-
PDF
- Split View
-
Views
-
Cite
Cite
R Ratan, D A Mason, B Sinnot, D S Goldfarb, R J Fleming, Drosophila Importin α1 Performs Paralog-Specific Functions Essential For Gametogenesis, Genetics, Volume 178, Issue 2, 1 February 2008, Pages 839–850, https://doi.org/10.1534/genetics.107.081778
Close - Share Icon Share
Abstract
Importin α's mediate nuclear transport by linking nuclear localization signal (NLS)-containing proteins to importin β1. Animal genomes encode three conserved groups of importin α's, α1's, α2's, and α3's, each of which are competent to bind classical NLS sequences. Using Drosophila melanogaster we describe the isolation and phenotypic characterization of the first animal importin α1 mutant. Animal α1's are more similar to ancestral plant and fungal α1-like genes than to animal α2 and α3 genes. Male and female importin α1 (Dα1) null flies developed normally to adulthood (with a minor wing defect) but were sterile with defects in gametogenesis. The Dα1 mutant phenotypes were rescued by Dα1 transgenes, but not by Dα2 or Dα3 transgenes. Genetic interactions between the ectopic expression of Dα1 and the karyopherins CAS and importin β1 suggest that high nuclear levels of Dα1 are deleterious. We conclude that Dα1 performs paralog-specific activities that are essential for gametogenesis and that regulation of subcellular Dα1 localization may affect cell fate decisions. The initial expansion and specialization of the animal importin α-gene family may have been driven by the specialized needs of gametogenesis. These results provide a framework for studies of the more complex mammalian importin α-gene family.
KARYOPHERINS are a multigene family of soluble nuclear transport receptors that ferry import and export signal-containing cargoes across nuclear pore complexes (NPCs) (reviewed in Mossammaparast and Pemberton 2004; Tran and Wente 2006; Stewart 2007). The nuclear import of proteins containing classical nuclear localization signals (cNLS's) is mediated by the importin α/β1 heterodimer (Goldfarb et al. 2004; Lange et al. 2007). Importin α functions as an adapter to link cNLS cargoes to the karyopherin importin β1. After the targeting complex passes through the central channel of the NPC, β1 binds to the small nuclear GTPase Ran-GTP, which induces a conformational change and causes the dissociation of importin α and the release of the cNLS cargo (Stewart 2007). Free importin α is then bound by the export karyopherin CAS/Cse1p and recycled as a complex with Ran-GTP through the NPC to the cytoplasm. Hydrolysis of the GTP in the cytoplasm releases importin α for a fresh cycle of import.
The animal importin α-gene family is diverse, having undergone multiple rounds of duplications and lineage-specific expansions. Most animal importin α's belong to one of three conserved clades, referred to here as α1, α2, and α3 (Kohler et al. 1997; Malik et al. 1997; Tsuji et al. 1997; Mason et al. 2002; Hogarth et al. 2006). Animal α1 genes are more similar to plant and fungal α1-like genes than to animal α2 or α3 genes, which arose from an animal α1-like progenitor to perform specialized roles in animal development and differentiation (Goldfarb et al. 2004). The evolution and maintenance of α1, α2, and α3 genes among animals is likely due to their specialized roles in conserved aspects of animal development. Although importin α1, α2, and α3 proteins are coexpressed in many adult tissues, they exhibit complex temporal and spatial expression patterns during development (see Kamei et al. 1999; Hogarth et al. 2006). Animal importin α1's, α2's, and α3's all mediate the import of classical NLS-containing cargoes and, in addition, each paralog is specialized to bind and mediate the import of distinct repertoires of NLS cargoes (for example, see Michaud and Goldfarb 1993; Prieve et al. 1996; Miyamoto et al. 1997; Prieve et al. 1998; Kohler et al. 1999; Talcott and Moore 2000; Fagerlund et al. 2002; Quensel et al. 2004; Lange et al. 2007). Importin α's may also mediate the import of a few cargoes independent of β1 (Hubner et al. 1999; Miyamoto et al. 2002; Kotera et al. 2005). Finally, importin α's play specialized roles in cell processes other than nuclear transport (Tabb et al. 2000; Gorjanacz et al. 2002; Hanz et al. 2003; Schatz et al. 2003; Harel and Forbes 2004).
The proliferation of the importin α-gene family in animals may have been driven in part by the specialized needs of gametogenesis (Goldfarb et al. 2004; Hogarth et al. 2005). Importin α1, α2, and α3 proteins are differentially expressed during spermatogenesis in both Drosophila (Giarre et al. 2002) and mouse (Hogarth et al. 2006). Two of the three Caenorhabditis elegans importin α's are expressed exclusively in the germline and are both required for gametogenesis (Geles and Adam 2001; Geles et al. 2002). Likewise, Drosophila lacking importin α2 (Dα2) develop normally to adulthood but have serious defects in gametogenesis (Gorjanacz et al. 2002; Mason et al. 2002, 2003). The spermatogenesis defect of Dα2 null flies is due to the loss of an activity shared by all three paralogs, since Dα1, Dα2, and Dα3 transgenes rescued the defect. In contrast, the role of Dα2 in oogenesis is unique since only Dα2 transgenes could rescue the phenotype. Like the C. elegans importin α3, Dα3 is required for somatic development and differentiation (Mason et al. 2003). Dα3 may also be required for gametogenesis but mutant animals die as larvae. Therefore, in addition to shared housekeeping roles in classical nuclear transport, importin α2's and α3's each have unique roles in animal-specific processes such as gametogenesis. What remains is to describe the consequences of mutating the single Drosophila importin α1 (Dα1).
Here, we describe the isolation and characterization of a Dα1 null mutation. Like Dα2 mutant flies, Dα1 null flies develop to adulthood with severe defects in gametogenesis. Spermatogenesis in Dα1 null flies is arrested and males are completely sterile. Oogenesis is morphologically less severely affected, but virtually all Dα1 null females are sterile. In addition, overexpression of Dα1 results in defects in tergite development and viability that are enhanced by mutations in the importin α-recycling factor CAS and suppressed by mutations in importin β. This is the first genetic analysis of an animal importin α1 mutant and completes our analysis on the null phenotypes of the conventional Drosophila α1, α2, and α3 gene family.
MATERIALS AND METHODS
Genetic stocks and markers:
Flies were raised on standard cornmeal–dextrose media at 25°. The y w1118; p{y+}Sup P or P−, CG8533 ry506 P-element insertion line, the X-linked P{w[+mC]=GAL4∷VP16-nos.UTR}MVD2, w1118 driver line, the y1 w; P{w[+mC]=Act5C-GAL4}25FO1/CyO, y+ driver line, the y1 w1; Ki1 P{ry[+t7.2]=Δ2-3}99B “jump starter” chromosome, and the multiply-marked rucuca (ru h th st cu sr e ca) chromosomes were obtained from the Bloomington Drosophila Stock Center (Indiana University).
The w1118; ru h th st cu sr e Δ2-3 chromosome was generated by meiotic recombination from a cross of w1118; rucuca (ru h th st cu sr e ca)/Ki1 P{ry[+t7.2]=Δ2-3}99B females to w1118; rucuca males and were maintained as homozygous stocks.
Male recombination screen for Dα1 deletions:
The male recombination screen was based on a similar analysis (Gray et al. 1996). Heterozygous y w1118/Y; p{y+} SupP or P−, CG8533 ry506 3/ ru h th st cu sr e Δ2-3 male progeny were collected and mated with homozygous w1118; rucuca females. Phenotypically white+, curled, stripe, ebony recombinant animals were recovered and crossed to w1118; TM3, Sb/TM6B animals to establish stocks of each individual recombinant line. Recombinant lines were “cleaned” of the cu sr e and Δ2-3 markers by crossing to w1118 flies with normal third chromosomes and isolating phenotypically w+ female progeny (expected genotype w1118; CG8533 *cu sr e Δ2-3/+, where * indicates a potential male recombination-generated deletion). These females were then crossed to homozygous w1118; rucuca males, and phenotypically white+ males that did not display the curled, stripe ebony or Δ2-3 characteristics were selected and crossed back to w1118; TM3, Sb/TM6B females to establish balanced stocks.
