial gene expression. Proteins involved in this process are localized in the matrix or form large molecular weight complexes on the matrix face of the inner mitochondrial membrane (Krause et al. 2004; Shadel 2004).
The purified Dmr1 protein expressed in bacteria specifically binds 15S rRNA in vitro:
A plasmid expressing the full-length Dmr1 protein, devoid of the N-terminal 26 amino acids containing the mitochondrial targeting sequence, was prepared as described in materials and methods. The protein was expressed as His6MBP fusion and initially purified on a NiNTA column (Tropea et al. 2007; Malecki et al. 2008). The entire His6MBP tag was subsequently removed by cleavage with the TEV protease (Kapust et al. 2001) and the resulting protein preparation was finally purified on a Superdex 200-size exclusion column. The obtained preparation contained protein of the apparent molecular mass corresponding to the expected Dmr127-864 monomer of 98.3 kDa. The size exclusion profile and the SDS–PAGE analysis indicated that a monomeric protein of good stability and solubility was obtained.
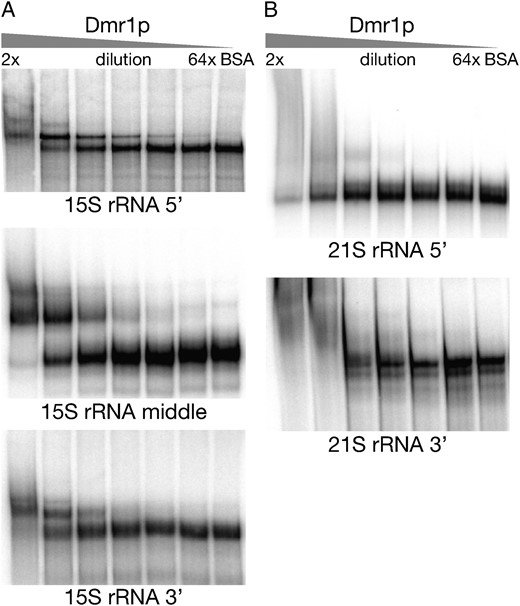
To test the RNA-binding capability of Dmr1p, we performed electrophoretic mobility shift assays (EMSA, gel shift) using varying concentrations of the protein and radiolabeled fragments of 15S and 21S rRNA obtained by in vitro transcription. Fragments of ∼500 nt were used due to the limitations the EMSA assay imposes on the maximum length of the nucleic acid molecule. The three 15S rRNA fragments used in the assays together covered the entire transcript and were designed to avoid disrupting any major secondary-structure domains of the molecule. Dmr1p displays clear dosage-dependent binding of all three 15S rRNA fragments (Figure 6A). Binding appears to be most efficient with the middle 15S rRNA fragment (positions 642–1119), but the 5′ terminal fragment (positions 7–680) also begins to show mobility shift at a relatively low protein concentration (32× dilution, corresponding to 18.75 ng of Dmr1p per reaction). The 3′ terminal fragment of 15S rRNA (positions 1107–1649) is also bound by Dmr1p, although a higher protein concentration is required. Additional bands of further decreased electrophoretic mobility become apparent at the highest protein concentrations (2× and 4×); it is not clear whether they represent a higher-order complex or nonspecific aggregation. At the 1× concentration (0.6 μg of Dmr1p per reaction) the protein and RNA formed aggregates that failed to enter the gel; it was therefore omitted from analysis.
Results of the gel shift (EMSA) assays performed with the purified heterologous Dmr1 protein and labeled fragments of 15S rRNA (A) and 21S rRNA (B). The protein concentration was decreased from left to right in a series of twofold dilutions, with 1× corresponding to 0.6 μg of Dmr1p per reaction. The 1× concentration was not included due to the formation of aggregates that failed to enter the gel. The lengths and locations of the RNA fragments are described in the text.
In contrast, neither of the two 21S rRNA fragments shows any shift in gel mobility with the increasing concentration of Dmr1p (Figure 6B). The 3′ fragment (positions 2600–3070 of the mature, intronless transcript) only displays nonspecific aggregation at highest protein concentrations, while for the 5′ terminal fragment (positions 1–485), adding Dmr1p has no discernible effect on the migration of RNA.
