-
PDF
- Split View
-
Views
-
Cite
Cite
Anne E Dodson, Jasper Rine, Donor Preference Meets Heterochromatin: Moonlighting Activities of a Recombinational Enhancer in Saccharomyces cerevisiae, Genetics, Volume 204, Issue 3, 1 November 2016, Pages 1065–1074, https://doi.org/10.1534/genetics.116.194696
Close - Share Icon Share
Abstract
In Saccharomyces cerevisiae, a small, intergenic region known as the recombination enhancer regulates donor selection during mating-type switching and also helps shape the conformation of chromosome III. Using an assay that detects transient losses of heterochromatic repression, we found that the recombination enhancer also acts at a distance in cis to modify the stability of gene silencing. In a mating-type-specific manner, the recombination enhancer destabilized the heterochromatic repression of a gene located ∼17 kbp away. This effect depended on a subregion of the recombination enhancer that is largely sufficient to determine donor preference. Therefore, this subregion affects both recombination and transcription from a distance. These observations identify a rare example of long-range transcriptional regulation in yeast and raise the question of whether other cis elements also mediate dual effects on recombination and gene expression.
CIS-ACTING elements regulate several processes in the nucleus, including gene expression, recombination, and DNA replication. Some elements act locally, whereas others act in some eukaryotes at distances ranging from several kilobase pairs to remarkable lengths of 1 or 2 Mbp (Pfeifer et al. 1999; Lettice et al. 2003; Smemo et al. 2014) or even an entire chromosome (Clemson et al. 1996; Lee and Jaenisch 1997). In budding yeast, a 2-kbp region referred to as the recombination enhancer (RE) acts in cis to promote the use of sites located throughout the left arm of chromosome III as donor templates during mating-type interconversion (Wu and Haber 1996). Through a separate, unknown mechanism, the RE also regulates the spatial conformation of chromosome III (Belton et al. 2015). Hence, this extraordinary locus is enriched for features that mediate long-range effects.
The RE functions with other loci on chromosome III to facilitate mating-type switching (Haber 2012). The MATa and MATα alleles of the mating-type (MAT) locus, located on the right arm of chromosome III, specify mating type. In addition, silent copies of the mating-type alleles reside on opposite ends of chromosome III; on the left end, the HML locus typically contains a silent copy of the α information, and on the right end, the HMR locus typically contains a silent copy of the a information. To switch mating types, haploids induce expression of the HO site-specific endonuclease, which creates a double-strand break at the MAT locus. Then, the homologous recombination machinery orchestrates a gene conversion event that replaces the original MAT sequence with a copy of the sequence stored at either HML or HMR. HMR serves as the default donor. In a cells, however, the RE somehow promotes the use of HML as the donor template. Therefore, a cells preferentially use HMLα as a donor, and α cells preferentially use HMRa as a donor.
Similar to transcriptional enhancers in metazoans, the RE interacts with transcription factors in a cell-type-specific manner. The left portion of the RE contains several binding sites recognized by the forkhead transcription factor Fkh1 (Sun et al. 2002). Fkh1 binds the RE in a cells but not in α cells, and is important for RE activity (Sun et al. 2002; Ercan et al. 2005; Coïc et al. 2006b; Li et al. 2012). The RE also contains an SCB (Swi4/Swi6 cell cycle box) sequence that binds the SBF (SCB binding factor) transcription factor (Coïc et al. 2006b). The SCB contributes to RE activity independently of Fkh1 (Coïc et al. 2006b). In α cells, the α2 protein encoded by MATα functions with Mcm1 to bind to and inactivate the RE and also repress the transcription of a-specific genes (Johnson 1995; Szeto and Broach 1997; Szeto et al. 1997). The α2-Mcm1 repressor complex binds two sites in the RE and positions highly ordered nucleosomes over the locus that presumably inhibit the binding of transcription factors (Weiss and Simpson 1997). Hence, the effect of the RE on recombination (and therefore donor preference) is specific to a cells.
The RE promotes recombination at sites located throughout the entire left arm of chromosome III, which is ∼110 kbp, and the efficacy of the RE is roughly proportional to its proximity to the locus serving as a donor template (Wu and Haber 1995, 1996; Coïc et al. 2006a). Given that a cells and α cells show similar patterns of DNase-I sensitivity throughout this chromosome arm, the RE does not dramatically alter the local chromatin structure (Ercan and Simpson 2004). Remarkably, the RE does effect a change in the higher-order folding of chromosome III; however, this function is genetically separable from the recombination function (Belton et al. 2015; Lassadi et al. 2015). The mechanism by which the RE acts at a distance remains unresolved.