Delimitation of Dα1 deletion lines:
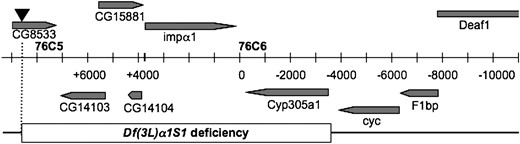
PCR was utilized to determine the extent of any deletions generated by the male recombination scheme described above. A DNA primer was generated complementary to the P-element sequence within the CG8533 P element (5′ CGACGGGACCACCTTATGTTAT 3′) and used in conjunction with antisense primers derived from genomic sequences (34). The genomic antisense primers (see Figure 1) were named relative to primer zero that lies just 3′ to Dα1 (5′ CTGGCCGCCTTCATTTAAATCC 3′). Two primers lying between Dα1 and CG8533 were generated. Their positions relative to primer zero are approximately +4000 (5′ GCATTTGTTCCACCTATTGGCC 3′) and +6000 (5′ GTATTAAAGTAGCGCTTGCCGG 3′). The remaining primers were generally spaced at ∼2-kb intervals from primer zero. Primer −2000 (5′ GCTGTTGGCGGCCAGCACATCC 3′), primer −4000 (5′ CCGCATCGAGTTGCTGGCGCCG 3′), primer −6000 (5′ TGTCGGCCTGCACAA ACTTCCG 3′), primer −8000 (5′ CTTGTGTTGGAACTACACAGTG 3′), and primer −10,000 (5′ ACTGGGCGTATGCCACTTGTCC 3′) were all made in the antisense orientation relative to the P-element primer located in the original CG8533 site (as determined from FlyBase). Each of the antisense primers was individually paired with the P-element primer and used on genomic DNA isolated from individual male recombinant lines. PCR products generated by these primer pairs were used to establish potential deletion borders existing in male recombinant lines within 10 kb of the 3′ limit of Dα1. Additional primers that are the reverse complement of the primers above were also generated so that ∼2-kb genomic intervals of homozygous viable recombinant lines could be surveyed for the presence or absence of the corresponding DNA region (e.g., primer −2000 INV is the complement of primer −2000. Its sequence is (5′ GGATGTGCTGGCCGCCAACAGC 3′).
PCR products generated with the primer pairs described above were cloned into the pCR II TOPO vector according to manufacturer's protocols (Invitrogen, Carlsbad, CA) and sequenced using a Beckman–Coulter CEQ80000 DNA sequencer.
Fertility assays:
For each female fertility assay, individual homozygous Dα1 deficiency female animals were mated to three w1118 (Dα1+) males. Control crosses utilized heterozygous Dα1−/Dα1+ females. A minimum of 20 crosses were performed for each trial with the number of fertile animals determined by the production of larval and/or adult progeny resulting in each vial. For male fertility assays, individual homozygous Dα1 deficiency male animals were crossed with three w1118 (Dα1+) females and the number of vials generating progeny determined. Heterozygous Dα1−/Dα1+ males were used as controls with ∼20 crosses used in each assay.
Fecundity assays:
Fecundity of Df(3L)α1S1 (Dα1−) females was measured by placing 50–100 females of each genotype along with 25–50 w1118 males in egg-laying cups. The females were allowed to lay eggs for three consecutive 24-hr periods on apple juice agar plates. Eggs were counted after each trial and the average number of eggs laid per female per day was calculated.
Transgene expression:
Crosses were performed between a UAS-α*/CyO; Df(3L)α1S1/TM6B line (* indicates Dα1, Dα2, or Dα3) and either a w1118;P{w[+mC]=GAL4∷VP16-nanos.UTR}MVD2, Df(3L)α1S1/TM6B line or a w1118; P{w[+mC]=Act5C-GAL4}25FO1/CyO; Df(3L)α1S1/TM6B line and homozygous Df(3L)α1S1/Df(3L)α1S1 animals carrying both UAS-α* and GAL4 driver chromosomes were isolated and tested for fertility. The statistical significance of differences in female fertility was determined by comparing the fertility of homozygous Dα1 mutant females to homozygous Dα1 mutant females expressing a Dα1, a Dα2, or a Dα3 transgene. P-values were calculated with the Fisher's exact test using Prism software (Graph Pad Software, San Diego).
Morphological analysis of Dα1 mutant testes and ovaries:
Testes were dissected from 1- to 2-day-old Dα1+/− and Dα1−/− males and ovaries were dissected from >2-day-old Dα1+/− and Dα1−/− females in 1× PBS buffer and stained with DAPI for ∼10 min in PBS buffer. Testes and ovaries were examined by DIC imaging and DAPI fluorescence using a Zeiss Axioplan 2 compound microscope equipped with a mercury lamp (Carl Zeiss, Jena, Germany). Images were collected using the Axiovision software and an ApoTome slider for optical sectioning (Carl Zeiss).
Kelch staining of ovaries was performed as previously described (Gorjanacz et al. 2002). Images were obtained by confocal microscopy using a Leica TCS NT microscope equipped with UV, Ar, Kr/Ar, and He/Ne lasers. The anti-Kelch antibody was developed by L. Cooley and provided by the Developmental Studies Hybridoma Bank (Iowa City). All digital images were processed using Adobe Photoshop software (Adobe Systems, San Jose, CA).
Ectopic overexpression of Dα1, -2, and -3:
To ectopically express the Drosophila importin α's, UASt Dα1, -2, or -3 transgenic males (Mason et al. 2002) were crossed to Gal4e22c/CyO females and UASt Dα1, -2, or -3/Gal4e22c offspring were examined for lethality and morphological defects. To generate a stock ectopically expressing Dα1, the UASt Dα1 transgene (Mason et al. 2002) was recombined onto the Gal4e22c chromosome. This was achieved by crossing UASt Dα1/Gal4e22c females to Sco/Cyo males and selecting for offspring which have tergite defects associated with ectopic expression of Dα1 (see text).
Genetic interactions were examined for ectopic expression of Dα1 with mutations in known nuclear transport genes by crossing Gal4e22c, UASt Dα1/Cyo females to the following males at 25° or 27.5°: (a) KetelRx41/Sco, (b) KetelRe34/Sco, (c) Dcasl(2)k03902/Sco, (d) Dcas16-1/Sco, (e) DcasDf(2L)H20/Sco, (f) UASt Dcas/Sco, (g) UASt cNLS GFP/UASt cNLS GFP, (h) UASt GFP/UASt GFP, and (i) UASt Dα2/UASt Dα2.
The Dcas and Ketel mutations were made heterozygous over Sco in the parental males to allow unambiguous identification and survival of all offspring genotypes. To quantify the ability of mutations to enhance or suppress the decreased viability of Dα1 overexpressing flies, a viability index was calculated from the ratio of the number of offspring that contain the UASt Gal4e22c, Dα1 chromosome compared to sibling controls that received the Cyo chromosome. P-values were calculated with Fisher's exact test using Prism (Graph Pad Software).
RESULTS
In an effort to determine the functional role of an animal importin α1, we wished to isolate a loss-of-function mutation in the Drosophila importin α1 paralog importin α1 (Dα1) (Mason et al. 2002). To this end we performed a male recombination screen to recover small P element-mediated deletions that incorporated Dα1 (supplemental data at http://www.genetics.org/supplemental/). Recombination in Drosophila males can occur by aberrant transposon mobilization events in animals heterozygous for a P-element insertion (Gray et al. 1996; Preston and Engels 1996). Recombinants recovered from these types of events often display small deletions at the site of the recombination event (Gray et al. 1996).
Among the strains isolated in this screen (see materials and methods and supplemental data) we chose to proceed with one containing a deletion that by DNA sequence analysis was shown to originate within the CG8533 P element and terminate 52 bp to the 5′ side of the Cyp305a1 gene lying downstream of the Dα1 locus (see Figure 1 and supplemental data). This deletion, named Df(3L)α1S1, removes most of CG8533 and part or all of several other predicted genes including Dα1 and results in homozygous viable adult animals.
Gene arrangement of the 76C5–76C6 interval. Predicted and defined genes in the interval are shaded with the direction of transcription denoted by the arrowheads. The central bar indicates the scale of the region marked in 1-kb intervals with the demarcation between the 76C5 and 76C6 cytological band marked by the heavy vertical line. The inverted triangle above the CG8533 gene represents the white+ bearing P-element insertion at that site. The names of the primers used for PCR amplification of genomic regions are shown at the bottom of the bar. The extent of Df(3L)α1S1 is denoted by the open box. Map modified from FlyBase (Crosby et al. 2007).
Homozygous Df(3L)α1S1 flies develop to adulthood but are sterile:
Although homozygous Df(3L)α1S1 flies developed to adulthood, the deletion could not be maintained as a homozygous stock because both sexes were sterile. The fertility of homozygous Df(3L)α1S1 males and females was quantified by crossing individual mutant flies to three essentially wild-type w1118 virgin females or males. As shown in Table 1, homozygous Df(3L)α1S1 males were completely sterile (23/23 animals tested) while females were almost completely sterile (32/33 animals tested). The single nonsterile female only produced three adult progeny. The only obvious defect in the soma of Df(3L)α1S1 flies was that the wings of both sexes, although of normal size and shape, appeared dull and also somewhat fragile, and many were damaged or improperly unfolded compared to those of sibling heterozygous animals (not shown). These results demonstrate that Dα1, the only Drosophila importin α1 paralog, is almost completely dispensable for development of the soma.