We can therefore conclude that Dmr1p specifically binds 15S rRNA in vitro; this binding is not, however, limited to any particular region of the molecule. The transcript 21S rRNA is not bound under these conditions. The results of in vitro binding experiments are consistent with the degradation phenotype obtained in vivo, which is also limited to 15S rRNA, but concerns the entire length of the molecule.
The putative 5′– 3′ exoribonuclease Pet127p may be involved in the degradation of 15S rRNA in the absence of Dmr1p:
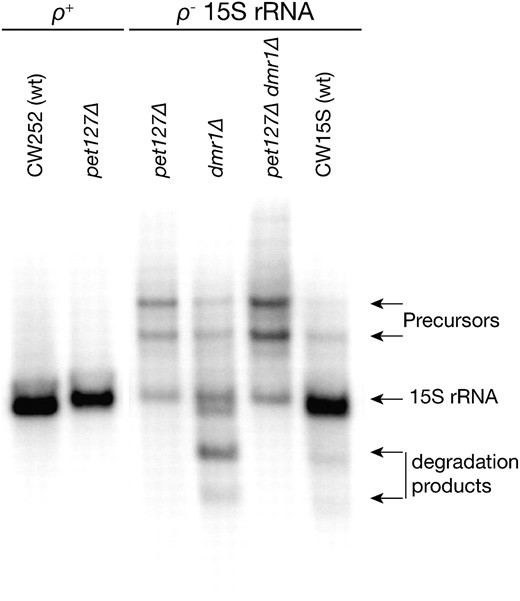
The only known mitochondrial 5′–3′ exoribonucleolytic activity depends on the protein encoded by the PET127 gene (Wiesenberger and Fox 1997; Fekete et al. 2007). As the deletion of DMR1 results in fragmentation of 15S rRNA, with the 3′ end left intact, Pet127p is an obvious candidate for the RNase activity responsible for this degradation. To test this possibility, we prepared a series of single and double deletants and performed Northern analysis of the 15S rRNA. Deletion of PET127 in a strain with wild-type ρ+ mtDNA (intron containing or intronless) results in a defect of 5′-end processing of several mitochondrial transcripts, including 15S rRNA (Wiesenberger and Fox 1997) in which the ∼80-nt 5′ terminal extension is not removed from the precursor. These defects do not, however, result in a complete loss of the mitochondrial gene expression, as the pet127Δ strains grow on nonfermentable carbon sources at normal temperature and exhibit only a temperature-sensitive respiratory deficiency (Haffter and Fox 1992). Our experiments confirmed these observations, as the ρ+ pet127Δ strain (DPET2) was respiratory competent at 30°, and Northern blot (Figure 7) with the oligonucleotide probe 15S_3ter (recognizing the 3′ terminal sequence of the 15S rRNA) showed a product which was slightly longer than in the wild-type control (CW252). No other effect of the pet127Δ deletion on the 15S rRNA was observed in the ρ+ background.
15S rRNA transcripts in single and double pet127 and dmr1 deletants. Hybridization with an oligonucleotide probe recognizing the 3′ fragment of the 15S rRNA shows that in the context of a ρ+ mtDNA (intronless) the deletion of PET127 (pet127Δ strain DPET2) the 15S rRNA transcript is only slightly longer than in the isogenic and isomitochondrial control (CW252), but no other defects are apparent. In a ρ− clone expressing the 15S rRNA transcript (all ρ− clones were isomitochondrial to the DPPR2/15S-7 strain) with the wild-type alleles of PET127 and DMR1 (CW15S) only a slight overaccumulation of precursors is observed. In the context of the same ρ− mtDNA, the single dmr1 deletant (DPPR2/15S-7) shows the typical 15S rRNA fragmetation pattern (cf. Figure 2), while both the single pet127Δ (DPETCW15S) and the double pet127Δ dmr1Δ (DDP/15S-7) mutant strains show a similar pattern of significant precursor overaccumulation with a decrease of the mature 15S rRNA level, but no detectable degradation products. RNA prepared from mitochondria purified by differential centrifugation was used in all the samples shown.
