-
PDF
- Split View
-
Views
-
Cite
Cite
Masaki Momose, Yutaka Abe, Yoshihiro Ozeki, Miniature Inverted-Repeat Transposable Elements of Stowaway Are Active in Potato, Genetics, Volume 186, Issue 1, 1 September 2010, Pages 59–66, https://doi.org/10.1534/genetics.110.117606
Close - Share Icon Share
Abstract
Miniature inverted-repeat transposable elements (MITEs) are dispersed in large numbers within the genomes of eukaryotes although almost all are thought to be inactive. Plants have two major groups of such MITEs: Tourist and Stowaway. Mobile MITEs have been reported previously in rice but no active MITEs have been found in dicotyledons. Here, we provide evidence that Stowaway MITEs can be mobilized in the potato and that one of them causes a change of tuber skin color as an obvious phenotypic variation. In an original red-skinned potato clone, the gene encoding for a flavonoid 3′,5′-hydroxylase, which is involved in purple anthocyanin synthesis, has been inactivated by the insertion of a Stowaway MITE named dTstu1 within the first exon. However, dTstu1 is absent from this gene in a purple somaclonal variant that was obtained as a regenerated plant from a protoplast culture of the red-skinned potato. The color change was attributed to reversion of flavonoid 3′,5′-hydroxylase function by removal of dTstu1 from the gene. In this purple variant another specific transposition event has occurred involving a MITE closely related to dTstu1. Instead of being fossil elements, Stowaway MITEs, therefore, still have the ability to become active under particular conditions as represented by tissue culturing.
COLOR mutation or variegation of grain, flower petals, or fruit skin represents a suitable visual marker for the identification of genes for both pigment production and transposable elements (Clegg and Durbin 2000; Winkel-Shirley 2001; Kobayashi et al. 2004). Recent large-scale genome analyses have uncovered numerous transposable elements occupying large portions of eukaryotic genomes. Approximately 45% of the human genome is composed of sequences originating from >3 million copies of transposable elements (International Human Genome Sequencing Consortium 2001). Even in rice, a plant with a relatively small genome, 20% of the genomic sequence can be derived from transposable elements (Turcotte et al. 2001; Goff et al. 2002; Yu et al. 2002). Although almost all of these insertions are thought to be inactive, these elements are suggested to have influenced the evolution of genomes and individual genes. They can rearrange a genome through transposition, insertion, excision, chromosome breakage, or ectopic recombination (Bennetzen 2000). Moreover, some can contribute to the emergence of a novel gene by conveying a poly(A) signal, a transcription start site, a TATA box, a splicing site, or an intron (Oki et al. 2008).
Bioinformatic analyses using data of genome projects found a miniature inverted-repeat transposable element (MITE) (Bureau and Wessler 1992, 1994), the copy number of which reaches over thousands in a genome (Feschotte et al. 2002). Characteristically, a MITE is not >600 bp, does not contain any coding sequences, and has imperfect terminal inverted repeats (TIRs) at the end of the element and its target site is duplicated upon insertion. The majority of MITEs in plants are divided into two groups, Tourist and Stowaway, on the basis of the sequences of TIRs and their target sites, TAA and TA, respectively. Tourist MITEs are found in grasses while Stowaway is present not only in monocotyledonous but also in dicotyledonous plants (Bureau and Wessler 1992, 1994; Feschotte et al. 2002). Although huge numbers of MITEs of each family have been found since their discovery in silico, their dynamic features remain largely unknown. The first mobile MITE, mPing, was identified in rice and belongs to the Tourist family. Its movement was activated during long-term cell culture (Jiang et al. 2003) and by anther culture (Kikuchi et al. 2003). When mPing was inserted into the gene for rice ubiquitin-related modifier-1 (Rurm1), its excision resulted in reversion of the mutable slender glume phenotype to wild type (Nakazaki et al. 2003). The identification of an active element made it possible to discover that the transposable elements Ping and Pong supplied the transposase acting on mPing (Yang et al. 2007). Movement of Stowaway MITEs in rice was also reported recently. These were mobilized in yeast cells by transposases of Mariner-like elements (MLEs) (Yang et al. 2009). Active copies of MITEs have been found only in rice. In dicotyledons the only indication that they can be mobilized has come from insertional polymorphisms between accessions or cultivars (Macas et al. 2005; Menzel et al. 2006).