Paralog-specific function of Dα1 in male and female fertility
Dα1a . | Transgeneb . | Gal4 driverc . | Fertile malesd (%) . | Fertile femalesd (%) . |
|---|---|---|---|---|
| −/− | − | − | 0 | 3 |
| (n = 23) | (n = 33) | |||
| Dα1 | + | 92.3 | 88 | |
| (n = 26) | (n = 25) | |||
| P < 0.0001e | ||||
| Dα2 | + | 0 | 8 | |
| (n = 19) | (n = 25) | |||
| P = 0.5791 | ||||
| Dα3 | + | 0 | 0 | |
| (n = 19) | (n = 14) | |||
| P = 1.0000 |
Dα1a . | Transgeneb . | Gal4 driverc . | Fertile malesd (%) . | Fertile femalesd (%) . |
|---|---|---|---|---|
| −/− | − | − | 0 | 3 |
| (n = 23) | (n = 33) | |||
| Dα1 | + | 92.3 | 88 | |
| (n = 26) | (n = 25) | |||
| P < 0.0001e | ||||
| Dα2 | + | 0 | 8 | |
| (n = 19) | (n = 25) | |||
| P = 0.5791 | ||||
| Dα3 | + | 0 | 0 | |
| (n = 19) | (n = 14) | |||
| P = 1.0000 |
Dα1−/− animals are homozygous for the Df(3L)α1S1 deficiency as described in the text.
Rescue of the sterility phenotype was assayed using the previously described UASp Dα1, α2, or α3 transgenes (Mason et al. 2003).
The Act5C Gal4 driver was used for male rescue and the VP16-nanos Gal4 driver was used for the female rescue.
Male and female fertility assays were performed by mating individual homozygous Df(3L)α1S1 animals with three w1118 animals of the opposite sex.
P-values were calculated as described in materials and methods by comparing the fertility of Dα1−/− control females to Dα1−/− females expressing Dα1, Dα2, or Dα3 transgene.
Paralog-specific function of Dα1 in male and female fertility
Dα1a . | Transgeneb . | Gal4 driverc . | Fertile malesd (%) . | Fertile femalesd (%) . |
|---|---|---|---|---|
| −/− | − | − | 0 | 3 |
| (n = 23) | (n = 33) | |||
| Dα1 | + | 92.3 | 88 | |
| (n = 26) | (n = 25) | |||
| P < 0.0001e | ||||
| Dα2 | + | 0 | 8 | |
| (n = 19) | (n = 25) | |||
| P = 0.5791 | ||||
| Dα3 | + | 0 | 0 | |
| (n = 19) | (n = 14) | |||
| P = 1.0000 |
Dα1a . | Transgeneb . | Gal4 driverc . | Fertile malesd (%) . | Fertile femalesd (%) . |
|---|---|---|---|---|
| −/− | − | − | 0 | 3 |
| (n = 23) | (n = 33) | |||
| Dα1 | + | 92.3 | 88 | |
| (n = 26) | (n = 25) | |||
| P < 0.0001e | ||||
| Dα2 | + | 0 | 8 | |
| (n = 19) | (n = 25) | |||
| P = 0.5791 | ||||
| Dα3 | + | 0 | 0 | |
| (n = 19) | (n = 14) | |||
| P = 1.0000 |
Dα1−/− animals are homozygous for the Df(3L)α1S1 deficiency as described in the text.
Rescue of the sterility phenotype was assayed using the previously described UASp Dα1, α2, or α3 transgenes (Mason et al. 2003).
The Act5C Gal4 driver was used for male rescue and the VP16-nanos Gal4 driver was used for the female rescue.
Male and female fertility assays were performed by mating individual homozygous Df(3L)α1S1 animals with three w1118 animals of the opposite sex.
P-values were calculated as described in materials and methods by comparing the fertility of Dα1−/− control females to Dα1−/− females expressing Dα1, Dα2, or Dα3 transgene.
Loss of Dα1 is responsible for the phenotypic defects of Df(3L)α1S1 flies:
Because Df(3L)α1S1 removes multiple open reading frames (Figure 1), we wished to determine if the mutant phenotypes could be attributed exclusively to the loss of Dα1. We therefore expressed the cDNA for Dα1 in the Df(3L)α1S1 background using the Gal4 system (Brand and Perrimon 1993). We initially used the Act5CGal4 promoter that is active in all cells. Expression of UAS Dα1 using Act5CGal4 in homozygous mutant Df(3L)α1S1 males efficiently restored fertility (Table 1). We conclude that the male sterility defect is due strictly to the loss of Dα1, and that the other deleted genes do not contribute significantly to the phenotype. In the same cross, the wing defect in both males and females was also rescued by the UAS Dα1 transgene using the Act5CGal4 driver (not shown).
In contrast to the efficient rescue of male sterility, the sterility of homozygous Df(3L)α1S1 females was only moderately rescued by a Act5CGal4-driven UAS Dα1 transgene (15/24 animals tested produced progeny), and the fecundity of the fertile females was low (3–4 progeny per productive cross). In contrast, the use of a VP16-nanos Gal4 promoter, which is specifically active in the female germ line and expressed in developing oocytes (Rorth 1998), rescued the sterility defect much more efficiently (Table 1). The fecundity of these females was nearly normal (Table 2). Thus both male and female sterility phenotypes were rescued by Dα1 transgenes, demonstrating that the loss of Dα1 is responsible for the male and female sterility and wing defects of homozygous Df(3L)α1S1 flies (referred to hereafter as Dα1−/−). We conclude that Dα1 is required for male and female fertility and for normal wing development.
Paralog-specific rescue of the Dα1−/− defect in egg laying
Dα1−/− animals are homozygous for the Df(3L)α1S1 deficiency as described in the text.
Rescue of the egg-laying defective phenotype was assayed using the previously described UASp Dα1, -α2, or -α3 transgenes (Mason et al. 2003). Transgene expression was driven using VP16-nanos Gal4.
Egg-laying assays were performed as described in materials and methods.
Paralog-specific rescue of the Dα1−/− defect in egg laying
Dα1−/− animals are homozygous for the Df(3L)α1S1 deficiency as described in the text.
Rescue of the egg-laying defective phenotype was assayed using the previously described UASp Dα1, -α2, or -α3 transgenes (Mason et al. 2003). Transgene expression was driven using VP16-nanos Gal4.
Egg-laying assays were performed as described in materials and methods.
The sterility defect of Dα1 null flies is due to a paralog-specific activity of Dα1:
All three conventional Drosophila importin α's (Dα1, Dα2, and Dα3) are expressed in testes, so it is important to determine if the sterility defect of Dα1 null flies is due to the loss of a paralog-specific or a shared activity. As summarized in Tables 1 and 2, Dα2 and Dα3 transgenes did not rescue the sterility/fecundity defects of Dα1−/− flies. Likewise, Dα2 and Dα3 transgenes did not rescue the minor wing defect (not shown). The Dα2 and Dα3 transgenes are functional as they are able to specifically rescue defects associated with mutations in Dα2 and Dα3, respectively (Mason et al. 2002, 2003). Therefore, we conclude that Dα1 performs paralog-specific activities that are required for oogenesis, spermatogenesis, and wing development.
Dα1 is required for the completion of spermatogenesis:
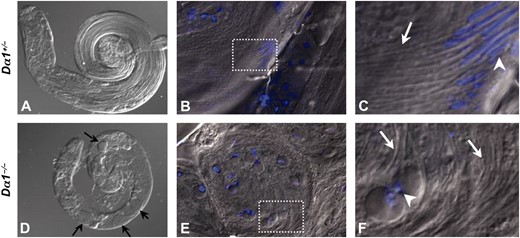
In an effort to determine the cause of the sterility of Dα1−/− males, testes were dissected from young adult males and examined by microscopy. Testes squashes from Dα1−/− males released no motile sperm (not shown) indicating a profound defect in spermatogenesis. This observation was substantiated by closer examination of mutant testes. As shown in Figure 2, A and D, 1- to 2-day-old Dα1−/− male testes were notably smaller than those from similarly aged heterozygous males. The testes dissected from heterozygous mutant animals were morphologically normal and filled with bundles of elongated sperm (Figure 2, A–C). In contrast, Dα1−/− testes contained numerous large round cysts filled with abnormal spermatocytes (arrows in Figure 2D). The flagella of mutant spematocytes appeared to have at least partially elongated, but they were not properly organized into bundles. In addition, the nuclei of the mutant spermatocytes appeared abnormal (white arrowhead in Figure 2, C and F). The large round shape of the Dα1−/− spermatocyte nuclei suggests that Dα1 may be required, either directly or indirectly, for chromatin condensation, which normally occurs after flagellar elongation and prior to individualization (Fuller 1998). This phenotype is clearly different from the incompletely penetrant defect in spermatogenesis observed in Dα2 null flies. Testes from Dα2 mutants contained numerous elongated well-organized sperm bundles but exhibited a defect in individualization (Mason et al. 2002). We conclude that Dα1 and Dα2 serve different important functions during spermatogenesis.