Next we analyzed the phenotype of the single and double deletions of PET127 and DMR1 genes in ρ− strains expressing the 15S rRNA transcript, isomitochondrial to the DPPR2/15S-7 strain used in previous experiments. When such ρ− mtDNA is introduced into a wild-type nuclear background (strain CW15S), almost no degradation of the transcript is observed (Figure 7, see also Figure 2A). The only differences from a ρ+ strain are the slight overaccumulation of unprocessed precursors containing the downstream tRNA-Trp (due to the lack of RNase P activity in ρ− mitochondria), and the presence of the ∼80-nt 5′ terminal extension of the 15S rRNA transcript, which is not removed in ρ− strains (Osinga et al. 1981; see also Figures 2A and 7), like in pet127Δ ρ+ cells. Deleting the PET127 gene in this background (the pet127Δ strain DPETCW15S) leads to a significant decrease in the amount of the 15S rRNA transcript, coupled with a much more pronounced overaccumulation of the precursors (Figure 7). When PET127 is deleted in an isomitochondrial ρ− strain, which also bears the dmr1Δ deletion (the pet127Δ dmr1Δ strain DDP1/15S-7), the pattern of 15S rRNA expression in the double deletant is very similar to that of a pet127Δ ρ− single deletant strain and differs significantly from the phenotype of a dmr1Δ ρ− strain DPPR2/15S-7 (Figure 7). The degradation products are no longer observed, but the amount of full-length 15S rRNA is severely reduced, and the unprocessed precursors clearly accumulate.
We can therefore conclude, that in the context of a ρ− mtDNA expressing the 15S rRNA transcript, the molecular phenotype of pet127Δ deletion is epistatic to the phenotype of the dmr1Δ deletion, as the pattern of transcripts containing the 15S rRNA sequence is similar in pet127Δ and pet127Δ dmr1Δ strains, while differing from that, which is observed in the dmr1Δ strain. This makes the Pet127 protein a plausible candidate for the activity responsible for the degradation of 15S rRNA observed in dmr1Δ strains. Deletion of PET127 does not, however, rescue the respiratory-deficient phenotype of dmr1Δ in the context of wild-type mtDNA. The results can, however, also be explained by the upstream processing defect in the pet127Δ ρ− strain reducing the amount of the 15S rRNA transcript so that the defect due to the lack of the Dmr1 protein is no longer observable.
DISCUSSION
The results of the present investigation provide evidence for the key role of the integral mitochondrial protein encoded by YGR150C in maintaining the stability of the small subunit mitochondrial 15S rRNA and thus in the expression of the mitochondrial genome. We therefore suggest the name DMR1 (for Degradation of Mitochondrial rRNA) for YGR150C. Deletion of DMR1 results in an irreversible petite phenotype, with the loss of ρ+ mtDNA accompanying the loss of respiratory competence. Although functional mtDNA is inevitably lost in dmr1Δ strains, fragments of it still remain in the form of ρ− genomes. Among possible causes of such phenotype, severe disruption of the mitochondrial genome expression, particularly translation, is a frequent occurrence (Myers et al. 1985; Contamine and Picard 2000; Duvezin-Caubet et al. 2006; Merz and Westermann 2009). Given the RNA-binding properties of known PPR proteins, this leads to the hypothesis that the product of DMR1 is an essential accessory to one of the RNA components of the mitochondrial gene expression system.
The ρ− cells containing the intact 15S rRNA gene are still capable of producing the full-length transcript, but in the absence of the functional DMR1 gene product it undergoes specific fragmentation. This fragmentation is reversed by the presence of wild-type DMR1 and is not an inherent feature of the ρ− genomes. Other mitochondrial transcripts in dmr1Δ strains, including the large subunit 21S rRNA, are not affected; the degradation effect is therefore specific to the 15S rRNA. The products of this fragmentation contain the intact 3′ terminal sequence of the transcript, which rules out premature transcription termination as an explanation of their origin. At this moment it is not entirely clear whether this degradation is brought about by endonucleases, 5′–3′ exoribonucleases or a combination of endo- and exoribonucleolytic activities. The main ribonucleolytic activity of yeast mitochondria is assured by the mtEXO (mitochondrial degradosome) complex, which is a 3′–5′ exoribonuclease (Dziembowski et al. 2003; Rogowska et al. 2006; Malecki et al. 2007), but the products of the degradation of 15S rRNA in the absence of Dmr1p do contain an intact 3′ region. The only known mitochondrial 5′–3′ exo activity is governed by the Pet127 protein (Wiesenberger and Fox 1997; Fekete et al. 2007), and the absence of distinct 15S rRNA degradation products in the pet127Δ dmr1Δ strain may suggest that Pet127p is indeed responsible for the degradation. It should, however, be noted that the deletion of PET127 in the context of the ρ− mitochondrial DNA results in a significant perturbation in the processing of the 15S rRNA precursors, which can obfuscate the phenotype of the deletion of DMR1.
