-
PDF
- Split View
-
Views
-
Cite
Cite
Ying Zhou, Mario Loeza-Cabrera, Zheng Liu, Boanerges Aleman-Meza, Julie K Nguyen, Sang-Kyu Jung, Yuna Choi, Qingyao Shou, Rebecca A Butcher, Weiwei Zhong, Potential Nematode Alarm Pheromone Induces Acute Avoidance in Caenorhabditis elegans, Genetics, Volume 206, Issue 3, 1 July 2017, Pages 1469–1478, https://doi.org/10.1534/genetics.116.197293
Close - Share Icon Share
Abstract
It is crucial for animal survival to detect dangers such as predators. A good indicator of dangers is injury of conspecifics. Here we show that fluids released from injured conspecifics invoke acute avoidance in both free-living and parasitic nematodes. Caenorhabditis elegans avoids extracts from closely related nematode species but not fruit fly larvae. The worm extracts have no impact on animal lifespan, suggesting that the worm extract may function as an alarm instead of inflicting physical harm. Avoidance of the worm extract requires the function of a cGMP signaling pathway that includes the cGMP-gated channel TAX-2/TAX-4 in the amphid sensory neurons ASI and ASK. Genetic evidence indicates that the avoidance behavior is modulated by the neurotransmitters GABA and serotonin, two common targets of anxiolytic drugs. Together, these data support a model that nematodes use a nematode-specific alarm pheromone to detect conspecific injury.
DETECTING danger is crucial for animal survival. Alarm pheromones are used to communicate danger by many animal species such as sea anemones, insects, fishes, and mammals (Wyatt 2003). Even humans have alarm pheromones (Mujica-Parodi et al. 2009). In these animals, chemical cues are released from injured or stressed animals and detected by conspecifics or closely related species to invoke innate alarm responses such as fleeing. Chemical compositions of alarm pheromones are often species specific, e.g., anthopleurine in sea anemone (Howe and Sheikh 1975), CO2 in fruit flies (Suh et al. 2004), chondroitin fragments in zebrafish (Mathuru et al. 2012), and 2-sec-butyl-4,5-dihydrothiazole in mice (Brechbühl et al. 2013b). The olfactory pathways that detect alarm pheromones largely consist of odorant receptors, G proteins (e.g., Gαq in flies, Gαi in fish, and Gαo and Gαi in mice), and a second messenger (e.g., cAMP in fish and cGMP in mice) (Enjin and Suh 2013).
Surprisingly, it remains unclear whether there is an alarm pheromone in nematodes, considering that alarm pheromones exist in a wide variety of animals (Wyatt 2003) and that nematodes are the most abundant animals on earth (Lorenzen 1994). Nematodes are known to use a class of small molecules called ascarosides as pheromones to regulate behaviors such as mate-finding and aggregation (Ludewig and Schroeder 2013). However, there is no published report of an alarm pheromone in the nematodes.
Here we present evidence of a potential nematode alarm pheromone in the internal fluid released from injured worms. The fluid induces an acute avoidance without inflicting physical harm. This avoidance signal appears ascaroside independent and conserved among multiple nematode species. In Caenorhabditis elegans, detection of this signal requires a cGMP signaling pathway. Together, these data suggest the existence of a nematode alarm signal.
Methods
Animal maintenance
C. elegans strains were cultured on nematode growth medium (NGM) with OP50 Escherichia coli at 20° as previously described (Stiernagle 2006). N2 (Bristol) was used as the wild-type strain. All worm strains were obtained from the Caenorhabditis Genetics Center (CGC) except daf-37(ttTi3058) from the Centre National de la Recherche Scientifique, goa-1(sy192) from the Sternberg laboratory, srbc-64(tm1946) and srbc-66(tm2943) from the Sengupta laboratory, and Steinernema carpocapsae from the Hallem laboratory. Unoutcrossed strains that showed as a hit in chemoavoidance assays were outcrossed six times and tested again. Detailed information of all mutant strains is listed in Supplemental Material, Table S1 in File S4.
Unless otherwise specified, day-1 adult hermaphrodites were used in our behavioral assays. Synchronized L1s were collected by bleaching gravid adults as described (Stiernagle 2006) and cultured on OP50 plates until they reached adulthood.
To obtain starved worms, we washed well-fed young adult worms off the plates into M9 buffer. The worms were washed three additional times in M9 and placed in M9 at a concentration of one worm/μl. Control worms were placed in M9 with 1% OP50. Both groups were incubated at 20° and tested after 1, 3, and 5 hr of starvation.
To collect dauers, C. elegans plates were starved for 5 additional days after the worms cleared the bacterial lawn. Five holes were made on the wall of each plate above the agar level using a flamed needle. Five 100-μl drops of sterile water were placed on each lid where the plate wall touched. The plates were placed upside down sitting on the lids overnight. The water drops on the lids were then collected and examined for dauers.
S. carpocapsae were cultured as described (Ehlers and Shapiro-Ilan 2005). Five waxworms (PetSmart) were placed in a 60-mm Petri dish lined with filter paper (55 mm, Whatman, Maidstone, UK). A total of 200 μl of water containing ∼100 infective juveniles (IJs) was dropped on top and around each waxworm. Waxworms were examined 48 hr after infection to ensure they were dead. The Petri dish was kept in the dark at room temperature for 5–8 more days until all waxworms flattened and dried.