The RE locus is atypical for yeast in that its effects extend across a large region. Other cis elements in budding yeast tend to act locally. In contrast to metazoan enhancers, which often activate promoters at a distance of multiple kbp (Smith and Shilatifard 2014), upstream activation sequences in yeast typically reside within the promoter region (Hahn and Young 2011) and are limited in their ability to activate transcription when moved further away from the TATA box (Dobi and Winston 2007; Reavey et al. 2015). Although silencer elements have the potential to act distally, boundary elements typically prevent them from doing so in their native context (Fourel et al. 1999; Pryde and Louis 1999; Donze and Kamakaka 2001; Meneghini et al. 2003). Therefore, long-range effects on gene expression are rarely observed in yeast.
We recently developed an assay to capture transient events of expression and have since utilized this assay to identify factors that modify the dynamics of heterochromatin-mediated gene silencing (Dodson and Rine 2015). Unexpectedly, our analysis revealed that the RE mediates a long-range, cis effect on the stability of heterochromatic repression. The identification and characterization of this effect, described here, adds a new dimension to our understanding of the RE and provides an exceptional example of long-range regulation in Saccharomyces cerevisiae.
Materials and Methods
Yeast strains
The strains used in this study were derived from the W303 background and are listed in Supplemental Material, Table S1. Deletions were made using one-step integration of gene-disruption cassettes (Goldstein and McCusker 1999; Gueldener et al. 2002) with the primers listed in Table S2, and were confirmed by PCR of the 5′ and 3′ junctions. The full RE locus was defined as chr III: 28,961–31,213, whereas RE-left was defined as chr III: 28,961–29,852 and RE-right was defined as chr III: 29,853–31,213. Strains containing the reSCB allele (JRY10721 and JRY10722) were made by first replacing the native RE with Kluyveromyces lactis URA3, followed by replacement of K. lactis URA3 with the reSCB sequence (amplified from pJR3396) through selection on 5-fluoroorotic acid and confirmation by sequencing. ARS305 was defined as chr III: 39,239–39,591 and ARS306 was defined as chr III: 74,458–74,677. All chromosome coordinates refer to version R64-2-1 of the S288C genome sequence in the Saccharomyces Genome Database (Engel et al. 2014).
The plasmids used in this study are listed in Table S3. For the construction of pJR3389, the RE locus (chr III: 28,961–31,213) was amplified from genomic DNA (W303) using the REEagI forward and REXhoI reverse primers and inserted into pRS313 following digestion of both the insert and backbone with EagI and XhoI. For the construction of pJR3396, the RE locus (chr III: 28,961–31,213) was first amplified from genomic DNA (W303) using the REEagI forward and REXhoI reverse primers and inserted into pRS303 following digestion of both the insert and backbone with EagI and XhoI. Then, the TC-to-GA mutation in the SCB was created using site-directed mutagenesis.
Colony growth and imaging
For tetrad analysis, JRY10656 was streaked out onto YPD containing both G418 (Geneticin; Life Technologies) and Hygromycin B [Sigma (Sigma Chemical), St. Louis, MO] to select for cells expressing red fluorescent protein (RFP), which were then sporulated for 4–5 days on 1% potassium acetate. Tetrads were dissected on Complete Supplement Mixture (CSM) –Trp (Sunrise Science Products) plates and imaged after 3 days of growth at 30°. For all other image analyses, strains were first streaked out onto YPD +G418 (or CSM –His +G418 for strains containing HIS3-marked plasmids) to select for RFP-expressing cells. Cells were then grown to log phase in CSM –Trp and plated onto CSM –Trp, 1% agar at a density of ∼10 cells/plate to allow for uniformly large colonies. Cells containing HIS3-marked plasmids (JRY10668–JRY10673) were grown to log phase in CSM –His –Trp and plated onto CSM –His –Trp 1% agar at a density of ∼10 cells/plate. Colonies were imaged after 6 days of growth at 30°. Images of at least 12 colonies per genotype were acquired with a Zeiss Axio Zoom.V16 microscope equipped with ZEN software (Zeiss), a Zeiss AxioCam MRm camera, and a PlanApo Z 0.5 × objective. Images were assembled in Photoshop (Adobe Systems).