How a transposable element becomes active is an interesting question since it is potentially an endogenous mutagen and could represent a force for evolution through rearrangement of a genome or production of novel genes. Cell culture is known to activate transposable elements. For example, Ac and Spm/En of class II (DNA) elements were mobilized under such conditions (Peschke et al. 1987; Peschke and Phillips 1991) and tissue culturing resulted in a vast increase of copy number of retrotransposons belonging to class I (RNA) elements (Hirochika 1993). The activation of transposable elements by culture can cause genetic and phenotypic variation in clonal plants, which is one of the reasons for somaclonal variation (Lee and Phillips 1988; Kaeppler et al. 2000).
The active Stowaway MITEs reported here induced somaclonal variation and provide a tool to investigate how MITEs have propagated to become a major component of the plant genome and under which conditions they become active.
MATERIALS AND METHODS
Plant materials:
Commercial triploid potato cultivars named “Jaga kids purple” (JKP) and “Jaga kids red” (JKR) were developed by Kirin Brewery Co. (Japan) from leaf protoplasts of a red-skinned, triploid clone “72218,” which was obtained by a cross between a tetraploid cultivar “Early rose” (Solanum tuberosum) and a diploid related species S. phureja (Tomida and Kawakami 1989). Tubers of 72218, generally designated “Neo-delicious” or “Akadake,” were kindly provided by Kazuyoshi Hosaka of Kobe University.
Pigment analysis:
Pigment was extracted from tuber skin with 50 ml 50% (v/v) acetic acid. After filtration, 200 ml of water was added to the extract and this solution was passed over an ODS resin column (Wakosil 25C18, i.d. 15 × 100 mm; Wako Pure Chemical Industries, Osaka, Japan) equilibrated with aqueous 10% (v/v) acetic acid. The column was washed with 10% acetic acid, and the fraction with anthocyanins was eluted by methanol containing 0.1% hydrochloric acid. The eluate was dried and the residue was separated by mass TLC [TLC Cellulose (10 × 10 cm); Merck KGaA, Darmstadt, Germany] using t-buthanol:acetic acid:water (TBA) 3:1:1 as the solvent. The anthocyanins, migrating as a colored band, were cut out and extracted by methanol containing 0.1% hydrochloric acid. After evaporation of the solvents, the anthocyanin was dissolved in 1 ml of 1% hydrochloric acid. An equal volume of concentrated hydrochloric acid was added and the solution was heated at 100° for 20 min to release the anthocyanidins that were extracted by isoamyl alcohol. Anthocyanidins in the resulting isoamyl alcohol layer were identified by HPLC/MS analysis; HPLC/MS (1525 Binary HPLC Pump, 996 Photodiode Array Detector, 2767 Sample Manager, Micromass ZQ; Waters, Milford, MA) was equipped with a Synergi 4-m Fusion-RP 80-Å column (4.6 × 100 mm; Phenomenex, Torrance, CA) operated at 30°. The mobile phase consisted of 1% aqueous formic acid as solvent A and methanol as solvent B, and the gradient program was 20% B to 70% B (20 min) and 100% B isocratic (10 min) at a flow rate of 1 ml/min.
Southern blot analysis:
Genomic DNA was isolated from the leaves by a Nucleon Phytopure Genomic DNA extraction kit (GE Healthcare, Uppsala, Sweden). Approximately 10 μg of genomic DNA was digested with EcoRV and then separated by 1% agarose gel electrophoresis. The DNAs were transferred to Hybond N+ (GE Healthcare) and then hybridized to PCR-amplified cDNA for F3′5′H∷rev as a probe. Probe labeling and signal detection were carried out with AlkPhosDIRECT (GE Healthcare).
PCR primers and the reaction condition for cDNA and genomic DNA analyses:
PCR primers used in this study are listed in supporting information, Table S1 with their approximate positions shown in Figure S1. Most PCR reactions were carried out nested, with two primer sets, to increase specificity and yield. Each PCR consisted of an initial denaturation step at 95° for 3 min, followed by 30 cycles at 95° for 30 sec, 56° for 30 sec, and extension at 72° for 2 or 5 min with a final 3 min extension at 72°. Gel-purified PCR products using MagExtractor (Toyobo, Shiga, Japan) were sequenced directly or after cloning into pCR 4-TOPO using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA) on an ABI PRISM 310 genetic analyzer (Applied Biosystems, Foster City, CA).