The only known mitochondrial endonucleases are the RNases involved in tRNA processing (RNase P and the, yet unidentified, RNase Z homolog) and intron-encoded endonucleases (Chevalier and Stoddard 2001; Schäfer 2005), which have very specific activities and are unlikely to act on the 15S rRNA. The protein encoded by the NUC1 gene (Zassenhaus et al. 1988), once thought to be the main mitochondrial nuclease, was found not to be involved in RNA degradation (Dziembowski et al. 1998) and the activity of Ynt20p appears to be limited to degradation of very short single-stranded oligonucleotides (Nguyen et al. 2000).
Thus, while Pet127p is the most plausible candidate for the activity responsible for the fragmentation of 15S rRNA in the absence of Dmr1p, we cannot make any strong assumptions about the mechanism of the degradation or the involvement of known nucleases.
While the presence of distinct degradation products indicates that certain regions of the 15S rRNA are particularly vulnerable in the absence of Dmr1p, no clear structural or sequence pattern is evident in the breakage sites. Dmr1p expressed and purified in a heterologous system shows RNA-binding activity in vitro that appears to be specific to the 15S rRNA. Different regions of the 15S rRNA molecule can bind to Dmr1p, but the strength of interaction seems to vary. This suggests that Dmr1p recognizes broader structural features, rather than particular sequences in the substrate. Binding of Dmr1p to different regions of the 15S rRNA correlates with the multiple degradation fragments, corresponding to breakages occurring at specific sites located across the length of the transcript.
The pleiotropic phenotype of the DMR1 deletion, with the concomitant loss of functional mtDNA, together with the demonstrated effect on 15S rRNA stability and binding of the purifed Dmr1p to 15S rRNA in vitro, suggest a role for the product of the DMR1 gene in the biogenesis and/or functioning of the mitochondrial ribosome. Even though a direct or indirect involvement of Dmr1p in mitochondrial translation cannot be proven in the context of the highly pleiotropic ρ− defect, it remains the most plausible explanation of the observed phenotypes, as such a severe disruption of 15S rRNA stability would almost certainly have a profound effect on the functioning of the ribosome. DMR1/YGR150C (referred to as RRG2 in that study) was classified as a gene essential for mitochondrial translation in a recent high-throughput study (Merz and Westermann 2009) based on the ρ−/ρ0 phenotype and the lack of mitochondrial translation in the deletant strain.
A recent report (Moreno et al. 2009) suggested that the protein encoded by the YGR150C ORF is required for the splicing of two group I introns: the fourth intron of COX1 and COB, and named it CCM1 (for COB and COX1 mRNA maturation). This does not, however, explain the severe and pleiotropic phenotype of the YGR150C deletion. In principle, disruption of COB and COX1 mRNA maturation would lead to respiratory deficiency, but the stability of the mitochondrial genome should not be affected. There are several examples of nuclear-encoded factors involved in the splicing of COB and/or COX1 introns, such as Mss18p (Seraphin et al. 1988), Mrs1p (Bousquet et al. 1990), or Cbp2p (Gampel et al. 1989), and while they are required for respiratory competence in strains containing their target introns, their dysfunctions do not lead to the loss of mtDNA integrity. An interesting exception is the NAM2 gene, which encodes the mitochondrial leucyl-tRNA synthetase, which is also a splicing factor for the excision of several group I introns (Labouesse et al. 1985; Labouesse 1990), but in this case, it is the function in mitochondrial translation that is essential for the integrity of the mitochondrial DNA.
At this point it is worth mentioning that functional translation is required for correct splicing of several mitochondrial introns, namely those that depend on intron-encoded maturases (Lazowska et al. 1980; De La Salle et al. 1982). The splicing defect described in DMR1 (a.k.a. CCM1) deletants (Moreno et al. 2009) can therefore be explained by deficient translation, as the fourth introns of both COX1 and COB require a mitochondrially encoded (by the fourth intron of COB) maturase for splicing (Labouesse et al. 1984).