RNA preparation for quantitative RT-PCR
Cells were grown to log phase in YPD and then harvested for RNA isolation. Total RNA was isolated using hot acidic phenol and chloroform (Collart and Oliviero 2001). Following treatment with DNase I (Roche Diagnostics), RNA was purified by phenol–chloroform extraction. Complementary DNA (cDNA) was synthesized using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) and oligo(dT) primers. Quantitative PCR of cDNA was performed using the primers listed in Table S2, the Thermo Scientific DyNAmo HS SYBR Green qPCR Kit (Fisher Scientific), and an Mx3000P machine (Stratagene, La Jolla, CA). Standard curves were generated from the cDNA of a sir4∆ mutant (JRY10663). Three independent RNA preparations were performed for each sample. A two-tailed Student’s t-test was used to determine the raw P-values presented in the text.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Strains and plasmids are available upon request.
Results
An assay designed to capture transient lapses of silencing recently revealed a dynamic dimension to heterochromatin at the silent mating-type loci HML and HMR (Dodson and Rine 2015). On rare occasions, a cre reporter gene under control of the native α2 promoter at either HMLα or HMRα escapes heterochromatin-mediated gene silencing and triggers a recombination-based switch from RFP expression to GFP expression. By converting transient transcription into permanent changes in genotype and phenotype, the Cre-Reported Altered States of Heterochromatin (CRASH) assay preserves a historical record of silencing loss that manifests as GFP-expressing sectors within an otherwise red colony.
Sas2 stabilized the silencing of HML
With the ability to now monitor the dynamics of silencing, we set out to identify factors that modify these dynamics. Sas2 was a particularly interesting candidate, as it both promotes and antagonizes the silenced state. Sas2 catalyzes the addition of an acetyl group on lysine 16 of histone H4 (H4 K16), which Sir2 must remove to establish and maintain silencing (Tanny et al. 1999; Imai et al. 2000; Shia et al. 2005). Consistent with the antisilencing role of H4 K16 acetylation, deletion of SAS2 improves silencing at HMR and the telomeres (Ehrenhofer-Murray et al. 1997; Kimura et al. 2002; Suka et al. 2002). At HML, however, sas2 mutations delay the de novo establishment of silencing and enhance the silencing defect of sir1 mutants (Reifsnyder et al. 1996; Xu et al. 2006; Osborne et al. 2009).
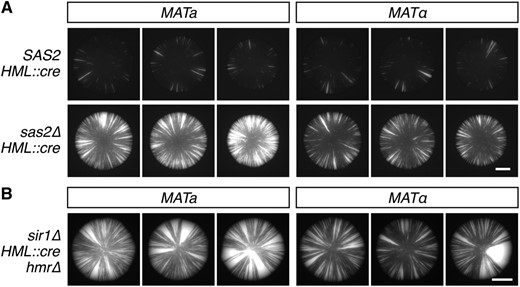
To determine the effects of Sas2 on the stability of silencing, we deleted one copy of SAS2 in a diploid homozygous for HML::cre and the RFP-GFP cassette, induced sporulation, dissected tetrads, and then imaged the fluorescence of the resulting colonies. Within each tetrad, two of the four colonies showed sectoring patterns indicative of frequent silencing loss (Figure S1). Genotyping of each tetrad revealed that the high-sectoring phenotype cosegregated consistently with the sas2∆ mutation (Figure 1A and Figure S1). Therefore, silencing of HML was less stable in colonies derived from the sas2∆ spores than in colonies derived from the SAS2 spores.
Contributions of SAS2 and MAT to the dynamics of heterochromatic repression. (A) GFP fluorescence of colonies grown from tetrad dissections of a sas2 hemizygote (JRY10656). All genotypes contain a cre gene at HML that, upon expression, triggers the permanent activation of GFP expression. See Figure S1 for complete tetrads. Bar, 1 mm. (B) GFP expression of sir1∆ mutants containing HML::cre and the RFP-GFP cassette. Bar, 2 mm. RFP, red fluorescent protein.
Silencing of HML was less stable in a cells than in α cells
Curiously, sas2∆ colonies seemed to fall into one of two phenotypic classes. Qualitatively, half of the sas2∆ colonies exhibited high rates of silencing loss compared to wildtype, whereas the other half exhibited even higher rates of silencing loss (Figure S1). The 1:1 ratio of the two sas2∆ phenotypes suggested that a second modifier of silencing (the first being SAS2) was segregating in the cross. Surprisingly, sas2∆ colonies with the highest levels of sectoring contained the MATa mating-type allele, whereas sas2∆ colonies with relatively lower sectoring levels contained the MATα mating-type allele (Figure 1A and Figure S1). This observation strongly suggested that the MAT genotype affected the stability of silencing at HML.