Isolation and sequence determination of the cDNAs for the F3′5′H gene:
Total RNA was isolated from ∼100 mg of tuber skin by using an RNeasy Plant Mini Kit (QIAGEN, Hilden, Germany). To obtain the sequence of the cDNA for the flavonoid 3′,5′-hydroxylase (F3′5′H) gene of JKP, a 5′-RACE experiment was performed using a GeneRacer kit (Invitrogen) with supplied and gene-specific primers [no. 1 (5′-AACATTTTTGTCAATAAAKCATCAAA-3′) and no. 2 (5′-CCTTGTAAATCCATCCAAGCTA-3′) for the first and the second amplifications, respectively] that anneal to two highly conserved regions among P450 or F3′5′H genes of S. melongena (GenBank accession no. X70824) (Toguri et al. 1993,b) and Petunia hybrida (GenBank accession nos. Z22544, Z22545, and X71130) (Holton et al. 1993; Toguri et al. 1993a). The gene-specific primers for 3′-RACE [no. 3 (5′-CCGAATTCAAGCTTTATATTATATCTTCGATTTT-3′) for the first and no. 4 (5′-GGCATTACGTATTAGTGAGTTG-3′) for the second amplification] were based on the sequence obtained by the 5′-RACE experiment. The outcome of both RACE experiments enabled the design of primers [no. 5 (5′-CCTTCTACTTCATTCTCACTCT-3′) and no. 6 (5′-AGCAAATATGTTGCACTATAAATG-3′) for the first and nos. 3 and 6 for the second amplification] to amplify the full-length cDNAs for the F3′5′H gene by RT–PCR using first-strand cDNAs prepared from 72218, JKR, and JKP as templates. The extension time for all PCRs was 2 min.
Isolation and sequence determination of the genomic DNA for F3′5′H genes:
Genomic DNA was isolated from ∼100 mg of leaves as described previously (Walbot and Warren 1988). Genomic DNA of the F3′5′H gene was amplified (using a 5-min extension time) with primer nos. 5 and 6. The methods for the isolation of the other F3′5′H pseudogenes, f3′5′h2 and f3′5′h3, are described in File S1.
Isolation of dTstu1-2 and the sequence determination proximal to the insertion site in JKP:
PCR with a primer specific for the internal sequence of dTstu1 [no. 25 (5′-ATTCATTTTGGACCACAAGTTTTA-3′)] yielded a JKP-specific product of 2.5 kb that enabled the design of two new primers [no. 26 (5′-TGTTTTTTGCAGTTATCTTATTTCA-3′) and no. 27 (5′-CAAGGGGAGACATTTAGG-3′)]. Inverse PCR on MboI-digested JKP genomic DNA followed by self-ligation [primer nos. 26 and 27 for the first and nos. 26 and 28 (5′-AGACATTTCATAGGCAAATTGTTA-3′) for the second PCR] produced a JKP-specific ∼1-kb fragment containing the flanking sequences of the dTstu1-2 insertion. Here, primer no. 28 was designed from dTstu1 internal sequence. PCR with primer nos. 29 (5′-AGCTGAAATATGAGATTGAAATTAG-3′) and 30 (5′-ATTTTGCTATATCCACAATGACTT-3′) annealing to these flanking regions amplified the dTstu1-2 insertion locus from genomic DNAs of 72218 and JKP. The extension time of all PCR reactions was 5 min.
MITE display:
Transposon display was carried out using primers designed from the sequence of dTstu1 and dTstu1-2 according to the procedure of Casa et al. (2000). Approximately 250 ng of genomic DNA was digested with MseI and ligated to an adaptor. Aliquots of the reactions were diluted 4-fold with 0.1 × TE. Preselective amplification was performed with a primer complementary to the adapter [Mse + 0 (5′-GACGATGAGTCCTGAGTAA-3′)] and another primer complementary to an internal dTstu1and dTstu1-2 sequence [no. 31 (5′-CATTCTTTTTGGGACTGACTA-3′)]. PCR consisted of 25 cycles at 94° for 30 sec, 56° for 30 sec, and extension at 72° for 1 min with a final 5-min extension at 72°. Aliquots of the reactions were diluted 20-fold with 0.1 × TE. Selective amplification was carried out with a selective primer [Mse + N (5′-GACGATGAGTCCTGAGTAA+N-3′)] and another primer specific for the TIR and target site duplication (TSD) sequence of dTstu1 and dTstu1-2 [no. 32 (5′-ATAAAWTGGGACRGAGGGAGTA-3′)]. The latter primer was labeled at the 5′ end with 6-FAM. Temperature cycling conditions were 94° for 5 min; 10 touchdown cycles of 94° for 30 sec, 66° for 30 sec (−1° each cycle), and extension at 72° for 1 min; followed by 25 cycles of 94° for 30 sec, 56° for 30 sec, and extension at 72° for 1 min with a final 5-min extension at 72°. The products were analyzed on an ABI PRISM 310 genetic analyzer (Applied Biosystems, Foster City, CA).