Steinernema IJs were harvested using the White trap method (White 1927). A 70-mm filter paper (Whatman) was placed on a raised island in a 100-mm Petri dish. Distilled water was added to the level of the filter paper. Dried infected waxworms were placed in the middle of the wet filter paper and left for 7–10 days. The water containing IJs was then collected. Freshly collected IJs were used immediately for experiments or washed three times in water, resuspended in 10 ml water in a 25-cm2 culture bottle (Falcon, cat. no. 353014), and stored at 15° as stock.
Worm extract
Animals were washed off from NGM plates for small-scale experiments or collected from liquid culture (Stiernagle 2006) for large-scale experiments. Animals were washed more than three times in M9 buffer (0.3% KH2PO4, 0.6% Na2HPO4, 0.5% NaCl, 1 mM MgSO4). The wash was to remove the culture media because they are known to repel C. elegans hermaphrodites and attract males because they contain ascarosides (Simon and Sternberg 2002). Unless noted otherwise, worms were put in a 100° water bath to be instantly killed. They were then homogenized using a pestle or sonication. The mixture was centrifuged and the supernatant was collected as worm extract. The worm extract was filtered using a 0.22-μm syringe filter (Millipore, Bedford, MA), and stored in aliquots at −20°. To obtain fly extract, wandering third instar Drosophila melanogaster larvae were collected, washed three times in M9 buffer, and homogenized using the same procedure.
Total organic carbon measurement
Total organic carbon (TOC) was measured using a TOC-VCSH total organic carbon analyzer (Shimadzu, Kyoto, Japan) operating in nonpurgeable organic carbon mode. A five-point calibration curve (0–20 ppm organic carbon) was constructed using potassium phthalate monobasic (Fluka, >99.5%) as the standard. Prior to measurement, aqueous worm extract samples were filtered through a Millex-GP 0.22 μm-pore-size PES syringe filter (Millipore, Bedford, MA). Filters were prerinsed with ultrapure water before use, and the first few milliliters of sample eluent were discarded. Samples were measured three to five times with the machine determining the average and variance values for the data. A 10-mg/liter organic carbon standard solution was run with each series of samples to ensure the standard curve remained accurate. In our experiments, 100 ppm TOC was equivalent to aqueous content from ∼2.6 mg dry weight of worms dissolved in 1 ml of buffer.
Population assay
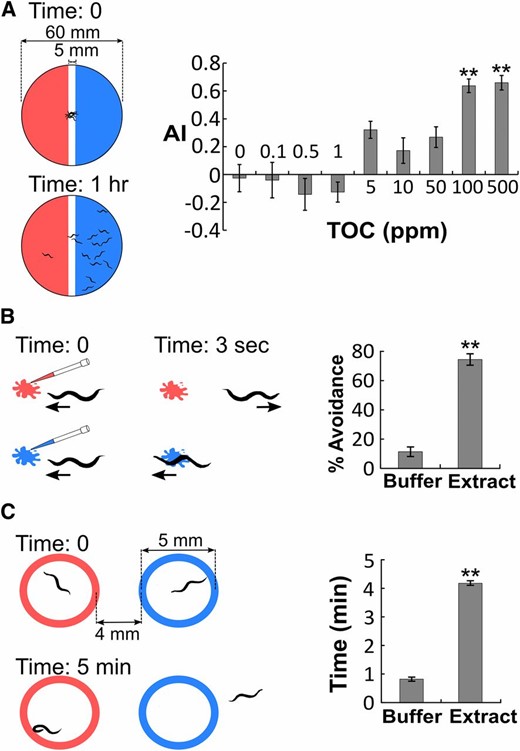
Chemotaxis plates were prepared by pouring 8 ml of CTX agar [CTX buffer (5 mM KH2PO4/K2HPO4 at pH 6, 1 mM CaCl2 and 1 mM MgSO4) with 1.6% agar] into 6-cm Petri dishes. The plates were spread with 10 μl worm extract (at 100 ppm TOC or an otherwise indicated concentration) on one side and 10 μl M9 buffer on the other side (Figure 1A). Worms were washed three times with CTX buffer and once with water. About 100 worms were dropped in the center of each chemotaxis plate. Excess liquid was withdrawn using a Kimwipe. The plates were then placed in a 20° incubator. After 1 hr (or otherwise indicated length of time) in the 20° incubator, chloroform was added to the lid of the plates to instantly immobilize and kill the animals as previously described (Ward 1973). The plates were then scanned using the QuantWorm imaging system (Jung et al. 2014) and the images were analyzed using the Java program WormCounter (see Image processing below). Animals that remained in the center 0.5-cm-wide strip were not used in calculation of avoidance index (AI) (Figure 1A) because they may have mobility issues. Plates with <50 worms counted were considered invalid.
Three assays to quantify nematode alarm response. (A) Population test. Plates were spread with worm extract (red) on one side and buffer (blue) on the other. Approximately 100 worms were dropped at the center, immobilized after 1 hr to evaluate the distribution of worms. In the dose response, 10 μl of different concentrations of worm extracts were tested. AI, avoidance index. n ≥ 10 plates for each data point. TOC, total organic carbon content. (B) Drop assay. A drop of worm extract (red) or buffer (blue) was applied in front of the head of a moving worm. A reversal within 3 sec indicated avoidance. Percentage avoidance was scored. n = 35 worms for each group. (C) Trap assay. Two unfilled circles were drawn using worm extract (red) and buffer (blue). A total of 1–3 worms were placed into each circle and recorded for 5 min to measure the average time each worm stayed inside the circle. n = 26 tests for each group. Bar graphs represent mean ± SEM * P < 0.05, ** P < 0.01, one-way ANOVA, and Scheffé post hoc analysis.