Morphology of Dα1 mutant testes. Testes dissected from 1- to 2-day-old males of the genotype Dα1+/− (A–C) and Dα1−/− (D–F) were DAPI stained and examined with DIC imaging. An overlay of DAPI and DIC is shown in B, C, E, and F. Low magnification of Dα1+/− and Dα1−/− testes demonstrate that mutant testes (D) were smaller than testes from heterozygous sibling males (A) and contained multiple round cysts of defective spermatocytes (black arrows in D). High magnification of elongated sperm bundles in Dα1+/− (B) and defective spermatocyte cysts from Dα1−/− (E). C and F correspond to a blowup of boxed areas in B and E. White arrows in C and F highlight elongated sperm tails in both heterozygous and homozygous mutant animals and white arrowheads mark nuclei positions. Note the characteristic condensed canoe-shaped nuclei in heterozygous spermatocytes (C) and the uncondensed round nuclei in homozygous mutant spermatocytes (F).
Dα1 is not required for Kelch localization to ring canals:
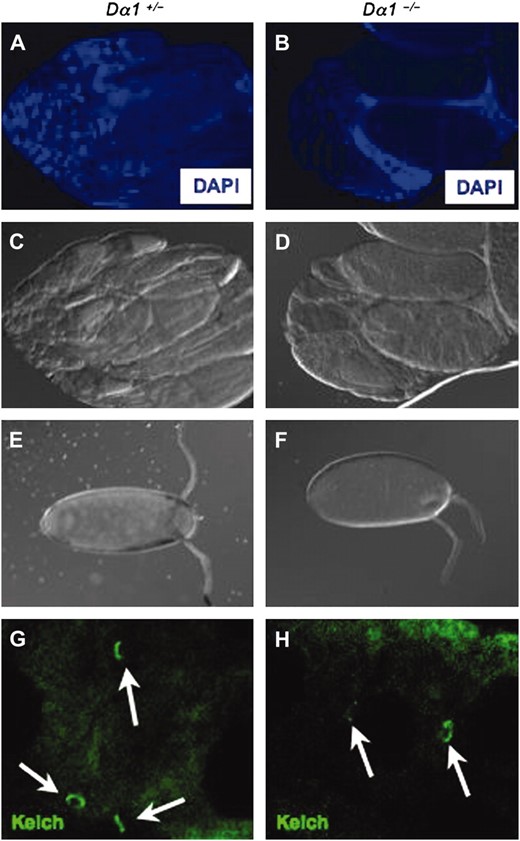
In contrast to the profound defect in spermatogenesis, Dα1−/− ovaries displayed only relatively minor defects. Specifically, mutant ovaries were smaller than wild-type ovaries and contained fewer ovarioles (Figure 3, A–D). The mature eggs in Dα1−/− exhibited only slight defects in shape and size, indicating that Dα1 plays a minor but important role in oogenesis (Figure 3, E and F). In contrast, oocytes from Dα2−/− mutant females exhibited a deflated morphology that corresponded to a defect in the targeting of the protein Kelch to the ring canals, through which the contents of nurse cells are dumped into the developing oocyte (Gorjanacz et al. 2002; Mason et al. 2002). Although Dα1 transgenes were unable to rescue the Kelch localization defect in Dα2−/− ovaries (Mason et al. 2003), it is possible that both importin α's are required for distinct steps leading to proper Kelch localization. A minor dumping defect could explain why Dα1−/− eggs are slightly smaller than wild type (Figure 3). Thus we investigated whether Dα1 is also required for proper Kelch localization. As shown in Figure 3, Kelch is correctly targeted to ring canals in Dα1 mutant ovaries, although we cannot rule out a minor defect (Figure 3, G and H). Dα1−/− females also displayed a severe egg-laying defect (Table 2). Specifically, Dα1−/− females lay on average <1 egg/day as opposed to the ∼30 eggs/day laid by Dα1+/− control females. This phenotype was rescued with the Dα1 transgene, but not the Dα2 and Dα3 transgenes (Table 2). Therefore the sterility of Dα1−/− females may be confounded by additional defects in egg-laying behavior or defects in the oviducts. Alternatively the egg-laying defect may be an indirect result of the subtle oogenesis defect. We conclude that the sterility defect of Dα1 null females is due either to some important but morphologically subtle defect in oogenesis or, possibly, could involve factors such as egg-laying or mating behavior.
Morphology of Dα1 mutant ovaries. Ovaries dissected from >2-day-old females of the genotype Dα1+/− (A and C) or Dα1−/− (B and D) were DAPI stained (A and B) and examined with DIC imaging (C and D). Dα1−/− mutant ovaries contained fewer ovarioles than ovaries from Dα1+/− siblings. Unlaid mature eggs from Dα1+/− (E) and Dα1−/− (F) ovaries revealed only minor defects in overall egg morphology in mutant ovaries. Kelch immunofluorescence of Dα1+/− (G) and Dα1−/− (H) ovaries demonstrated that Kelch localizes to ring canals (arrows) in Dα1 mutant ovaries.
Ectopic expression of Dα1 and Dα3 result in abdominal defects:
In conjunction with our previous work, it has now been demonstrated that all three conserved Drosophila importin α's, Dα1, Dα2, and Dα3, perform distinct paralog-specific functions in vivo (Mason et al. 2002, 2003). These results raise the possibility that regulated activity of specific importin α-forms may be a mechanism by which cell fate decisions are controlled. Consistent with this, it has been found that the switch in expression from a mouse importin α2 paralog to an α1 paralog regulates neural differentiation of embryonic stem cells (Yasuhara et al. 2007). To further explore this possibility we examined the phenotypes associated with ectopic overexpression of Drosophila importin α's. Previous results demonstrated that overexpression of Dα1 during development decreases viability (Mason et al. 2003). For this reason, we hypothesized that Dα1 overexpression might alter the activity of nuclear proteins that are specific targets of an importin α1, resulting in a partial lethal phenotype. To begin to test this hypothesis we examined the phenotypes associated with ectopic expression of Dα1, Dα2, and Dα3 using the Gal4e22c driver.
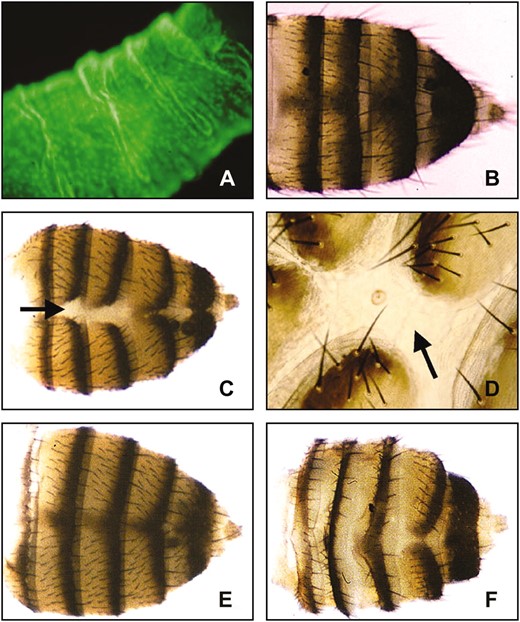
The Gal4e22c transgenic line expresses Gal4 constitutively in the embryo (McCartney et al. 1999). Using a UASt GFP transgene crossed to Gal4e22c we were also able to observe a high level of expression in the larval epidermis (Figure 4) and the developing pupal abdomen (not shown). Ectopic expression of UASt Dα1 and Dα3 at 25° via Gal4e22c caused partial lethality and, among surviving adults, produces an incompletely penetrant defect in adult abdominal development (Table 3, Figure 4). These defects were not observed with expression of UASt Dα2 (Table 3). Expression of Dα1 (Table 5) and Dα3 (not shown) at 27.5°, was almost completely lethal, while UASt Dα2-expressing progeny did not display any defects when raised at temperatures up to 29° (not shown). The UASt Dα2 transgene is functional because it rescues the sterility defect of Dα2−/− flies (Mason et al. 2002). Dα2 can contribute to the lethality phenotype since offspring expressing both Dα1 and Dα2 at 25° have lower viability than offspring only expressing Dα1 (not shown). The lack of a defect associated with ectopic expression of Dα2 could be due to lower expression levels from the Dα2 transgene.