To test whether the apparent MAT effect was due to the MAT genotype or rather to a de novo mutation that was closely linked to one of the MAT copies, we analyzed the two MAT genotypes in a different strain background. Consistent with the tetrad analysis of the sas2∆ hemizygote, MATa cells showed more frequent losses of silencing than MATα cells in the absence of Sir1, another protein that contributes to the stability of silencing at HML (Figure 1B) (Dodson and Rine 2015). HMR was deleted in both sir1∆ strains to prevent HMR expression from influencing mating-type identity by mimicking the a/α cell type. Therefore, the MAT genotype affected the stability of silencing at HML in both sas2∆ and sir1∆ mutants, and this effect did not depend on the presence of HMR. The phenotype showed incomplete penetrance in both mutants, as a small fraction of MATa colonies exhibited sectoring levels comparable to the majority of MATα colonies and vice versa (data not shown).
Mating type affected silencing through the RE locus
Only a few features distinguish a cells from α cells. MATα encodes α1, an activator of α-specific gene expression, as well as α2, a repressor of both a-specific gene expression and RE activity. By contrast, MATa encodes a1, which functions with α2 in diploids to repress haploid-specific gene expression, as well as a2, a protein of unknown function. In principle, any of the MAT products or any of the loci regulated by a MAT product could be responsible for the effect on silencing.
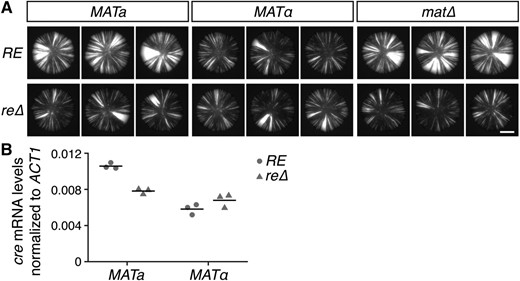
Given that most mating-type-specific genes encode components of the pheromone signaling pathway and therefore seemed unlikely to affect heterochromatin, we instead turned our attention to the RE locus. We deleted the RE region (chr III: 28,961–31,213) in both sas2∆ MATa and sas2∆ MATα strains and analyzed the stability of silencing. Deletion of the RE had no detectable effect on silencing in MATα cells (Figure 2A). In MATa cells, however, deletion of the RE reduced the frequency of silencing loss to a level indistinguishable from the levels observed in MATα RE and MATα re∆ cells. Thus, in the absence of the RE, the stability of silencing was similar between a and α cells.
Effects of mating type and the RE on HML::cre expression. (A) GFP images of colonies. All strains contained the sas2∆ mutation. Bar, 2 mm. (B) Quantitative RT-PCR of cre mRNA levels normalized to ACT1 mRNA levels. Horizontal lines represent the means (n = 3). All strains contained the sas2∆ mutation and exhibited ∼100-fold repression of HML::cre relative to a mutant lacking Sir4, an essential component of heterochromatin (data not shown). mRNA, messenger RNA; RE, recombination enhancer.
Although the strains used in this study do not contain a functional HO gene and therefore do not induce mating-type interconversion, the cre gene at HML could, in principle, be copied into the transcriptionally active MAT locus at a very low rate. If the RE were to promote the occurrence of this event as it normally does during mating-type interconversion, then the RE could potentially increase the frequency of RFP-to-GFP switches in a cells without affecting the stability of silencing. To test this possibility, we removed the sequences shared between HML and MAT by deleting the entire MAT locus, thus blocking all potential for mating-type interconversion. Since a-specific genes are constitutively expressed in the absence of α2, mat∆ cells mate as a. Sectoring levels were similar between MATa and mat∆ colonies, suggesting that the majority of RFP-to-GFP switches observed in MATa colonies did not arise from a transposition of cre into the MAT locus (Figure 2A). Furthermore, the re∆ mutation reduced sectoring levels in mat∆ colonies as it did in MATa colonies and therefore acted independently of the MAT locus (Figure 2A).
The RE could conceivably increase the frequency of RFP-to-GFP switches by enhancing recombination between the two loxP sites in the RFP-GFP cassette rather than by modifying the expression of HML::cre. However, the RE promotes recombination only in cis, and the RFP-GFP cassette was located on a different chromosome (chromosome V).