Drop assay
A single animal was placed on a chemotaxis plate at room temperature and allowed to rest for 5–10 min. A total of 0.4 μl 100 ppm TOC worm extract or M9 buffer was dropped ∼1 mm in front of the head of the moving worm. Once the worm reached the drop, it would either move into the drop or reverse to avoid the drop. A reversal within 3 sec of contact was counted as an avoidance response. Each animal was tested with worm extract and M9 buffer drops alternatively with an interval of at least 1 min between successive drops. Each animal was tested with not more than 15 drops.
Trap assay
Young adult animals were collected and washed three times in CTX buffer. Two platinum loops of 5-mm diameters were dipped into M9 buffer and worm extract (200 ppm TOC), respectively. The loops were then used to briefly touch the surface of a chemotaxis plate to print two ring-shaped liquid marks. Three worms were placed inside each ring and video recorded for 5 min. The videos were analyzed using the Java program WormTrap (see Image processing below).
While the three assays (population, drop, and trap assays) gave similar results, each had a unique strength. The population assay had the highest throughput and was used as the default method in this study. The other two assays required many fewer animals and were used when the number of animals was limited, e.g., laser-ablated animals, or the animals had certain locomotion defects. For example, the drop assay was used for mutants that crawled slowly; the trap assay was used for male worms that tend to touch other worms and have excessive spontaneous reversals.
Image processing
Two programs, WormTrap and WormCounter, were developed for automatic image video processing. More details, including source codes, executable files, user manuals, and sample images, are available at www.quantworm.org and figshare.com/articles/Potential_nematode_alarm_pheromone_induces_acute_avoidance_in_C_elegans_Source_code_executable_files_and_sample_images_/4989776.
WormCounter analyzes images of worm plates from population assays. It assembles tiled images taken by the QuantWorm imaging system to create one image for a plate and binarizes the image using an empirically determined threshold. Worms are detected by region extraction, and their areas are determined as number of pixels. As most worms do not overlap on the image, the median worm area is used as the size of a single worm to calculate the total number of worms.
WormTrap analyzes videos from trap assays. It extracts time-lapse images for every 2 sec of a video, then binarizes the images using local adaptive thresholding (Bradley and Roth 2007). Median particle area is used as the size for a single worm. The number of worms in each trap is calculated for each image. The average trapped time, Tr (second), is calculated as where C(t) is the normalized worm count , Nt is the worm count at time t, Nt = 0 is the initial worm count at time = 0, and Δt is the measurement interval (2 sec in this case).
Lifespan assay
Lifespan assays were carried out at 20° as described (Gandhi et al. 1980). A total of 50–70 synchronized L1 larvae were dropped onto seeded 60-mm NGM plates. A total of 80 μl 2.5 mM 5-fluoro-2′-deoxyuridine (FUdR) (Sigma, St. Louis, MO, cat. no. 50-91-9) was added to each plate when the worms reached the L4 stage to prevent progeny from hatching. After the worms reached L4, 80 μl 100 ppm worm extract or M9 was added every other day to each test and control plate, respectively. Two independent trials were performed, with triplicates used in each trial. Dead worms were removed every day and the number of dead worms on each plate was recorded. The first day of adulthood was counted as day 1.
Laser ablation of neurons
Cell ablations were done using the standard protocol (Bargmann and Avery 1995). The operation was conducted using a Spectra Physics VSL-337ND-S Nitrogen Laser (Mountain View, CA) attached to an Olympus BX51 microscope. L1 worms were operated on 5% agar pads containing 0.5 µl of 0.1 µm diameter polystyrene microspheres and covered with a coverglass. The mock-ablated animals were placed on the same agar pad for the same amount of time to rule out the possibility that behavioral changes are due to pressure applied on the worms by the coverglass. Animals were then recovered on regular culture plates and assayed when they were 1-day adults.
Transgenic animals
The ASI- and AWC-genetically ablated (via caspase expression) worms (Beverly et al. 2011) were kindly provided by Piali Sengupta. After the drop assay, the animals were mounted on an agar slide and observed under the microscope to confirm the loss of the neurons. tax-4(p678) worms with transgenes expressing tax-4 complementary DNA (cDNA) sequences under the promoter ceh-36 or srbc-65 (Beverly et al. 2011) were kindly provided by Piali Sengupta. Plasmids with wild-type tax-4 cDNA sequences under the promoter sra-13, str-3, or srg-8 (Olofsson 2014) were kindly provided by Birgitta Olofsson. The plasmids were microinjected into tax-4(p678) worms at 50 ng/µl together with 50 ng/µl Pmyo-2::dsRED as an injection marker to generate transgenic worms.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. All strains and plasmids are available upon request. Relevant Java codes for image processing are available at figshare.com/articles/Potential_nematode_alarm_pheromone_induces_acute_avoidance_in_C_elegans_Source_code_executable_files_and_sample_images_/4989776.