Tergite defects associated with overexpression of importin α1. (A) Gal4e22c/UASt-GFP third instar larvae showing GFP expression at a high level in the larval epidermis. (B) Standard w1118 flies not expressing importin α-transgenes show normal tergite development. (C) Ectopic expression of UASt Dα1 with Gal4e22c at 25° results in a partially penetrant defect in tergite development. (D) Higher magnification of abdomen shown in C. Adult hemitergites fail to properly fuse at the dorsal midline, leaving a stripe of presumptive larval tissue (arrows in C and D). (E) These phenotypes are suppressed by mutations in the Drosophila importin β1 homolog, Ketel. The majority of Gal4e22c, UASt Dα1/KetelRe34 (E) or Gal4e22c, UASt Dα1/KetelRx41 (not shown) adults display normal hemitergite fusion or very slight tergite defects (not shown). (F) Mutations in the Drosophila CAS homolog, Dcas, enhance the defect in abdominal development. Gal4e22c, UASt Dα1/Dcasl(2)k03902 surviving flies display abdomens with much thinner tergites and appear to have an abundance of presumptive larval tissue in the intertergal area.
Ectopic expression of importin α-transgenes at 25°
Genotypea . | % tergite defectb . | Viability indexc . |
|---|---|---|
| UASt Dα1/Gal4e22C | 96 | 0.35 |
| (n = 111) | (111/310) | |
| UASt Dα2/Gal4e22C | 0 | 1.5 |
| (n = 169) | (169/115) | |
| UASt Dα3/Gal4e22C | 65 | 0.8 |
| (n = 162) | (162/202) |
Genotypea . | % tergite defectb . | Viability indexc . |
|---|---|---|
| UASt Dα1/Gal4e22C | 96 | 0.35 |
| (n = 111) | (111/310) | |
| UASt Dα2/Gal4e22C | 0 | 1.5 |
| (n = 169) | (169/115) | |
| UASt Dα3/Gal4e22C | 65 | 0.8 |
| (n = 162) | (162/202) |
Gal4e22C/CyO females were crossed to homozygous UASt Dα1, Dα2, or Dα3 males at 25°.
Percentage of flies with dorsal clefts in their tergites was determined for offspring expressing the UASt importin α-transgenes.
Viability indexes were calculated by dividing the number of offspring of the genotype UASt Dα1, -α2, or -α3/Gal4e22C by the number of sibling offspring of the genotype UASt Dα1, -α2, or -α3/CyO.
Ectopic expression of importin α-transgenes at 25°
Genotypea . | % tergite defectb . | Viability indexc . |
|---|---|---|
| UASt Dα1/Gal4e22C | 96 | 0.35 |
| (n = 111) | (111/310) | |
| UASt Dα2/Gal4e22C | 0 | 1.5 |
| (n = 169) | (169/115) | |
| UASt Dα3/Gal4e22C | 65 | 0.8 |
| (n = 162) | (162/202) |
Genotypea . | % tergite defectb . | Viability indexc . |
|---|---|---|
| UASt Dα1/Gal4e22C | 96 | 0.35 |
| (n = 111) | (111/310) | |
| UASt Dα2/Gal4e22C | 0 | 1.5 |
| (n = 169) | (169/115) | |
| UASt Dα3/Gal4e22C | 65 | 0.8 |
| (n = 162) | (162/202) |
Gal4e22C/CyO females were crossed to homozygous UASt Dα1, Dα2, or Dα3 males at 25°.
Percentage of flies with dorsal clefts in their tergites was determined for offspring expressing the UASt importin α-transgenes.
Viability indexes were calculated by dividing the number of offspring of the genotype UASt Dα1, -α2, or -α3/Gal4e22C by the number of sibling offspring of the genotype UASt Dα1, -α2, or -α3/CyO.
In addition to causing lethality, the ectopic overexpression of Dα1 and Dα3 caused defects in the development of the abdominal cuticle. The dorsal cuticle of the adult abdomen is composed of six or seven (in females) rectangular plates called tergites, which are decorated with posterior-pointing bristles (microchaetae and macrochaetae) (Madhavan and Madhavan 1980). In surviving Dα1- and Dα3-expressing flies, a common phenotype is the failure of the hemitergites to fuse, leaving a visible stripe of colorless tissue that could be either intersegmental cuticle or persistent larval tissue (Table 3; Figure 4, C and D). The patterning within each tergite appears relatively normal since the anterior, central, and posterior regions of each tergite segment are visible, appropriately pigmented, and decorated with bristles (Struhl et al. 1997). In conclusion, ectopic expression of Dα1 and Dα3, but not Dα2, causes partial lethality and, in surviving flies, defects in tergite development. These defects are more severe in Dα1- than in Dα3-overexpressing flies.
Mutations in Dcas and Ketel (importin β1) interact with Dα1 ectopic expression phenotypes:
To our knowledge the preceding experiments are the first to describe deleterious effects associated with the ectopic expression of an animal importin α. To further explore the underlying mechanism we examined the effects of ectopic Dα1 expression in genetic backgrounds that have altered levels of the two karyopherins that mediate the import (importin β/Ketel) and export (CAS/Dcas) phases of the importin α-targeting cycle. As described below, circumstances that are expected to promote the import or inhibit the export of Dα1 (possibly increasing nuclear Dα1 levels) enhance the defects, and those expected to inhibit import or enhance export (possibly decreasing nuclear Dα1 levels) suppress the defects.
We first examined flies expressing UASt Dα1 under Gal4e22c control in heterozygous Dcas mutant genetic backgrounds and assayed for enhancement or suppression of the lethality and tergite phenotypes. The Df(2L)H20 deficiency completely removes Dcas (I. Davis, personal communication) and is viable when heterozygous. Heterozygous Df(2L)H20 decreases the viability of Gal4e22c, UASt Dα1 flies such that complete lethality is observed at 25° (Table 4). Since heterozygous Df(2L)H20 animals are less viable than wild-type animals (not shown), it is possible that its effects on Gal4e22c-mediated Dα1 expression may simply be additive rather than specific. We therefore obtained a P element that disrupts Dcas (Dcasl(2)k03902) (Tekotte et al. 2002) and acts as a simple recessive lethal mutation. Significantly, when Dcasl(2)k03902 is heterozygous in the presence of Gal4e22c, UASt Dα1 expression, the associated lethality is substantially increased at 25° (Table 4). In addition, surviving Dα1-expressing, Dcasl(2)k03902 heterozygous flies exhibited a new defect in tergite development such that tergites appear thinner and retained only the posterior pigmented band, which resulted in an apparent expansion of the intertergal region (Figure 4F). Significantly, the enhancement of the Dα1 ectopic expression phenotypes is eliminated by precise excision of the P element within Dcas (Dcas16-1) (Tekotte et al. 2002) demonstrating that the enhancement by Dcasl(2)k03902 is specific for the P-element insertion (Table 4). To further test whether Dcas interacts with the Dα1 overexpression phenotype we created a UASt Dcas transgene. Consistent with the observation that mutations in Dcas enhance Dα1 expression phenotypes, we found that ectopic expression of Dcas and Dα1 at 27.5° caused a significant suppression of lethality (Table 5). However, the expression of UASt Dcas at 25° with the Gal4e22c driver also caused a failure of the tergites to fuse (not shown). Therefore, Dcas seems to interact with the lethality phenotype in a consistent manner, but it is still unclear as to the precise nature of the interaction of Dcas with the tergite phenotype.
Mutations in Dcas enhance the lethality associated with Dα1 expression
Crossa . | Genotypeb . | Viability indexc . | . |
|---|---|---|---|
| 1 | UASt Dα1, Gal4e22C/Sco | 0.65 (63/97) | P < 0.0001 |
| UASt Dα1, Gal4e22C/DcasDf(2L)H20 | 0 (0/72) | ||
| 2 | UASt Dα1, Gal4e22C/Sco | 0.83 (158/189) | P < 0.0001 |
| UASt Dα1, Gal4e22C/DcasI(2)k03902 | 0.12 (28/229) | ||
| 3 | UASt Dα1, Gal4e22C/Sco | 0.76 (97/127) | P < 0.8538 |
| UASt Dα1, Gal4e22C/Dcas16-1 | 0.79 (111/140) |
Crossa . | Genotypeb . | Viability indexc . | . |
|---|---|---|---|
| 1 | UASt Dα1, Gal4e22C/Sco | 0.65 (63/97) | P < 0.0001 |
| UASt Dα1, Gal4e22C/DcasDf(2L)H20 | 0 (0/72) | ||
| 2 | UASt Dα1, Gal4e22C/Sco | 0.83 (158/189) | P < 0.0001 |
| UASt Dα1, Gal4e22C/DcasI(2)k03902 | 0.12 (28/229) | ||
| 3 | UASt Dα1, Gal4e22C/Sco | 0.76 (97/127) | P < 0.8538 |
| UASt Dα1, Gal4e22C/Dcas16-1 | 0.79 (111/140) |
UASt Dα1, Gal4e22C/CyO females were crossed to DcasDf(2L)H20/Sco (cross 1), Dcasl(2)k03902/Sco (cross 2), or Dcas16-1/Sco (cross 3) males at 25°.