To test whether the RE directly affected silencing, we measured the levels of cre mRNA using quantitative RT-PCR (Figure 2B). Consistent with the sectoring phenotypes in the CRASH assay, cre mRNA levels were significantly higher in MATa RE cells than in MATα RE cells (P = 2 × 10−4). Deletion of the RE reduced the levels of cre mRNA in MATa cells (P = 4 × 10−4), but did not significantly alter cre mRNA levels in MATα cells (P = 0.2). Moreover, cre mRNA levels were not significantly different between MATa cells and MATα cells in the absence of the RE (P = 0.1). Therefore, mating type directly affected HML::cre expression, and this effect depended on the RE.
The left region of the RE mediated the effect on silencing
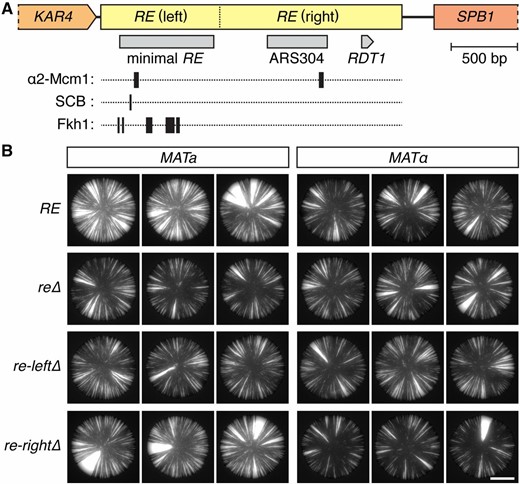
The RE locus contains several features of interest. A 700-bp sequence in the left portion of the RE, often referred to as the minimal RE, is necessary and largely sufficient to promote the usage of HML as a donor in MATa cells (Wu and Haber 1996). The minimal RE contains an SCB site, multiple Fkh1 binding sites, and one of the two α2-Mcm1 operators located within the RE (Figure 3A). The right portion of the RE contains the second α2-Mcm1 operator, an autonomously replicating sequence (ARS) element, and a putative open reading frame (Figure 3A). In addition, a cells transcribe multiple RNA species from the central and right portions of the RE (Szeto et al. 1997; Ercan et al. 2005). A recent study shows that the conformation of chromosome III differs between a and α cells, and that this difference depends on the right portion of the RE, but not on the left portion containing the minimal RE (Belton et al. 2015). Given that the right portion makes only a small contribution to the effect on recombination, the RE is roughly divisible into two subregions with separate functions.
RE features and their effects on HML::cre expression. (A) Map of the RE locus. The left region contains the minimal RE and several protein binding sites, whereas the right region contains an ARS element (ARS304) and a putative open reading frame (RDT1). (B) GFP images of colonies. All strains contained the sas2∆ mutation. Bar, 2 mm. RE, recombination enhancer.
To determine which features were required for the mating-type effect on silencing, we made partial deletions that removed either the left (chr III: 28,961–29,852) or right (chr III: 29,853–31,213) portion of the RE (Figure 3). Deletion of the left portion, which contained the minimal RE, eliminated the difference in sectoring between MATa and MATα colonies (Figure 3B). By contrast, MATa colonies lacking the right RE portion still exhibited high levels of sectoring relative to their MATα counterparts (Figure 3B). Therefore, the mating-type effect on silencing depended only on the left region of the RE locus. Interestingly, deletion of the right side slightly stabilized silencing in α cells, suggesting that the RE affected silencing in more than one way (Figure 3B).
The RE did not act through Fkh1 or the SCB to antagonize silencing
The effect of the RE on donor preference depends on the transcription factor binding sites enriched in the left portion of the RE (Sun et al. 2002; Coïc et al. 2006b). Since the left portion of the RE was responsible for the effect on silencing, we tested whether this effect depended on the transcription factors known to bind this region.
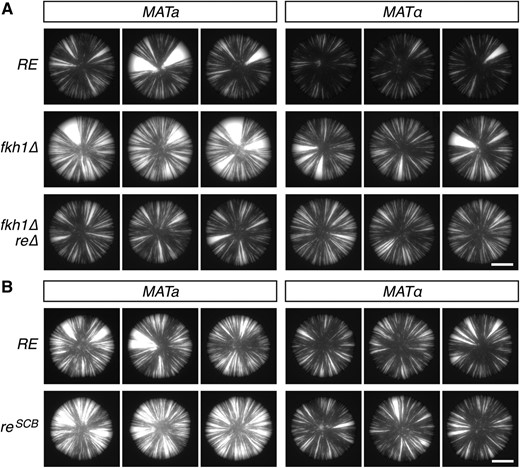
Fkh1, which recognizes several sites within the RE, serves a positive role in Sir-mediated silencing of HMR (Hollenhorst et al. 2000). Consistent with this observation, we found that Fkh1 also contributes to the stability of silencing at HML, as deletion of FKH1 increased the rate of silencing loss in both a and α cells (Figure 4A). In the absence of Fkh1, however, silencing was still less stable in a cells than in α cells, suggesting that the mating-type effect on silencing did not depend on Fkh1. Furthermore, the RE antagonized silencing independently of Fkh1, as deletion of the RE stabilized silencing in MATa fkh1∆ cells (Figure 4A). Thus, unlike the effect of the RE on donor preference, the effect of the RE on silencing did not require Fkh1.