RESULTS
Key enzyme of the color variation:
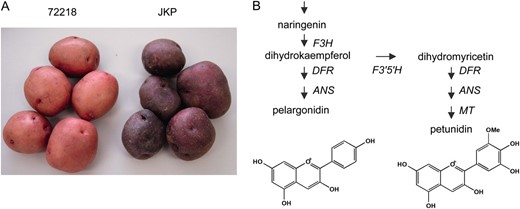
JKP is a potato cultivar with purple tubers that was obtained as a somaclonal variant of skin color after selection from plants regenerated from leaf protoplasts of clone 72218 with red tubers (Figure 1A) (Okamura 1991, 1994). Analysis of the anthocyanin aglycones revealed that the crucial difference between these purple and red potatoes was the presence of petunidin in the tuber skin of JKP as one of the major anthocyanidins, whereas in 72218 this was pelargonidin. The difference between petunidin and pelargonidin is the number of hydroxyl and methoxyl groups at the B-ring of these molecules. Addition of two hydroxyl groups to dihydrokaempferol, which is the precursor of pelargonidin, produces dihydromyricetin, a precursor of petunidin. This reaction is catalyzed by flavonoid 3′,5′-hydroxylase (F3′5′H) (Figure 1B). Therefore, the cause of the color variation from red (72218) to purple (JKP) was attributed to gain of F3′5′H function in the tuber skin of JKP. Recovery of the F3′5′H gene itself would most likely explain the restoration of enzyme activity since genetic analysis had revealed that the dominant allele for F3′5′H in the P locus is solely responsible for determination of the purple color phenotype (Jung et al. 2005).
Tuber pigmentation of 72218 and Jaga kids purple (JKP). (A) Tuber appearance of 72218 and JKP. (B) Schematic pathway of anthocyanidin biosynthesis. Enzyme abbreviations are as follows: F3H, flavanone 3-hydroxylase; F3′5′H, flavonoid 3′,5′- hydroxylase; DFR, dihydroflavonol 4-reductase; ANS, anthocyanidin synthase; MT, anthocyanin 3′-methyltransferase.
Analysis of F3′5′H genes:
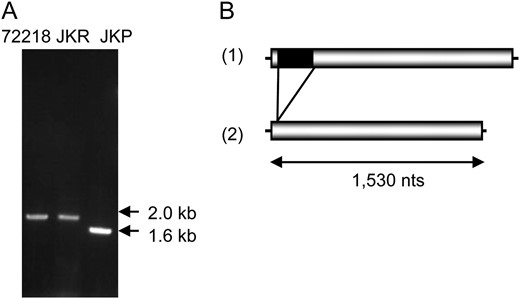
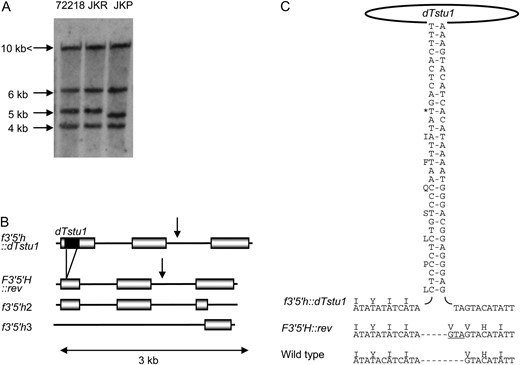
The possibility that disruption of the F3′5′H gene of 72218 was involved in the coloration of its tuber skin was assessed by RT–PCR analysis of the F3′5′H transcript. Sequencing of the obtained cDNA product revealed the presence of a MITE belonging to Stowaway, named dTstu1. This element was absent from the F3′5′H transcript in JKP, which was analyzed in parallel (Figure 2). In support of this, Southern blot analysis with F3′5′H cDNA from JKP as a probe demonstrated a reduction in size in JKP of a 5-kb EcoRV fragment present in 72218 and JKR, which is a somaclonal cultivar with red tubers simultaneously obtained from the leaf protoplast culture of 72218 that yielded JKP (Okamura 1991, 1994). Genomic sequence analysis of F3′5′H genes from 72218 and JKP revealed that the only difference between the full-length genes is the insertion of dTstu1 into the first exon of F3′5′H in 72218 (designated f3′5′h∷dTstu1, DDBJ accession no. AB496977). This element was not present in F3′5′H of JKP (named F3′5′H∷rev, DDBJ accession no. AB496976), which explained the size difference observed in Southern blot analysis (Figure 3, A and B). As the result of a stop codon within dTstu1, f3′5′h∷dTstu1 should produce a truncated protein of only 24 amino acid residues in 72218, whereas F3′5′H∷rev codes for a functional full-length protein of 510 amino acid residues, one residue longer than predicted for the wild type that was reported as a functional F3′5′H gene of diploid potato clone W5281.2 (GenBank accession no. AY675558) (Jung et al. 2005).