DcasDf(2L)H20 is a deficiency with breakpoints 36A8–9; 361, Dcasl(2)k03902 is a P element in the 5′ region of Dcas, and Dcas16-1 is a precise excision of Dcasl(2)k03902.
Viability indexes were calculated for the Sco (control) or Dcas (experimental) mutant backgrounds by dividing the number of offspring that inherited the UASt Dα1, Gal4e22C chromosome (non-CyO) by the number of sibling offspring that did not inherit the UASt Dα1, Gal4e22C (CyO). P-values were calculated as described in materials and methods.
Mutations in Dcas enhance the lethality associated with Dα1 expression
Crossa . | Genotypeb . | Viability indexc . | . |
|---|---|---|---|
| 1 | UASt Dα1, Gal4e22C/Sco | 0.65 (63/97) | P < 0.0001 |
| UASt Dα1, Gal4e22C/DcasDf(2L)H20 | 0 (0/72) | ||
| 2 | UASt Dα1, Gal4e22C/Sco | 0.83 (158/189) | P < 0.0001 |
| UASt Dα1, Gal4e22C/DcasI(2)k03902 | 0.12 (28/229) | ||
| 3 | UASt Dα1, Gal4e22C/Sco | 0.76 (97/127) | P < 0.8538 |
| UASt Dα1, Gal4e22C/Dcas16-1 | 0.79 (111/140) |
Crossa . | Genotypeb . | Viability indexc . | . |
|---|---|---|---|
| 1 | UASt Dα1, Gal4e22C/Sco | 0.65 (63/97) | P < 0.0001 |
| UASt Dα1, Gal4e22C/DcasDf(2L)H20 | 0 (0/72) | ||
| 2 | UASt Dα1, Gal4e22C/Sco | 0.83 (158/189) | P < 0.0001 |
| UASt Dα1, Gal4e22C/DcasI(2)k03902 | 0.12 (28/229) | ||
| 3 | UASt Dα1, Gal4e22C/Sco | 0.76 (97/127) | P < 0.8538 |
| UASt Dα1, Gal4e22C/Dcas16-1 | 0.79 (111/140) |
UASt Dα1, Gal4e22C/CyO females were crossed to DcasDf(2L)H20/Sco (cross 1), Dcasl(2)k03902/Sco (cross 2), or Dcas16-1/Sco (cross 3) males at 25°.
DcasDf(2L)H20 is a deficiency with breakpoints 36A8–9; 361, Dcasl(2)k03902 is a P element in the 5′ region of Dcas, and Dcas16-1 is a precise excision of Dcasl(2)k03902.
Viability indexes were calculated for the Sco (control) or Dcas (experimental) mutant backgrounds by dividing the number of offspring that inherited the UASt Dα1, Gal4e22C chromosome (non-CyO) by the number of sibling offspring that did not inherit the UASt Dα1, Gal4e22C (CyO). P-values were calculated as described in materials and methods.
Suppression of lethality associated with Dα1 expression at 27.5°
Crossa . | Genotypeb . | Viability indexc . | . |
|---|---|---|---|
| 1 | UASt Dα1, Gal4e22C/+ | 0.02 (4/196) | |
| 2 | UASt Dα1, Gal4e22C/Sco | 0.07 (6/85) | P < 0.0001 |
| UASt Dα1, Gal4e22C/KetelRe34 | 0.52 (65/125) | ||
| 3 | UASt Dα1, Gal4e22C/Sco | 0.04 (5/135) | P < 0.0001 |
| UASt Dα1, Gal4e22C/KetelRx41 | 0.42 (80/190) | ||
| 4 | UASt Dα1, Gal4e22C/Sco | 0.07 (6/85) | P < 0.0001 |
| UASt Dα1, Gal4e22C/UASt Dcas | 0.88 (99/112) |
Crossa . | Genotypeb . | Viability indexc . | . |
|---|---|---|---|
| 1 | UASt Dα1, Gal4e22C/+ | 0.02 (4/196) | |
| 2 | UASt Dα1, Gal4e22C/Sco | 0.07 (6/85) | P < 0.0001 |
| UASt Dα1, Gal4e22C/KetelRe34 | 0.52 (65/125) | ||
| 3 | UASt Dα1, Gal4e22C/Sco | 0.04 (5/135) | P < 0.0001 |
| UASt Dα1, Gal4e22C/KetelRx41 | 0.42 (80/190) | ||
| 4 | UASt Dα1, Gal4e22C/Sco | 0.07 (6/85) | P < 0.0001 |
| UASt Dα1, Gal4e22C/UASt Dcas | 0.88 (99/112) |
UASt Dα1, Gal4e22C/CyO females were crossed to w1118/y; +/+ (Cross 1), KetelRe34/Sco (Cross 2), KetelRx41/Sco (Cross 3), or UASt Dcas/Sco males at 27.5°.
KetelRe34 and KetelRx41 are loss-of-function alleles of the Drosophila importin β1 gene. UASt Dcas contains the full-length Dcas cDNA in the pUASt vector.
Viability indexes were calculated for the Sco (control) or Ketel mutant or UASt Dcas (experimental) backgrounds by dividing the number of offspring that inherited the UASt Dα1, Gal4e22C chromosome (non-CyO) by the number of sibling offspring that did not inherit the UASt Dα1, Gal4e22C chromosome (CyO). P-values were calculated as described in materials and methods.
Suppression of lethality associated with Dα1 expression at 27.5°
Crossa . | Genotypeb . | Viability indexc . | . |
|---|---|---|---|
| 1 | UASt Dα1, Gal4e22C/+ | 0.02 (4/196) | |
| 2 | UASt Dα1, Gal4e22C/Sco | 0.07 (6/85) | P < 0.0001 |
| UASt Dα1, Gal4e22C/KetelRe34 | 0.52 (65/125) | ||
| 3 | UASt Dα1, Gal4e22C/Sco | 0.04 (5/135) | P < 0.0001 |
| UASt Dα1, Gal4e22C/KetelRx41 | 0.42 (80/190) | ||
| 4 | UASt Dα1, Gal4e22C/Sco | 0.07 (6/85) | P < 0.0001 |
| UASt Dα1, Gal4e22C/UASt Dcas | 0.88 (99/112) |
Crossa . | Genotypeb . | Viability indexc . | . |
|---|---|---|---|
| 1 | UASt Dα1, Gal4e22C/+ | 0.02 (4/196) | |
| 2 | UASt Dα1, Gal4e22C/Sco | 0.07 (6/85) | P < 0.0001 |
| UASt Dα1, Gal4e22C/KetelRe34 | 0.52 (65/125) | ||
| 3 | UASt Dα1, Gal4e22C/Sco | 0.04 (5/135) | P < 0.0001 |
| UASt Dα1, Gal4e22C/KetelRx41 | 0.42 (80/190) | ||
| 4 | UASt Dα1, Gal4e22C/Sco | 0.07 (6/85) | P < 0.0001 |
| UASt Dα1, Gal4e22C/UASt Dcas | 0.88 (99/112) |
UASt Dα1, Gal4e22C/CyO females were crossed to w1118/y; +/+ (Cross 1), KetelRe34/Sco (Cross 2), KetelRx41/Sco (Cross 3), or UASt Dcas/Sco males at 27.5°.
KetelRe34 and KetelRx41 are loss-of-function alleles of the Drosophila importin β1 gene. UASt Dcas contains the full-length Dcas cDNA in the pUASt vector.
Viability indexes were calculated for the Sco (control) or Ketel mutant or UASt Dcas (experimental) backgrounds by dividing the number of offspring that inherited the UASt Dα1, Gal4e22C chromosome (non-CyO) by the number of sibling offspring that did not inherit the UASt Dα1, Gal4e22C chromosome (CyO). P-values were calculated as described in materials and methods.
The findings that mutations in Dcas increase and overexpression of Dcas decreases the severity of Dα1 overexpression phenotypes suggest that high nuclear levels of Dα1 may cause developmental defects. If true, then mutations that reduce the entry of Dα1 into the nucleus should suppress the Dα1 ectopic expression phenotype. To examine this possibility, genetic interactions with mutations in the Drosophila importin β1 homolog Ketel were examined (Erdélyi et al. 1997).