The RE destabilized silencing independent of Fkh1 and the SCB. (A and B) GFP expression within colonies of cells containing HML::cre and the sas2∆ mutation. Bar, 2 mm. The reSCB allele in (B) is a TC-to-GA mutation in the SCB sequence within the RE. RE, recombination enhancer; SCB, Swi4/Swi6 cell cycle box.
The SBF (Swi4/Swi6) complex binds the SCB located in the left portion of the RE, and a TC-to-GA mutation in the SCB, referred to hereinafter as reSCB, reduces the preference for HML as a donor during mating-type switching (Coïc et al. 2006b). To determine whether the SCB contributes to the effect of the RE on silencing, we replaced the native RE locus with the reSCB allele and assayed loss-of-silencing events. Whereas deletion of the RE stabilized silencing in a cells (Figure 2), mutation of the SCB slightly destabilized silencing in a cells (Figure 4B). This observation suggested that the RE did not act through the SCB to affect silencing; rather, the RE seemed to be even more effective at weakening silencing in the absence of an intact SCB.
The RE destabilized the silencing of HML in cis
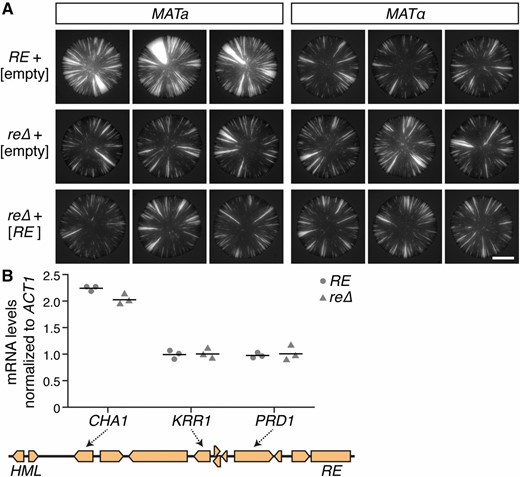
Since the RE activates recombination in cis and resides ∼17 kbp away from HML (Wu and Haber 1996), we tested whether the RE affected silencing in cis by introducing a plasmid-borne copy of the RE into re∆ mutants. If the RE were to act in cis, then the plasmid-borne RE should not be sufficient to restore the difference in silencing between a and α cells. As expected, MATa re∆ cells containing an empty plasmid showed fewer losses of silencing than MATa RE cells (Figure 5A). MATa re∆ cells containing RE on a plasmid phenocopied the empty-plasmid control and therefore also showed fewer losses of silencing than MATa cells containing the RE at its native locus (Figure 5A). These observations suggested that the RE must be on the same molecule of DNA as HML to affect HML expression.
The RE affected specific loci in cis. (A) Comparison between the native RE and a plasmid-borne RE. Images show GFP expression within colonies. Brackets indicate plasmid contents. All strains contained HML::cre and the sas2∆ mutation. Bar, 2 mm. (B) RNA measurements corresponding to genes that lie between HML and the RE. CHA1, KRR1, and PRD1 mRNA levels were normalized to ACT1 mRNA levels. Horizontal lines represent the means (n = 3). All strains contained the sas2∆ mutation. mRNA, messenger RNA; RE, recombination enhancer.
The effect of the RE on gene expression was not widespread
Approximately 10 genes lie between HML and the RE (Figure 5B). If the RE were to activate a large domain for transcription as it does for recombination, then the RE should increase the expression levels of these genes, as well. Since quantitative RT-PCR was sensitive enough to detect a difference in HML::cre expression between MATa RE cells and MATa re∆ cells (P = 4 × 10−4) (Figure 2B), we used this approach to sample a subset of the other nearby genes. CHA1 mRNA levels were slightly higher in RE cells than in re∆ cells, whereas KRR1 and PRD1 mRNA levels were not significantly different between RE and re∆ cells (PCHA1 = 0.03, PKRR1 = 0.9, and PPRD1 = 0.7) (Figure 5B). Collectively, these measurements suggested that the effect of the RE on gene expression was locus-specific rather than widespread.