Flavonoid 3′,5′-hydroxylase (F3′5′H) transcripts in 72218, JKR, and JKP. (A) RT–PCR products specific for the F3′5′H gene using cDNAs synthesized from RNAs prepared from tuber skins of 72218, JKR, and JKP as templates. The migration of molecular weight markers is shown on the right. (B) Schematic structure of cDNAs for the F3′5′H gene in 72218, JKR (1), and JKP (2). Shaded boxes indicate the coding regions of F3′5′H genes and thin lines the noncoding regions. The black box depicts the insertion of dTstu1.
Flavonoid 3′,5′-hydroxylase (F3′5′H) genes in 72218 and JKP. (A) Southern blot analysis of genomic DNA digested with EcoRV and probed with a labeled RT–PCR product of F3′5′H∷rev. Approximate sizes are given on the left. The largest band represents f3′5′h2 since the EcoRV recognition site is absent in 7.8 kb of determined sequence. The 6.3-kb fragment is derived from f3′5′h3. The rest of the bands represent f3′5′h∷dTstu1 or F3′5′H∷rev since both f3′5′h∷dTstu1 and F3′5′H∷rev have an EcoRV recognition site at the middle of the gene. (B) Structure comparison of F3′5′H genes. Both f3′5′h2 and f3′5′h3 are incomplete genes, f3′5′h2 lacks the latter half of the third exon, and f3′5′h3 contains only the latter half of the third exon. Triploid red 72218 has only pseudogenes, f3′5′h∷dTstu1, f3′5′h2, and f3′5′h3. Triploid purple JKP, a somaclonal variant of 72218, has F3′5′h∷rev, f3′5′h2, and f3′5′h3. Coding regions (shaded boxes) are separated by introns (lines) with the dTstu1 insertion depicted by a solid bar. Arrows indicate the EcoRV recognition site in f3′5′H∷dTstu1 and F3′5′H∷rev. (C) Structure of dTstu1 and the nucleotide and amino acid sequences of F3′5′H genes proximal to the dTstu1 insertion site. Wild type is the previously reported functional F3′5′H gene (Jung et al. 2005). A pair of vertical sequences shows the TIRs where complementary sequences are hyphened. An asterisk indicates a stop codon present in f3′5′h∷dTstu1. The footprint remaining after dTstu1 excision (including the duplicated TA target site) is underlined.
At most, three copies of F3′5′H were deduced to exist in 72218 and JKP on the basis of the results of Southern blot and genomic sequence analyses. Apart from the full-length F3′5′H, the triploid 72218 and JKP possess two truncated copies of this gene (f3′5′h2 and f3′5′h3, DDBJ accession nos. AB496978 and AB496979) (Figure 3B). The sequences of each pseudogene were completely identical between 72218 and JKP. Both f3′5′h∷dTstu1 and F3′5′H∷rev have an EcoRV recognition site at the middle of the gene, which is absent in 7.8 kb of determined f3′5′h2 sequence. Therefore, the largest band in Figure 3A represents f3′5′h2, while the 6.3-kb fragment is derived from the third allele, f3′5′h3, which contains only the latter half of the third exon, encoding the P450 signature motif conserved among all known plant F3′5′H genes. This motif is lacking in f3′5′h2, which strongly suggests that transcripts of this copy do not function properly. Triploid red 72218 has only pseudocopies of the gene, f3′5′h∷dTstu1, f3′5′h2, and f3′5′h3. Its purple somaclonal variant, JKP, has three copies of the gene, F3′5′h∷rev, f3′5′h2, and f3′5′h3.
As F3′5′H∷rev is the only allele able to produce a full-length, nondefective protein, we conclude that excision of dTstu1 from f3′5′h∷dTstu1 during the establishment of JKP is the major reason for the color change from red to purple.