As predicted, mutations in Ketel produced effects on ectopic Dα1 expression in the opposite direction to those of Dcas. Expression of the Dα1 transgene in flies heterozygous for the KetelRe34 or KetelRx41 loss-of-function alleles resulted in a slight increase in viability of animals at 25° (not shown) and a significantly increased viability at 27.5° (Table 5). In addition, less severe defects in tergite morphology were present in Dα1-overexpressing flies heterozygous for Ketel mutations (Figure 4). In flies where Gal4e22c, UASt Dα1 is expressed in a wild-type background, almost 70% of progeny displayed defects in two or more abdominal segments while only 20% had normal tergites. In contrast, sibling controls that express Dα1 in a heterozygous KetelRx41 background resulted in nearly 70% of progeny with normal abdominal morphology. A third allele of Ketel, KetelRx22, does not suppress the Dα1 expression phenotypes (not shown), indicating that this allele behaves differently for unknown reasons. Nonetheless, we conclude that animals with heightened levels of Dα1 expression fare better if they have a simultaneous reduction in the level of importin β1.
To further test the model that high levels of nuclear importin α are deleterious, we attempted to drive importin α into the nucleus by increasing the expression of cNLS cargo. The ectopic expression of an importin α-cargo enhanced the lethality associated with ectopic expression of Dα1. Specifically, coexpression of a UASt cNLS GFP construct and UASt Dα1 caused complete lethality at 25° [viability index = 0 (0/189), P < 0.0001 compared to viability index of 0.76 calculated from the pooled Gal4e22c, UASt Dα1/Sco at 25° data set]. This appears to be specific for cNLS–GFP and is not a consequence of overexpressing GFP since coexpression of a UASt GFP construct and Dα1 did not significantly enhance the lethality Dα1 overexpression at 25° [viability index = 0.7 (102/145), P = 0.5530 compared to viability index of 0.76 calculated from the pooled Gal4e22c, UASt Dα1/Sco at 25° data set]. In conclusion, genetic interactions between ectopic expression of Dα1 and altered expression of Dcas, Ketel, and cNLS cargo suggest that elevated nuclear levels of importin α are deleterious and cause death during pupation. These studies indicate that both the expression and nucleocytoplasmic trafficking of importin α's during development must be maintained under tight control.
DISCUSSION
Cargo adapters such as importin α may have evolved to provide a greater range of control over nuclear transport in response to variable environmental conditions (see Riddick and Macara 2007). The evolution of multiple importin α-genes would seem to extend the utility of these adapters by allowing the independent control of distinct sets of cargo repertoires. We have taken a genetic approach in Drosophila to analyze the in vivo function of the conserved family of animal importin α1's, α2's, and α3's. In addition to binding unique repertoires of NLS cargoes, all three types likely share housekeeping duties in cNLS cargo import. The contribution of individual importin α's to redundant activities is influenced by their differing temporal and spatial expression patterns in various cells and tissues. In this study we describe the first animal importin α1 mutant.
The key finding here is that Dα1 mutant flies develop (almost) normally to adulthood but both males and females are sterile due to defects in gametogenesis. Dα1 null flies also exhibit a minor wing defect, so Dα1's nonredundant activities extend in this small way to somatic development. In contrast to Dα1 and Dα2, Dα3 is required for somatic development and Dα3 mutants arrest as larvae. Interestingly, Dα1 and Dα2 mutants display distinct phenotypes in gametogenesis. Spermatogenesis is more severely affected than oogenesis in Dα1 mutants, while Dα2 mutants have more severe defects in oogenesis (Mason et al. 2002). Dα2 is not absolutely essential for spermatogenesis—some motile sperm and viable progeny are produced by mutant males—and the defect can be rescued by Dα1, Dα2, or Dα3 transgenes. In contrast, no viable sperm are produced in Dα1 mutants, and only a Dα1 transgene can rescue the defect. Therefore, Dα1 serves a paralog-specific role in spermatogenesis that is distinct from the role of Dα2 in this process.
Dα1 and Dα2 are both required for gametogenesis and have no significant roles in somatic development. It seems likely, therefore, that the evolutionary expansion of the importin α-gene family occurred to serve the uniquely complex processes of spermatogenesis and oogenesis, both of which involve the differentiation of germ-line stem cells using analogous signaling pathways (Gilboa and Lehmann 2004). Dα1 plays an especially important paralog-specific role in spermatogenesis. All three importin α's are expressed in the fly testes, although in distinct, partially overlapping patterns that correspond to different stages of spermatogenesis, which include stem cell division, spermatogonial divisions, growth, meiotic divisions, and spermatid differentiation (reviewed in Gilboa and Lehmann 2004; Hogarth et al. 2005). The expression of Dα1 overlaps with Dα2 expression during meiosis, and later with Dα3 during differentiation and individualization (Giarre et al. 2002). Dα1 is expressed at low levels in testes until the growth stage, when it appears cytoplasmic. Dα1 levels rise during meiosis when it accumulates in spermatid nuclei. Dα1 levels are lower during differentiation and, by the time spermatid heads become aligned toward the wall of the testes, are equally distributed between the nucleus and cytoplasm. Dα1 was not detectable in sperm with elongated heads. The defects exhibited by Dα1 and Dα2 mutants are manifested at different stages of sperm differentiation, although the timing and nature of these defects do not necessarily correspond to when and where during spermatogenesis these factors are actually required (see Fuller 1998).
The oogenesis defects of Dα1 and Dα2 mutant flies are also distinct from one another, and both phenotypes are due to paralog-specific activities. The cause of the severe Dα2 mutant phenotype (deflated oocytes) is likely related to the Dα2-dependent targeting of Kelch to ring canals, through which nurse cell cytoplasm is dumped into the developing oocyte (Gorjanacz et al. 2002). Kelch localization and dumping appear normal in Dα1 mutant females. Giarre et al. (2002) reported that Dα1 expression in ovaries is weaker than that of Dα2 or Dα3, and is, therefore, unlikely to play a major role. This prediction is partially supported by our finding that the ovaries and eggs of Dα1 null flies are only mildly defective. Still, Dα1 must have an important role in oogenesis since almost all mutant females are sterile. It remains possible that the female sterility is due to a behavioral phenotype in egg laying or mating or some other defect that was too subtle for us to notice.
The finding that two of the three conventional Drosophila importin α's are specialized to serve important roles in gametogenesis has a strong parallel in C. elegans (reviewed in Goldfarb et al. 2004; Hogarth et al. 2005). The C. elegans genome encodes three importin α's, IMA-1, IMA-2, and IMA-3, two of which (IMA-1 and IMA-2) localize exclusively to the germ line and are required for gametogenesis (Geles and Adam 2001; Geles et al. 2002). Therefore, two of the importin α's in both fly and worm are required for gametogenesis. IMA-3, a conventional α3 type, is expressed in both somatic and germ-line cells, and like Dα3, is required for somatic development (Geles and Adam 2001). Although IMA-1 and IMA-2 are highly divergent and dissimilar to any of the conventional types, their exclusive expression in the germ line and important role in gametogenesis suggest they may be functional homologs of Dα1 and Dα2. Also, like Dα2, IMA-2 displays cell cycle-dependent shifts between the nucleus and cytoplasm in the gonads, and both accumulate around chromosomes at the onset of nuclear envelope breakdown (Geles et al. 2002). Taken together, these results suggest the possibility that the special needs of gametogenesis may have driven the early expansion and specialization of the metazoan animal importin α-gene family. The complex temporal expression patterns of the five mouse importin α1's, α2's, and α3's in testes indicate that this role likely extends to mammalian spermatogenesis, which, in many ways, is similar to spermatogenesis in flies (Fuller 1998; Hogarth et al. 2005).
Because importin α1's are very similar both by sequence and gene structure to ancestral plant and fungal α1-like genes (A. Mason and D. Goldfarb, unpublished results), we originally expected that the loss of Dα1 would cause defects in the nuclear transport of many important proteins with catastrophic consequences. Therefore, we were initially surprised to find that Dα1 null flies developed normally to adulthood with only a slight wing defect. Phenotypically, then, Dα1 is more similar to Dα2, whose loss also primarily affects gametogenesis. At gene structure and primary sequence levels Dα2 is more similar to Dα3 (A. Mason and D. Goldfarb, unpublished results). Thus the evolutionary history of the three genes does not predict the nature of their mutant phenotypes. We hypothesize that the ancient and essential role the importin α's play in cNLS cargo import is redundantly supported in somatic tissues by the partially overlapping coexpression of the three paralogous proteins. The loss of any one is apparently masked by the activity of one or both of the others. Most of the phenotypes that appear in single gene mutants are likely due to paralog-specific functions that were divided among the genes following the duplications that gave rise to the extant importin α-gene family. An exception is the spermatogenesis defect of Dα2 mutant flies that is rescued by any of the three paralogs (Mason et al. 2002). It is established that importin α1's each have both shared and distinct cargo repertoires (Michaud and Goldfarb 1993; Prieve et al. 1996; Miyamoto et al. 1997; Prieve et al. 1998; Kohler et al. 1999; Talcott and Moore 2000; Fagerlund et al. 2002; Quensel et al. 2004; Lange et al. 2007). The simplest explanations for the paralog-specific phenotypes associated with Dα1, Dα2, and Dα3 mutants invoke deficiencies in the nuclear import of their distinct NLS cargoes.