Early-firing replication origins distal to HML affected the stability of silencing
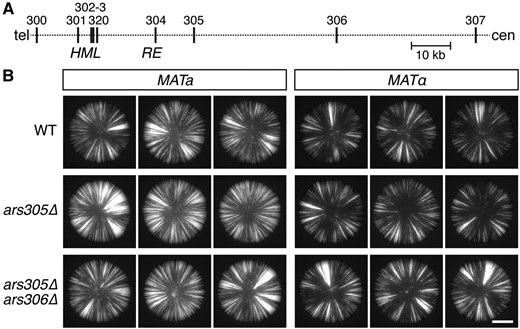
After identifying the long-distance, cis effect of the RE on silencing, we explored whether other distal elements affect silencing, as well. The firing of origins during DNA replication was of particular interest, as the silencers flanking HML contain ARS elements that become active origins of replication upon deletion of ARS305 and ARS306, two early-firing origins located 25- and 59 kbp centromere-proximal to HML, respectively (Figure 6A) (Vujcic et al. 1999; Wang et al. 2001). To test whether the timing or location of origin firing affects the stability of silencing, we deleted active origins on the left arm of chromosome III and analyzed the rate of silencing loss at HML. Deletion of ARS305, the left-most active origin on chromosome III, caused a slight destabilization of silencing in both mating types, as did deletion of both ARS305 and ARS306 (Figure 6B). However, neither deletion removed the striking difference in the stability of silencing at HML between the two mating types. Therefore, active origins of replication exerted subtle effects on the dynamics of heterochromatin, but were not related to the role of the RE.
Effects of distal replication origins on the stability of silencing. (A) Map of the left arm of chromosome III showing the ARS elements (vertical lines) located between the telomere (tel) and centromere (cen). (B) GFP expression within colonies of cells containing HML::cre and the sas2∆ mutation. Bar, 2 mm. ARS, autonomously replicating sequence; WT, wild-type.
Discussion
An evaluation of Sas2 and its contributions to heterochromatin dynamics led to the unexpected identification of a long-range effect on gene expression. We found that the RE, a 2-kbp intergenic region located on chromosome III, acted in cis at a distance of ∼17 kbp to antagonize the silencing of HML. Partial deletions of the RE locus revealed that this effect depended on an 891-bp subregion that contains an unusually high density of transcription factor binding sites. This subregion is also critical for the role of the RE in donor preference (Wu and Haber 1996). Similar to the effect of the RE on recombination, its effect on gene expression was specific to a cells. Thus, the RE locus, originally defined for its role in enhancing recombination, moonlighted as a cell-type-specific modifier of heterochromatin dynamics.
RE: transcriptional enhancer or silencing suppressor?
Whereas the RE promotes recombination throughout the entire left arm of chromosome III, the effects of the RE on gene expression were locus-specific. That is, the RE increased the expression of HML, but did not generally affect other genes in the vicinity. At least two explanations could account for this specificity. In the first scenario, the RE would somehow interfere with the ability of Sir proteins to silence transcription. KRR1 and PRD1 are euchromatic loci and would therefore be immune to any effect mediated by the RE. In support of this model, the RE showed a very modest effect on the expression of CHA1, which sits adjacent to HML and is partially repressed by the Sir proteins (Moreira and Holmberg 1998; Ellahi et al. 2015). In the second scenario, something specific about the α2 promoter at HML would render it sensitive to the RE’s activity. For example, the RE could physically interact with the α2 promoter and thereby activate transcription. Of these two models, we favor the one in which the RE antagonizes the silencing machinery, particularly because we were unable to detect a reproducible effect of the RE on HML expression in the absence of silencing (data not shown).
Action at a distance
One noteworthy difference between a cells and α cells is the spatial conformation of chromosome III (Belton et al. 2015; Lassadi et al. 2015). In comparison to α cells, a cells show more frequent interactions between the left arm of chromosome III, which contains HML and the RE, and a region on the right arm that extends from the centromere to the MAT locus. Upon deletion of the right portion of the RE locus, chromosome III adopts a configuration that is indistinguishable between the two mating types (Belton et al. 2015). This configuration is distinct from that of both MATa RE cells and MATα RE cells, suggesting that the RE regulates chromosome folding in both mating types.