Our results also demonstrate that the phenotype of YGR150C deletion in a strain devoid of mitochondrial introns is as severe as that observed in strains carrying wild-type mtDNA. It is therefore obvious that splicing defects are not a primary deficiency responsible for the deletion phenotype. The loss of functional mtDNA, observed in our study and reported previously (Ellis et al. 2004; Merz and Westermann 2009; Moreno et al. 2009) in strains carrying the null allele of YGR150C is, on the other hand, consistent with the disruption of mitochondrial translation, caused by degradation of the small subunit ribosomal RNA, being the initial dysfunction leading to the irreversible collapse of the entire mitochondrial gene expression system.
Evidence gathered so far leads to a conclusion that the product of the DMR1 gene is an RNA binding protein, with the small subunit mitochondrial 15S rRNA being its target. Its likely role may involve assisting in the proper assembly of the ribonucleoprotein complex of the ribosome and/or chaperoning the nascent 15S rRNA transcript before it can be fully processed and integrated into the ribosome. PPR proteins were found to interact with rRNAs and be involved in ribosome biosynthesis and translation in plants (Williams and Barkan 2003; Uyttewaal et al. 2008), Trypanosoma (Pusnik et al. 2007), and human cells (Davies et al. 2009). Our results indicate that Dmr1p is the first yeast PPR protein that has an rRNA target and is involved in mitoribosomal biogenesis and translation. This opens the way to apply the powerful techniques of yeast genetics and molecular biology to study the involvement of PPR proteins in those fundamental processes.
Footnotes
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.113969/DC1.
These authors contributed equally to this work.
Footnotes
Communicating editor: L. Pillus
Acknowledgements
We thank Aneta Kaniak-Golik for her assistance in constructing the DPET1 strain. We are extremely grateful to Agnieszka Chacinska for her assistance with mitochondrial protein localization experiments and fruitful discussions. We thank Roman Szczesny for his assistance with microscopy. We are grateful to Ewa Bartnik for critical reading of the manuscript.This work was supported by grant N N301 2386 33 from the Ministry of Science and Higher Education of Poland and by the CoE BioExploratorium project: WKP_1/1.4.3/1/2004/44/44/115/2005.
References
Andres, C., C. Lurin and I. D. Sluyter,
Blatch, G. L., and M. Lassle,
Bonnefoy, N., and T. D. Fox,
Bousquet, I., G. Dujardin, R. O. Poyton and P. P. Slonimski,
Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li et al.,
Brule, F., R. Marquet, L. Rong, M. A. Wainberg, B. P. Roques et al.,
Burke, D., D. Dawson and T. Stearns,
Cannone, J. J., S. Subramanian, M. N. Schnare, J. R. Collett, L. M. D'Souza et al.,
Chacinska, A., S. Pfannschmidt, N. Wiedemann, V. Kozjak, L. K. Sanjuan Szklarz et al.,
Chevalier, B. S., and B. L. Stoddard,
Chiron, S., A. Suleau and N. Bonnefoy,
Conde, J., and G. R. Fink,
Contamine, V., and M. Picard,
Das, A. K., P. W. Cohen and D. Barford,
Davies, S. M., O. Rackham, A. M. Shearwood, K. L. Hamilton, R. Narsai et al.,
De La Salle, H., C. Jacq and P. P. Slonimski,
Delannoy, E., W. A. Stanley, C. S. Bond and I. D. Sluyter,