Results
Quantitative assays were developed to study nematode alarm response
It was observed that when a C. elegans was punctured with a needle, worms within the radius of 1–2 mm would flee from the victim (J. Thomas and H. R. Horvitz, personal communication; Bargmann et al. 1990), suggesting that the internal fluid from the injured worms contains a potential alarm signal. We also observed the same phenomenon. To study this phenomenon, we designed assays to quantify both the signal and the response.
To collect a large amount of the signal molecule, we used a pestle or sonication to break the animals and collected the aqueous content (hereinafter referred to as “worm extract”). As the chemical identity of this avoidance signal is unknown, TOC content was used to measure the concentration of worm extracts.
We modified three standard chemotaxis assays (Hart 2006) to quantify the worm response to the worm extract (Figure 1). In the population assay (Figure 1A), we spread the worm extract on one side of an agar plate and buffer on the other side, placed live worms in the center, and measured the distribution of live worms after a given time. We let A and B denote the number of animals on the buffer side and worm extract side, respectively; the AI is calculated as (A − B)/(A + B). The avoidance index ranges from −1 to 1 with 1 being complete repulsion and −1 being complete attraction. In the drop assay (Figure 1B), a drop of buffer or worm extract was placed in front of a worm, and the percentage of times that the animal reversed its movement was calculated. In the trap assay (Figure 1C), individual worms were placed inside either a ring drawn with worm extract or a ring drawn with buffer, and the time the worms remained inside the circles was measured. We developed open-source software to automatically analyze images and videos for the population assay and the trap assay.
While the three assays gave similar results, each had a unique strength. The population assay had the highest throughput and was used as the default method in this study. The other two assays required much fewer animals and were more tolerant on animals with locomotion defects. We used these two assays for laser-ablated animals, male worms, and mutants that crawled slowly.
Existence of a potential nematode alarm pheromone
All three methods showed that the worm extract induced an acute avoidance behavior in C. elegans (Figure 1 and File S1, File S2, and File S3). The avoidance was dose dependent of the worm extract (Figure 1A) and was not due to residual bacterial food (Figure S1 in File S4).
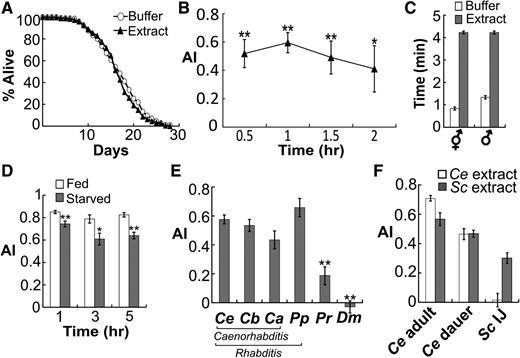
As C. elegans avoids many harmful chemicals, we asked whether the worm extract is harmful to the worms and thus induces nociception rather than an alarm response. We dosed C. elegans with the worm extract every other day and found that such constant exposure to the worm extract did not reduce their lifespan (Figure 2A, another independent experiment was shown in Figure S2 in File S4. In both experiments, P > 0.05 between buffer and extract, log-rank test). These data suggested that the worm extract did not induce any physical damage.
Evidence of a potential nematode alarm pheromone. (A) Worm lifespan was not affected by repeated doses of the worm extract. n ≥ 333 worms in each group. (B) Worms avoided the worm extract for over 2 hr in the population assay. * P < 0.05, ** P < 0.01 in comparison with AI = 0, Student’s t-test. n ≥ 10 plates for each data point. (C) Both males and hermaphrodites avoided the worm extract in the trap assay. n ≥ 21. (D) Worms starved in M9 buffer for 1–5 hr were less effective but still avoided the worm extract. * P < 0.05, ** P < 0.01, Student’s t-test, starved vs. fed. n ≥ 10. (E) C. elegans responses to extracts from different nematodes. The labels indicate extracts from the following species. Ce, Caenorhabditis elegans; Cb, Caenorhabditis briggsae; Ca, Caenorhabditis angaria; Pp, Pristionchus pacificus; Pr, Panagrellus redivivus; Dm, Drosophila melanogaster. * P < 0.05, ** P < 0.01 in comparison with the Ce group, one-way ANOVA, and Scheffé post hoc analysis. n ≥ 10. (F) C. elegans (Ce) and Steinernema carpocapsae (Sc) responses to Ce and Sc extracts. n ≥ 10. All bar graphs display mean ± SEM.
Consistent with the importance of an alarm response, avoidance of the worm extract is a very robust behavior in C. elegans. In the population assay, the worms remained “avoiding” for >2 hr (Figure 2B). Both males and hermaphrodites avoided the worm extract (Figure 2C, males vs. hermaphrodites, P > 0.05; buffer vs. worm extract, P < 0.0001, Student’s t-test). Starvation was known to modulate certain C. elegans chemotaxis responses (Hallem and Sternberg 2008), so we tested starved worms for their avoidance of the worm extract. Starved worms were less effective in avoiding the worm extract (Figure 2D), suggesting that the avoidance response is modulated by feeding status. However, worms starved for up to 4 hr still strongly avoided the worm extract (AI > 0.6, Figure 2D), demonstrating the robustness of this behavior.