Since the RE controls the three-dimensional organization of chromosome III, it is tempting to speculate that the RE antagonized silencing by either facilitating or disrupting certain long-range contacts involving HML. However, the role of the RE in chromosome folding depends largely on the right portion of the RE locus (Belton et al. 2015), whereas the difference in silencing between a and α cells depended only on the left portion of the RE. Deletion of the right portion slightly stabilized silencing in α cells, but this effect only heightened the overall difference between the two mating types. Therefore, the major effect of the RE on silencing could not be attributed to its role in chromosome folding.
Alternatively, the RE could relay a message to HML by establishing a particular chromatin state that then spreads in cis across the left arm of chromosome III. At face value, this model seems to conflict with the observation that the RE did not detectably affect the expression of more proximal loci such as KRR1 and PRD1. However, the nature of the chromatin state could be such that it only affects loci subject to silencing. This model predicts that a large, continuous domain on the left arm of chromosome III contains a mating-type-specific chromatin signature. Although no such domain is evident in genome-wide surveys of chromatin modifications to date, a comprehensive comparison between a and α cells remains to be tested.
Given that deletion of ARS305 and ARS306 affected the stability of silencing, it is possible that the RE destabilized silencing by regulating the firing of replication origins or by altering progression of the replication fork. Interestingly, Fkh1, which binds several sites in the left portion of the RE, regulates the timing of origin firing (Knott et al. 2012). However, Fkh1 was not required for the RE to antagonize silencing, and at this point there is no clear connection between the RE and ARS elements with respect to their effects on silencing.
At the intersection of heterochromatin and recombination
The effect of the RE on silencing had several aspects in common with the effect of the RE on recombination. For example, both effects occur in cis and depend specifically on the left portion of the RE (Wu and Haber 1996). Therefore, future insight into the mechanism of donor preference may provide additional clues for the mechanism through which the RE affects silencing. Determining which factors affect RE activity upstream of a double-strand break should be particularly informative given that the RE destabilized silencing in the absence of a programmed double-strand break. Interestingly, measurements made by chromatin immunoprecipitation show that the RE promotes a-cell-specific enrichment of yKu70 at HML in the absence of HO induction (Bystricky et al. 2009). Both yKu70 and yKu80, which together form a heterodimer involved in DNA repair, affect silencing and also contribute to donor preference (Ruan et al. 2005; Coïc et al. 2006b; Patterson and Fox 2008; Vandre et al. 2008; Bystricky et al. 2009). Given their connections to both silencing and RE activity, the Ku proteins could, in principle, contribute to the effect of the RE on silencing. The DNA helicase-like protein Chl1, another protein that affects both silencing and RE activity, is another promising candidate (Weiler et al. 1995; Das and Sinha 2005).
Notably, the RE did not depend on Fkh1 or the SCB to antagonize silencing as it does to enhance recombination. Although Fkh1 and Swi4/Swi6 both affect Sir-mediated silencing (Laman et al. 1995; Sussel et al. 1995; Hollenhorst et al. 2000), the RE did not act through either of these transcription factors to increase the rate of silencing loss. In fact, the destabilization of silencing resulting from mutation of the SCB suggested that the SCB may normally act to dampen the effect of the RE on silencing. Perhaps association of the SBF complex with the RE interferes with the binding or activity of some other protein that mediates the effect on silencing. Other proteins known to interact with the RE include Fkh2 and Ndd1 (Sun et al. 2002), and there may be additional RE-interacting proteins that have yet to be identified.
A newfound appreciation of mating type
Sir proteins preserve the mating-type identity of cells by silencing the transcription of HML and HMR. We found that mating type, in turn, affected the ability of Sir proteins to silence transcription. Specifically, cells that mated as a lost silencing at HML more often than cells that mated as α. This distinction had gone unnoticed until now, presumably because the effects of the RE are rare and transient. Deletion of SAS2 or SIR1 sensitized silencing to a level where the difference between a and α cells became apparent, although a close evaluation of wild-type cells showed a similar trend (data not shown). Overall, these observations highlight both the power of segregation analysis and the importance of maintaining consistent MAT genotypes when comparing the stability of silencing between strains.
Acknowledgments
We thank Jim Haber, Barbara Meyer, Job Dekker, and members of the Rine laboratory for helpful discussions. We thank Oskar Hallatschek for use of the Zeiss Axio Zoom.V16 microscope. This work was supported by the National Institutes of Health (GM-031105 to J.R.) and a National Science Foundation Graduate Research Fellowship (DGE1106400 to A.E.D.).
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.194696/-/DC1.
Communicating editor: O. J. Rando