An active Stowaway MITE, dTstu1:
The sequence of dTstu1 is short (239 bp), A/T rich (67%), and marked by TIRs corresponding to the consensus CTCCCTCYGTC and a duplication of the TA target sequence at the insertion site, all characteristics of Stowaway MITEs (Bureau and Wessler 1994). The formation of DNA secondary structure is predicted for this element as well (Figure 3C). Database searches retrieved sequences similar to dTstu1 not only in genomes of Solanum but also in the other Solanaceae plants, for example, Capsicum, Petunia, or Nicotiana (GenBank accession nos. DQ309518, AY136628, and AF277455).
Comparison of the wild-type F3′5′H gene with that of JKP confirmed the addition of one amino acid residue (valine) generated by a three-nucleotide insertion, GTA, in F3′5′H∷rev (Figure 3C). These nucleotides could be traced to consist of one base (G) derived from dTstu1 and two (TA) from the duplicated target site. This duplication was also present in the disrupted f3′5′h∷dTstu1 of 72218 and leading to the observed size difference of 238 bp between the transcripts derived from these genes. Therefore, the presence of these three nucleotides in F3′5′H∷rev of JKP strongly supports that the 239-bp dTstu1 was excised from f3′5′h∷dTstu1 in 72218 as a transposable element leaving a footprint that is normally associated with transposase-mediated excision. We conclude that the F3′5′H gene in 72218 (red) had become functionless as a result of dTstu1 insertion and then reverted in JKP (purple), presumably by transposition of dTstu1 during culturing.
Another active dTstu1-like Stowaway MITE, dTstu1-2:
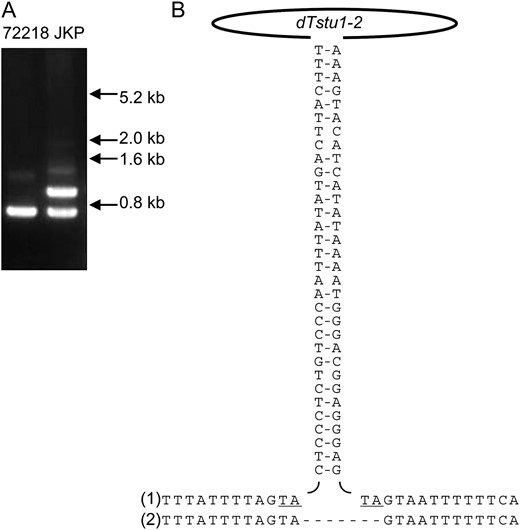
Excision of dTstu1 from the F3′5′H gene during culturing of leaf protoplasts derived from 72218 raised the possibility that other dTstu1-like Stowaway MITEs had undergone transposition under these conditions. In support of this, we isolated an extra dTstu1-like element specific for JKP by use of a DNA-fingerprinting technique adapted from a method with which inter-MITE polymorphisms were detected. With this method, multiple regions between MITEs had been amplified by PCR using a primer annealing to TIRs in the outer direction (Chang et al. 2001). By using primers specific for the dTstu1 internal sequence (instead of the TIR sequences), we obtained a product for JKP not observed for 72218 that contained an element almost identical to dTstu1, named dTstu1-2 (DDBJ accession no. AB496980). After identification of the flanking regions, PCR amplification of the region containing the site of integration of dTstu1-2 in JKP produced in 72218 a fragment of one size, not containing the transposable element. In JKP, however, two fragments, one with and the other without dTstu1-2, were detected (Figure 4A), suggesting that no alleles of the locus carried the transposable element in 72218 and that dTstu1-2 had been newly inserted in an allele. Comparison of the sequence surrounding the insertion site confirmed the presence of a duplicated TA dinucleotide, which is the target sequence of Stowaway MITEs (Figure 4B). Compared to dTstu1, dTstu1-2 had a similar length, 239 bp, but contained four base changes, two of which were in the TIRs (Figure 5). These changes made the TIRs of dTstu1-2 more complementary to each other than in the case of dTstu1. Therefore, in view of a comparable propensity for transposition, this Stowaway MITE conceivably was mobilized under the same conditions that caused dTstu1 to be excised from the F3′5′H gene. If this is the case, activation of transposition of these MITEs was induced by culturing.
Insertion of dTstu1-2 in JKP occurring as a somaclonal variation. (A) PCR-amplified genomic region proximal to the dTstu1-2 target site in JKP in comparison with 72218. Insertion of dTstu1-2 yielded the larger amplified fragment in JKP. The migration of molecular weight markers is shown on the right. (B) Nucleotide sequences around the dTstu1-2 insertion site in JKP (1) and 72218 (2). The pair of vertical sequences represents the TIRs where hyphens connect complementary nucleotides. The target sequence TA and its duplication are underlined.