Dimmer, K. S., S. Fritz, F. Fuchs, M. Messerschmitt, N. Weinbach et al.,
Ding, Y. H., N. Y. Liu, Z. S. Tang, J. Liu and W. C. Yang,
Dujardin, G., P. Pajot, O. Groudinsky and P. P. Slonimski,
Duvezin-Caubet, S., M. Rak, L. Lefebvre-Legendre, E. Tetaud, N. Bonnefoy et al.,
Dziembowski, A., M. Malewicz, M. Minczuk, P. Golik, A. Dmochowska et al.,
Dziembowski, A., J. Piwowarski, R. Hoser, M. Minczuk, A. Dmochowska et al.,
Ellis, T. P., K. G. Helfenbein, A. Tzagoloff and C. L. Dieckmann,
Fekete, Z., T. P. Ellis, M. S. Schonauer and C. L. Dieckmann,
Gampel, A., M. Nishikimi and A. Tzagoloff,
Ghaemmaghami, S., W. K. Huh, K. Bower, R. W. Howson, A. Belle et al.,
Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles et al.,
Gietz, R. D., and A. Sugino,
Gietz, R. D., and R. A. Woods,
Goldstein, A. L., and J. H. McCusker,
Groudinsky, O., G. Dujardin and P. P. Slonimski,
Haffter, P., and T. D. Fox,
Hoffman, C. S., and F. Winston,
Huh, W. K., J. V. Falvo, L. C. Gerke, A. S. Carroll, R. W. Howson et al.,
Jacquier, A., and B. Dujon,
Kapust, R. B., J. Tozser, J. D. Fox, D. E. Anderson, S. Cherry et al.,
Karpenahalli, M. R., A. N. Lupas and J. Soding,
Krause, K., R. Lopes de Souza, D. G. Roberts and C. L. Dieckmann,
Kuhn, J., and S. Binder,
Labouesse, M.,
Labouesse, M., P. Netter and R. Schroeder,
Labouesse, M., G. Dujardin and P. P. Slonimski,
Lazowska, J., C. Jacq and P. P. Slonimski,
Lightowlers, R. N., and Z. M. Chrzanowska-Lightowlers,
Lurin, C., C. Andres, S. Aubourg, M. Bellaoui, F. Bitton et al.,
Malecki, M., R. Jedrzejczak, P. P. Stepien and P. Golik,
Malecki, M., R. Jedrzejczak, O. Puchta, P. P. Stepien and P. Golik,
Mancebo, R., X. Zhou, W. Shillinglaw, W. Henzel and P. M. Macdonald,
Manthey, G. M., and J. E. McEwen,
Merz, S., and B. Westermann,
Moreno, J. I., K. S. Buie, R. E. Price and M. A. Piva,
Morimoto, R., J. Locker, R. M. Synenki and M. Rabinowitz,
Mulero, J. J., and T. D. Fox,
Myers, A. M., L. K. Pape and A. Tzagoloff,
Nakamura, T., and M. Sugita,
Nguyen, L. H., J. P. Erzberger, J. Root and D. M. Wilson, 3rd,
Osinga, K. A., R. F. Evers, J. C. Van der Laan and H. F. Tabak,
Perez-Martinez, X., S. A. Broadley and T. D. Fox,
Pusnik, M., I. Small, L. K. Read, T. Fabbro and A. Schneider,
Reinders, J., R. P. Zahedi, N. Pfanner, C. Meisinger and A. Sickmann,
Rogowska, A. T., O. Puchta, A. M. Czarnecka, A. Kaniak, P. P. Stepien et al.,
Saint-Georges, Y., N. Bonnefoy, J. P. di Rago, S. Chiron and G. Dujardin,
Schmitz-Linneweber, C., and I. D. Sluyter,
Seraphin, B., A. Boulet, M. Simon and G. Faye,
Seraphin, B., M. Simon and G. Faye,
Shadel, G. S.,
Small, I. D., and N. Peeters,
Steinmetz, L. M., C. Scharfe, A. M. Deutschbauer, D. Mokranjac, Z. S. Herman et al.,
Szczepanek, T., and J. Lazowska,
Tabak, H. F., J. van der Laan, K. A. Osinga, J. P. Schouten, J. H. van Boom et al.,
Tavares-Carreon, F., Y. Camacho-Villasana, A. Zamudio-Ochoa, M. Shingu-Vazquez, A. Torres-Larios et al.,
Tropea, J. E., S. Cherry, S. Nallamsetty, C. Bignon and D. S. Waugh,
Uyttewaal, M., H. Mireau, M. Rurek, K. Hammani, N. Arnal et al.,
Wiesenberger, G., and T. D. Fox,
Williams, P. M., and A. Barkan,
Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson et al.,
Zassenhaus, H. P., T. J. Hofmann, R. Uthayashanker, R. D. Vincent and M. Zona,