The avoidance factor is nematode specific and conserved in multiple nematode species
Some animals such as fishes can detect alarm pheromones released by not only conspecifics but also related species (Wyatt 2003). To test the species specificity of the avoidance factor, we exposed C. elegans to worm extracts from other free-living terrestrial nematodes. C. elegans strongly avoided not only the conspecific extract, but also extracts from three other nematodes in the Rhabditis genus (Figure 2E). An extract from a more distant nematode, Panagrellus redivivus, was also able to invoke a significant (P < 0.001, Student’s t-test), yet much milder avoidance response from C. elegans (Figure 2E). In contrast, despite the fact that Caenorhabditis and Drosophila often share the same habitat of rotting fruits (Félix and Duveau 2012), extract from the fruit fly larvae had no effects on C. elegans (Figure 2E), suggesting that the avoidance signal is nematode specific.
The Rhabditis genus also contains families of parasitic nematodes. To examine whether the avoidance factor is also conserved in these parasitic nematodes, we collected extract from the insect parasite S. carpocapsae (Sc). C. elegans avoided both the conspecific and the Sc extracts, however, Sc IJs avoided only the Sc (AI > 0, P < 0.001, Student’s t-test) but not the C. elegans extract (P = 0.75, Figure 2F). This difference in the avoidance behaviors is unlikely due to difference in developmental stages, because C. elegans dauers (an IJ-equivalent developmental stage) also avoided both C. elegans and Sc extracts (Figure 2F). These data suggested that the avoidance signals in different nematode species are similar but not identical, and that parasitic and free-living nematodes have different responses to various avoidance signals.
The avoidance factor is a novel nematode repellent
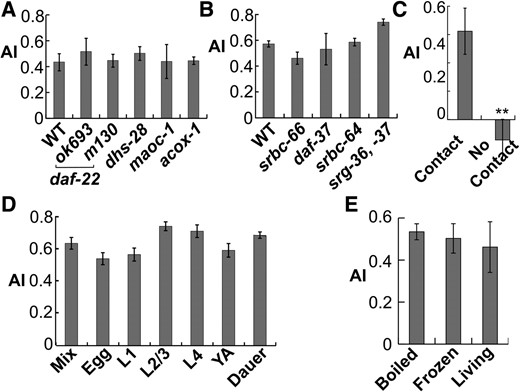
The avoidance signal is unlikely an ascaroside, the best-known nematode pheromone. Worm extracts from C. elegans mutants defective of ascaroside synthesis (e.g., daf-22, maoc-1, acox-1.1) (Ludewig and Schroeder 2013) functioned effectively as avoidance signals (Figure 3A). In addition, mutants of known ascaroside receptors (daf-37, srbc-64, srbc-66, srg-36, and srg-37) (Ludewig and Schroeder 2013) successfully avoided the worm extract (Figure 3B). These results suggested that the avoidance factor is not an ascaroside or at least contains ascaroside-independent factors.
Properties of the nematode alarm pheromone. (A) Worm extracts from ascaroside synthesis mutants repelled C. elegans. n ≥ 7. (B) Ascaroside receptor mutants avoided worm extract. n ≥ 9. (C) The alarm pheromone was not volatile. A total of 500 ppm worm extract was used in the “no contact” group whereas the default 100 ppm was used in the “contact” group. n ≥ 10. (D) The alarm chemical existed in different developmental stages of worms. Mix, mixed stages. YA, young adults. n ≥ 10. (E) Effects of differently prepared worm extracts. n ≥ 10. All bar graphs display mean ± SEM * P < 0.05, ** P < 0.01 in comparison with the first group, one-way ANOVA, and Scheffé post hoc analysis.
The avoidance factor appeared to be none of the known nematode repellents because C. elegans mutants defective in avoiding known repellents such as acid, osmolarity, benzaldehyde or quinine, still efficiently avoided the worm extract (Figure S3A in File S4). Glycosaminoglycan (GAG) chondroitin has been reported as the fish alarm pheromone (Mathuru et al. 2012). RNAi of C. elegans chondroitin synthesis gene mig-22 or sqv-5 (Hwang et al. 2003; Suzuki et al. 2006) produced extracts with normal alarm efficacy (Figure S3B in File S4), suggesting that chondroitin is also not the nematode alarm pheromone.
Our preliminary efforts to fractionate the crude extract using reversed phase and size exclusion chromatography indicate that the avoidance signal consists of at least three distinct components of medium polarity. While the chemical identity of the components remains unknown, we have characterized several properties of the avoidance signal.
The avoidance factor is a nonvolatile endogenous factor
The avoidance factor appeared nonvolatile. In a modified population assay, we poured agar on both lids and plates of Petri dishes, spread the worm extract and buffer on the lid agar, and placed the worms on the plate agar. In that way, the worms were not in direct contact but a short distance (1–2 mm) away under the signal. Worms showed no avoidance under these conditions, even with a fivefold increase in the amount of the worm extract (Figure 3C), suggesting that the avoidance signal is not volatile.
Alarm pheromones can be actively secreted by stressed animals (e.g., flies and mice) or passively diffused from internal cells that become exposed to the environment by tissue damage (e.g., zebrafish) (Enjin and Suh 2013). The nematode avoidance factor likely belongs to the second class because it existed in all developmental stages, including embryos in which secretion to the environment is hindered by egg shells (Figure 3D).