The genetic interactions between coectopic expression of Dα1 and Dcas and Ketel are consistent with the idea that the tergite defects and lethality are the result of increases in the levels of importin α in nuclei. Genetic manipulations that would be expected to decrease nuclear levels of Dα1 (overexpression of Dcas or loss-of-function Ketel mutants) mitigated the effects of overexpressing Dα1. Likewise, manipulations that would be expected to increase nuclear levels of Dα1 (overexpression of Ketel or loss-of-function Dcas mutants) enhanced Dα1 overexpression phenotypes. Interestingly, an increase in cNLS cargo levels also enhanced the Dα1 overexpression defects. Here, higher cNLS cargo levels could be expected to recruit more Dα1 into targeting complexes with importin β1 (Ketel), resulting in higher steady state nuclear levels of Dα1. Taken together, these results argue that higher than normal nuclear levels of Dα1 are deleterious, and that the nucleocytoplasmic trafficking of nuclear transport factors must be carefully balanced during development.
The defect in tergite development observed in Dα1-overexpressing flies may lend insight into the mechanisms underlying the deleterious effects of excess nuclear importin α. Development of the tergites involves a tightly coordinated process of epithelial cell sheet replacement during which the adult tergites arise from histoblast nests that proliferate and spread to replace larval epidermal cells during pupal morphogenesis (Madhavan and Madhavan 1980; Ninov et al. 2007). The tergite defects observed in Dα1-expressing abdomens may be attributable either to the failure of the adult histoblast nests to proliferate or spread correctly or to a failure of the larval epidermal cells to undergo apoptosis since both of these processes are thought to be codependent (Madhavan and Madhavan 1980; Ninov et al. 2007). The genetic interactions between Dcas and Dα1 may be especially relevant to understanding the tergite phenotypes associated with Dα1 overexpression. Expression of CAS antisense RNA in MCF-7 breast carcinoma cells, which likely leads to increased nuclear levels of importin α, inhibits apoptosis (Brinkmann et al. 1995). It is possible, then, that elevated levels of nuclear importin α inhibit apoptosis in these cells. By analogy, it is possible that elevated levels of nuclear Dα1 interfere with the apoptosis of larval epidermal cells, the persistence of which might impair the ability of the adult cuticle to properly proliferate and spread. Consistent with this hypothesis, blocking cell death in the larval epidermal cells of the abdomen result in defects in spreading of the histoblast nests and resulted clefts in the abdominal cuticle (Ninov et al. 2007). Alternatively, these tergite phenotypes may be caused by defects in tergite development since thermocautery of histoblast nests also produces similar tergite defects (Bryant 1978). Nonetheless, it is intriguing to speculate that the regulated subcellular localization of importin α-proteins affects susceptibility to proapoptotic signals.
This analysis of Dα1 complements our previous analyses of Dα2 and Dα3 (Mason et al. 2002, 2003). We can now say that two of the three conserved Drosophila importin α-genes are required almost exclusively for gametogenesis (Dα1 and Dα2), and only one (Dα3) is required for general viability. The larger picture emerges of a gene family that likely arose by gene duplication to serve the newly evolving requirements of gametogenesis. Following their initial establishment, each of the three paralogous genes was available to evolve specialized (derived) roles and, in mammals, undergo further gene duplications and specializations. It is curious that Dα1, which is more similar to ancient plant and fungal importin α1-like genes than to Dα2 or Dα3, only exhibits paralog-specific phenotypes in derived processes such as gametogenesis and wing development. We hypothesize that α1 genes are not functionally constrained; rather, ancestral α2/α3 genes simply diverged. Why ancestral α2 and α3 genes evolved more rapidly remains a mystery, although important clues no doubt lie among their largely unexplored NLS cargo repertoires. It will be extremely interesting to learn if these roles and relationships are conserved in the more complex mouse and human importin α-gene family.
Footnotes
These authors contributed equally to this study.
Present address: Center for Neural Development and Disease, University of Rochester Medical Center, Rochester, NY 14627.
Footnotes
Communicating editor: K. Golic
Acknowledgement
This research was supported in part by National Institutes of Health grant RO1 GM067838 (D.S.G.) and National Research Foundation grant IBN-0234751 (R.J.F).
References
Brand, A. H., and N. Perrimon,
Brinkmann, U., E. Brinkmann, M. Gallo and I. Pastan,
Bryant, P. J.,
Crosby, M. A. J. L. Goodman, V. B. Strelets, P. Zhang, W. M. Gelbart and The FlyBase Consortium,
Erdélyi, M., E. Máthé and J. Szabad,
Fagerlund, R., K. Melen, L. Kinnunen and I. Julkunen,
Fuller, M. T.,
Geles, K. G., and S. A. Adam,
Geles, K. G., J. J. Johnson, S. Jong and S. A. Adam,
Giarre, M., I. Torok, R. Schmit, M. Gorjanacz, I. Kiss et al.,
Gilboa, L., and R. Lehmann,
Goldfarb, D. S., A. H. Corbett, D. A. Mason, M. T. Harreman and S. A. Adam,
Gorjanacz, M., G. Adam, I. Torok, B. M. Mechler, T. Szlanka et al.,
Gray, Y., M. Tanaka and J. A. Sved,
Hanz, S., E. Perlson, D. Willis, J. Q. Zheng, R. Massarwa et al.,
Harel, A., and D. J. Forbes,
Hogarth, C., C. Itman, D. A. Jans and K. L. Loveland,
Hogarth, C. A., S. Calanni, D. A. Jans and K. L. Loveland,
Hubner, S., H. M. Smith, W. Hu, C. K. Chan, H. P. Rihs et al.,
Kamei, Y., S. Yuba, T. Nakayma and Y. Yoneda,
Kohler, M., S. Ansieau, S. Prehn, A. Leutz, H. Haller et al.,
Kohler, M., C. Speck, M. Christiansen, F. R. Bischoff, S. Prehn et al.,
Kotera, I., T. Sekimoto, Y. Miyamoto, T. Saiwaki, E. Nagoshi et al.,
Lange, A., R. E. Mills, C. J. Lange, M. Stewart, S. E. Devine et al.,
Madhavan, M. M., and K. Madhavan,
Malik, H. S., T. Eickbush and D. S. Goldfarb,
Mason, D. A., R. J. Fleming and D. S. Goldfarb,
Mason, D. A., E. Mathe, R. J. Fleming and D.S. Goldfarb,
McCartney, B. M., H. A. Dierick, C. Kirkpatrick, M. M. Moline, A. Baas et al.,
Michaud, N., and D. S. Goldfarb,
Miyamoto, Y., N. Imamoto, T. Sekimoto, T. Tachibana, T. Seki et al.,
Miyamoto, Y., M. Hieda, M. T. Harreman, M. Fukumoto, T. Saiwaki et al.,
Mosammaparast, N., and L. F. Pemberton,
Ninov, N., D. A. Chiarelli and E. Martin-Blanco,
Preston, C. R., and W. R. Engels,
Prieve, M. G., K. Guttridge, J. E. Munguia and M. L. Waterman,
Prieve, M. G., K. Guttridge, J. E. Munguia and M. L. Waterman,
Quensel, C., B. Friedrich, T. Sommer, E. Hartmann and M. Kohler,
Riddick, G., and I. G. Macara,
Rorth, P.,
Schatz, C. A., R. Santarella, A. Hoenger, E. Karsenti, I. W. Mattaj et al.,
Stewart, M.,
Struhl, G., D. A. Barbash and P. A. Lawrence,
Tabb, M. M., P. Tongaonkar, L. Vu and M. Nomura,
Talcott, B., and M. S. Moore,
Tekotte, H., D. Berdnnik, T. Torok, M. Buszczak, L. M. Jones et al.,
Tran, E. J., and S. R. Wente,
Tsuji, L., T. Takumi, N. Imamoto and Y. Yoneda,
Yasuhara, N., N. Shibazaki, S. Tanaka, M. Nagai, Y. Kamikawa et al.,