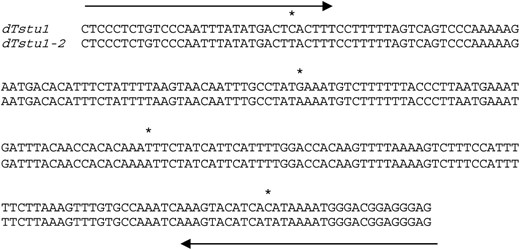
Sequence comparison between dTstu1 and dTstu1-2. Arrows indicate the sequences of TIRs. Nucleotide differences are marked by asterisks.
To survey the active MITE copies related to dTstu1, we carried out MITE display using primers designed from the sequences of dTstu1 and dTstu1-2. More than 50 peaks were detected but slight differences existed among 72218, JKR, and JKP. JKR revealed 3 new peaks and JKP exhibited 3 new peaks and a missing peak as compared with 72218 when using a primer with selective nucleotide T (Figure S2). The insertion of dTstu1-2 in JKP was visualized as a new peak at the expected position of 315 bases in size but the excision of dTstu1 in JKP was not detected at the expected position of 50 bases due to the signal of the other putative insertion at the same position. Although most of the peaks were identical, a few polymorphisms were detected among the three clones.
DISCUSSION
In this study we found the first active Stowaway MITEs in dicotyledons and presented the evidence of their movement. Excision of dTstu1 caused a somaclonal variation of skin color in potato tubers. Insertion of dTstu1-2 was observed at another locus in the genome of the same somaclonal variant, JKP. It became obvious that two major groups of MITEs, Stowaway and Tourist, have the potential to transpose in plants. Movement of MITEs was not proved for a long time because most of them are not inserted into genes (Oki et al. 2008) with the possibility to cause an altered phenotype and because the high copy number of MITEs in the genome precludes analysis of their individual movements. “Fingerprints” of MITE abundance, obtained by Southern hybridization with MITE DNA probes (Naito et al. 2006), showed differences among strains, which suggested movement of MITEs but did not provide direct evidence for their transposition. Previously, a case in which MITE transposition resulted in a phenotypic change was reported. A MITE named mPing, belonging to Tourist, was found to be inserted in the rice Rurm1 gene causing the slender glume phenotype that reverted to wild type by excision of the mobile element (Nakazaki et al. 2003). We present in this report another rare case of a MITE giving rise to an altered phenotype, namely that of dTstu1 belonging to Stowaway. We found this MITE to disrupt the F3′5′H gene of a potato clone (72218), resulting in a red tuber color. Due to the excision of dTstu1 tuber color changed to purple in the somaclonal variant. Thus, in two cases, visible phenotypes, the grain shape for mPing and the tuber color for dTstu1, provided strong evidence for the movement of MITEs belonging to Tourist and Stowaway, respectively.
As described in this report, the duplication of the target sequence TA at the insertion site of dTstu1 was observed for the F3′5′H gene of 72218. The footprint left behind in F3′5′H∷rev in JKP suggests that the excision is catalyzed by a transposase. By lack of any open reading frame, the short Stowaway MITEs of both dTstu1 and dTstu1-2 are not able to code for such a transposase, which has to originate from other, unrelated transposable elements as found in the case of mPing. This Tourist MITE was mobilized by transposases derived from the Ping and Pong transposable elements (Yang et al. 2007). Mobile dTstu1 and dTstu1-2 enable us to search for transposases that control Stowaway MITEs. The Mariner-like element (MLE) is one of the most widely distributed transposable elements in eukaryotes and its transposase can interact in vitro with TIRs of a Stowaway MITE (Feschotte et al. 2005). Using yeast cells, MLE transposases of rice were proved to actually activate transposition of Stowaway MITEs of rice (Yang et al. 2009). MLE is a good candidate for a source of transposase for dTstu1 movement.