We further tested whether the avoidance factor is synthesized when animals are stressed or whether it is an endogenous chemical that constantly exists but is released upon injury. We prepared worm extracts from animals that were killed instantly in boiling water bath or liquid nitrogen. Extracts from instantly killed worms induced similar avoidance behaviors as those from living worms (Figure 3E), suggesting that injury did not induce synthesis of the avoidance factor but rather released an endogenous factor that was already present inside worms.
Worm extract avoidance requires cGMP signaling
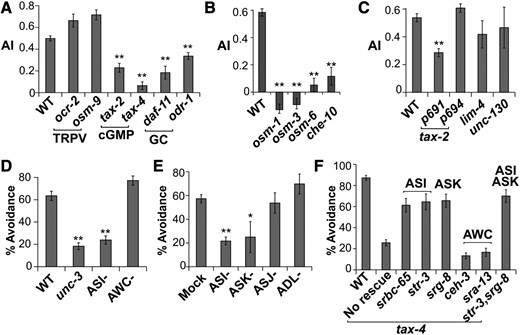
Most C. elegans sensory neurons signal through the cGMP-gated ion channel, encoded by the tax-2 and tax-4 genes, and the transient receptor potential (TRPV) channel, encoded by the osm-9 and ocr-2 genes (Bargmann 2006). We tested mutants of these genes and found that TAX-2 and TAX-4, but not OSM-9 or OCR-2, are required for avoidance of the worm extract (Figure 4A). Consistent with this observation, mutants of daf-11 and odr-1, two guanylyl cyclases that have been linked to chemosensation (Birnby et al. 2000; L’Etoile and Bargmann 2000), also showed defective avoidance of the worm extract.
Avoidance of the worm extract requires cGMP signaling in the ASI and ASK neurons. (A) cGMP-channel mutants as well as guanylyl cyclase (GC) mutants were defective in avoiding the worm extract, while TRPV-channel mutants exhibited normal avoidance. (B) Mutants with cilia defects showed defective avoidance of worm extract. n ≥ 10. (C and D) Among worm strains with defective neurons, genetic ablation of the ASI neurons showed defective avoidance of the worm extract. ASI− and AWC− indicate cell ablation via caspase expression. unc-3 mutants were tested using the drop assay because of motor defects. n ≥ 10 plates for population assay in C and n ≥ 10 animals for drop assay in D. (E) Avoidance response of animals after laser ablation of amphid neurons. n ≥ 10 animals. (F) Cell-specific rescue of tax-4. Labels indicate promoters used to drive neuron-specific expression. Restoring tax-4 in the ASI and/or ASK neurons showed significant rescue effects of the avoidance behavior (P < 0.01, Student’s t-test, in comparison with the no-rescue group). n ≥ 10 animals for each group. In all bar graphs, bars and error bars represent mean and SE, respectively. * P < 0.05, ** P < 0.01, one-way ANOVA, and Scheffé post hoc analysis. WT, wild-type.
Worm extract avoidance requires the ASI and ASK neurons
Next we sought to identify the sensing neurons in the neural circuit mediating the avoidance of the worm extract. C. elegans has two types of chemosensory organs, amphids in the anterior of the worm and phasmids in the posterior, that have sensory cilia exposed to the environment (Inglis et al. 2006). Mutations that caused structural defects in these cilia (Inglis et al. 2006) abolished the avoidance of the worm extract (Figure 4B), suggesting that the worm extract is detected through these ciliated neurons.
Because TAX-2 and TAX-4 are required for avoidance of the worm extract (Figure 4A), we focused on the 12 neurons where tax-2 and tax-4 are expressed: AWC, AFD, ASE, ASG, ASJ, ASI, AWB, ASK, BAG, AQR, PQR, and URX (Coburn and Bargmann 1996). We tested the tax-2 allele tax-2(p694), which has a mutation in cis-regulatory elements and only disrupts tax-2 expression in the AQR, AFD, ASE, and BAG neurons. tax-2(p694) mutants showed normal avoidance of the worm extract (Figure 4C). Therefore, we focused on the remaining eight neurons. Observation from our drop assay and trap assay showed that the worm head could sense the alarm pheromone (File S2 and File S3), indicating that amphid neurons were involved. Among the remaining tax-2/tax-4-expressing neurons, six were amphid neurons: ASG, ASI, ASJ, ASK, AWB, and AWC (Bargmann 2006). Two mutants, lim-4 and unc-130, with defects in the development of the AWB and ASG neurons, respectively (Hobert 2005), did not show significant defects in worm extract avoidance (Figure 4C), leaving four neurons, ASI, ASJ, ASK, and AWC, as candidates.
To examine whether the ASI neurons are required for avoidance of the worm extract, we tested strains in which the ASI neurons were genetically ablated using either a mutation of unc-3, which encodes a transcription factor required for the ASI neurons (Prasad et al. 1998), or ASI-specific expression of caspases (Beverly et al. 2011). These strains displayed strong defects in avoiding the worm extract (Figure 4D). In contrast, AWC expression of caspases (Beverly et al. 2011) did not cause significant defects in worm extract avoidance (Figure 4D).
Laser ablation of the ASI neurons also caused defective avoidance of the worm extract (Figure 4E), confirming that the ASI neurons are involved in the avoidance of the worm extract. Laser ablation of the ASK neurons caused similar defects (Figure 4E), suggesting that the ASK neurons are also part of the avoidance neural circuit. In contrast, laser ablation of the ASJ neurons did not produce any avoidance defect (Figure 4E). We also tested the ADL neurons because they have been reported to be involved in nociception and chemoavoidance (Bargmann 2006). We found that they were not required for avoidance of the worm extract (Figure 4E), consistent with the fact that ADL neurons do not express TAX-2/TAX-4 (Bargmann 2006) and our observation that TAX-2/TAX-4 are required for the worm extract detection.
cGMP signaling is required in the ASI and ASK neurons for avoidance of the worm extract
The genetic and laser ablation experiments revealed that the ASI and ASK neurons are required for the avoidance of the worm extract. To examine whether TAX-2/TAX-4 function in these neurons to modulate the avoidance behavior, we performed cell-specific rescue experiments with tax-4 by expressing tax-4 cDNA under various promoters in tax-4(p678) mutants. tax-4 mutants in which tax-4 is rescued in the ASI neurons either through the srbc-65 promoter or the str-3 promoter (Beverly et al. 2011; Olofsson 2014) showed significantly higher avoidance of the worm extract than the mutants without rescue (Figure 4F). Similar effects were achieved by restoring tax-4 in the ASK neurons (Figure 4F). In contrast, tax-4 expression in the AWC neurons failed to rescue the avoidance defects (Figure 4F). These data support our model that the ASI and ASK neurons function in direct sensing of the avoidance factor.
Other neurons may also be involved in sensing the avoidance factor. Restoring TAX-4 function in either ASI or ASK neurons did not restore the avoidance to wild-type levels (Figure 4F, P < 0.01 in comparison with wild type, Student’s t-test), suggesting that more than one neuron is needed in wild-type sensing. This is consistent with the genetic and laser ablation experiment, showing that missing either ASI or ASK caused avoidance defects (Figure 4, D and E). Restoring TAX-4 in both ASI and ASK still did not fully reach wild-type avoidance (Figure 4F, P < 0.05, Student’s t-test). This could be a result of varying levels of transgene expression or may suggest that additional neurons are involved in worm extract sensing.
Worm extract avoidance is modulated by γ-aminobutyric acid and serotonin
Currently there are two major classes of drugs for treating anxiety: (1) benzodiazepines that target the neurotransmitter γ-aminobutyric acid (GABA), and (2) monoamine-altering drugs, which are also antidepressants (Griebel and Holmes 2013; Murrough et al. 2015). The second class of drugs includes tricyclic antidepressants (TCAs) that modulate the neurotransmitters serotonin and norepinephrine; monoamine oxidase inhibitors (MAOIs) that modulate monoamine neurotransmitters, including dopamine, serotonin, melatonin, epinephrine, and norepinephrine; and selective serotonin reuptake inhibitors (SSRIs) that modulate serotonin levels (Griebel and Holmes 2013; Murrough et al. 2015).
As all existing anxiolytic drugs target certain neurotransmitters, we examined whether these neurotransmitters are involved in C. elegans avoidance of the worm extract. C. elegans has seven types of neurotransmitters: acetylcholine (ACh), serotonin (5-HT), dopamine (DA), tyramine (TA), octopamine (OA), glutamate (Glu), and GABA (Loer and Rand 2016).
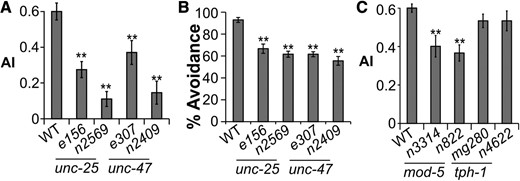
We first examined GABA, which is the target of benzodiazepine anxiolytic drugs. Mutants of the GABA biosynthetic enzyme glutamic acid decarboxylase UNC-25 or the membrane GABA transporter UNC-47 displayed reduced avoidance of the worm extract (Figure 5, A and B), suggesting that the avoidance is modulated by GABA levels.
Neurotransmitters modulate avoidance of the worm extract. (A) Avoidance of GABA mutants tested by the population assay. n ≥ 17. (B) Avoidance response of GABA mutants tested by the drop assay. n ≥ 11. (C) Avoidance response of serotonin mutants. n ≥ 16. In all panels, bars and error bars represent mean and SEM, respectively. ** P < 0.01, one-way ANOVA, and Tukey post hoc analysis.
The neurotransmitter 5-HT is a common target of monoamine-altering drugs. Mutants of the tryptophan hydroxylase TPH-1, an enzyme required for 5-HT biosynthesis, had normal avoidance of the worm extract (Figure 5C). Mutants of the serotonin reuptake transporter (SERT) MOD-5 displayed mild defects in worm extract avoidance (Figure 5C). These data suggested that increased but not decreased 5-HT levels have a mild influence on the avoidance behavior.
Discussion
We present evidence of a potential nematode alarm pheromone. First, the worm extract does not cause pain or physical harm, considering that the worm extract did not reduce animal lifespan (Figure 2A), and that the nociceptive ADL neurons and the TRPV channels OSM-9/OCR-2 (Bargmann 2006) were not required for worm extract avoidance (Figure 4). Second, unlike most worm repellents that require the ADL neurons, the acute avoidance of the worm extract is sensed by the ASI and ASK neurons (Figure 4), two neurons that are also involved in detection of the pheromone ascaroside, suggesting that the worm extract may differ from generic repulsive signals and contain a pheromone. While chemical identification of the avoidance factor is needed to definitively answer whether it is an alarm pheromone, existing data consistently support the model of an alarm pheromone in the worm extract.
Avoidance of the worm extract requires the cGMP-gated TAX-2/TAX-4 channels in the amphid ASI and ASK neurons (Figure 4). The behavior is susceptible to modulation of GABA and serotonin levels (Figure 5). As our assay does not detect functional redundancy, some molecules and cells that showed no effects in this study may still be involved.
There are some similarities between the nematode and the mouse alarm responses. First, the alarm pheromone detecting cells are similar. The mouse alarm pheromone-sensing organ, the Grueneberg ganglion, differs significantly from the canonical olfactory system in both cellular and molecular components (Enjin and Suh 2013), yet showed striking similarity to C. elegans amphid neurons in both neuron morphology (Brechbühl et al. 2008) and protein expression profiles (Brechbühl et al. 2013a). Second, the molecules mediating alarm pheromone detection are also similar between C. elegans and mice. Orthologs of TAX-4 and DAF-11 are expressed in mouse Grueneberg ganglion (Brechbühl et al. 2013a). Both C. elegans and mouse use a cGMP-dependent pathway in alarm pheromone sensing, whereas zebrafish use cAMP. Finally, similar to mice, the C. elegans alarm response is also susceptible to modulation of GABA and 5-HT levels.
A likely function for the nematode alarm pheromone is to signal the presence of a nematode-feeding predator so that other nematodes can escape. In their natural habitat, C. elegans live in large populations in rotting fruits (Félix and Duveau 2012). Because of such high-density aggregation of animals, the alarm pheromone does not need to be volatile to cover a long range. The same rotting vegetal environments are often shared by multiple Caenorhabditis species and Drosophila (Félix and Duveau 2012). C. elegans can distinguish injured nematodes from Drosophila larvae (Figure 2E), enabling them to avoid nematode-specific dangers.
Similar to fish alarm pheromones, the nematode alarm pheromone is likely an endogenous signal that is stored and released only upon injury (Figure 3D), instead of a product of acute synthesis upon stress or injury. For a nematode, an injury that penetrates the cuticle is likely to be fatal, as the worm is under internal hydrostatic pressure, and bursts when its cuticle is punctuated. Therefore, the alarm pheromone has little adaptive advantage for the sender. Using an endogenous factor as the alarm pheromone in this case brings no additional cost to the sender while benefiting the receivers.
Ascarosides and the worm alarm pheromone are similar in that both of them are nonvolatile, conserved in nematodes, and detected by the amphid neurons ASI and ASK in a cGMP-dependent pathway (Ludewig and Schroeder 2013). However, the alarm pheromone is likely not a member of ascaroside class of pheromones, as dauers synthesize fewer ascarosides (Kaplan et al. 2011) but have abundant alarm pheromone (Figure 3D); a handful of ascarosides have sexual dimorphic effects at certain concentrations (Srinivasan et al. 2008), but the alarm pheromone has no sexual dimorphism (Figure 2C); and the ascaroside C9 is sensed by the ADL neurons in addition to ASI and ASK neurons (Jang et al. 2012; Ludewig and Schroeder 2013), whereas ADL does not appear to be required for the alarm pheromone sensing (Figure 4E). However, because of the diversity of ascarosides, it remains possible that the alarm pheromone is a novel ascaroside that has not been well characterized. We also cannot exclude the possibility that the alarm pheromone contains both ascaroside and nonascaroside components. These questions can be revealed by future research on the chemical identity of the alarm pheromone.
Acknowledgments
We thank Birgitta Olofsson, Elisa Hallem, Piali Sengupta, Clifford Stephan, and Paul Sternberg for reagents; Qilin Li and Carrie Massielo for total organic carbon testing; Rene Garcia for laser ablation experiments; Celeste Riepe, Liming Wang, and James Thomas for discussion and critical reading of the manuscript; and Joaquina Nunez and Erin Yun Xiao for technical assistance. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). This research was funded by the NIH (HG004724 and DA018341), Department of Defense (W81XWH-16-1-0110), a Searle Scholar grant to W.Z., and a Sloan Fellowship to R.A.B.
Author contributions: W.Z. designed the research. Y.Z., M.L.-C., Z.L., J.K.N., Y.C., and W.Z. conducted the experiments. Q.S. and R.A.B. performed fractionation on alarm pheromone. B.A.-M. and S.-K.J. developed analytic tools. W.Z. analyzed and interpreted the data. W.Z. wrote the paper.
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.197293/-/DC1.
Communicating editor: P. Sengupta
Literature Cited
Bargmann, C.I. (2006) Chemosensation in C. elegans (October 25, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.123.1, http://www.wormbook.org.
Hart, A. C., (2006) Behavior (July 3, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.87.1, http://www.wormbook.org.
Hobert, O. (2005) Specification of the nervous system (August 8, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.12.1, http://www.wormbook.org.
Ludewig, A. H. and F. C. Schroeder (2013) Ascaroside signaling in C. elegans (January 15, 2013), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.150.1, http://www.wormbook.org.
Stiernagle, T. (2006) Maintenance of C. elegans (February 11, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.101.1, http://www.wormbook.org.