Our results show that the activation of Stowaway MITEs not only involves a transposase but also appears to occur under particular conditions. MITE displays of regenerated plants from protoplasts indicated that most of the MITE insertion sites were maintained, although a few differences emerged during tissue culture. The observed differences in sequences and in the insertion sites between the silent copies and the active ones should be investigated further as these may reveal the factors for transposition. Tissue culturing causes the activation of various transposable elements (Peschke et al. 1987; Grandbastien et al. 1989; Peschke and Phillips 1991; Hirochika 1993; Jiang et al. 2003; Kikuchi et al. 2003). It was observed that the conditions under which dTstu1 (and possibly dTstu1-2) was excised, i.e., at some time during the culturing of leaf protoplasts isolated from 72218, caused 7% of the regenerated plants to bear purple tubers instead of the parental red potatoes (Okamura 1991). Furthermore, red tubers with small purple sectors were found in some regenerated plants that originated from cultured leaf protoplasts of 72218 (Figure S3). Such chimeric tubers or purple tubers, however, have not been found in tuber-propagated 72218 plants, which are clonally reproduced as seed potatoes in the field. These facts also support the importance of cell culture conditions for the activation of dTstu1. It remains to be seen how tissue culturing confers the activation. Alteration of the epigenetic status by DNA demethylation of the element itself or of the genes encoding its transposase has been reported to activate a transposable element during tissue culture (Kaeppler et al. 2000; Cheng et al. 2006; Lisch 2009) and could therefore be part of the reason.
How MITEs have spread over various genomes and in such high numbers is still obscure but poses one of the important questions to be tackled to comprehend the evolution of the eukaryotic genome. Active MITEs, like dTstu1, can provide a tool for this investigation.
Footnotes
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.117606/DC1.
Present address: Division of Food Additives, National Institute of Health Sciences, Kamiyouga 1-18-1, Setagaya, Tokyo 158-8501, Japan.
Footnotes
Communicating editor: A. H. Paterson
Acknowledgements
We thank Kazuyoshi Hosaka for 72218 tubers; Yoshio Itoh, Takayasu Hirosawa, Toshihiro Toguri, Noboru Onishi, Naoyuki Umemoto, and Masachika Okamura for discussions; and Chika Aoyama for assistance with experiments. We are grateful to Atsuko Momose for critical reading of the manuscript. This work was partly supported by a grant from the “Technical Development Program for Making Agribusiness in the Form of Utilizing the Concentrated Know-how from the Private Sector” of the Ministry of Agriculture, Forestry and Fisheries, Japan.
References
Bennetzen, J. L.,
Bureau, T. E., and S. R. Wessler,
Bureau, T. E., and S. R. Wessler,
Casa, A. M., C. Brouwer, A. Nagel, L. Wang, Q. Zhang et al.,
Chang, R.-Y., L. S. O'Donoughue and T. E. Bureau,
Cheng, C., M. Daigen and H. Hirochika,
Clegg, M. T., and M. L. Durbin,
Feschotte, C., N. Jiang and S. R. Wessler,
Feschotte, C., M. T. Osterlund, R. Peeler and S. R. Wessler,
Goff, S. A., D. Ricke, T.-H. Lan, G. Presting, R. Wang et al.,
Grandbastien, M. A., A. Spielmann and M. Caboche,
Hirochika, H.,
Holton, T. A., F. Brugliera, D. R. Lester, Y. Tanaka, C. D. Hyland et al.,
International Human Genome Sequencing Consortium,
Jiang, N., Z. Bao, X. Zhang, H. Hirochika, S. R. Eddy et al.,
Jung, C. S., H. M. Griffiths, D. M. De Jong, S. Cheng, M. Bodis et al.,
Kaeppler, S. M., H. F. Kaeppler and Y. Rhee,
Kikuchi, K., K. Terauchi, M. Wada and H. Hirano,
Kobayashi, S., N. Goto-Yamamoto and H. Hirochika,
Lee, M., and R. L. Phillips,
Lisch, D.,
Macas, J., A. Koblížková and P. Neumann,
Menzel, G., D. Dechyeva, H. Keller, C. Lange and H. Himmelbauer,
Naito, K., E. Cho, G. Yang, M. A. Campbell, K. Yano et al.,
Nakazaki, T., Y. Okumoto, A. Horibata, S. Yamahira, M. Teraishi et al.,
Okamura, M.,
Okamura, M.,
Oki, N., K. Yano, Y. Okumoto, T. Tsukiyama, M. Teraishi et al.,
Peschke, V. M., and R. L. Phillips,
Peschke, V. M., R. L. Phillips and B. G. Gengenbach,
Toguri, T., M. Azuma and T. Ohtani,
Toguri, T., N. Umemoto, O. Kobayashi and T. Ohtani,
Tomida, Y., and K. Kawakami,
Turcotte, K., S. Srinivasan and T. Bureau,
Walbot, V., and C. Warren,
Winkel-Shirley, B.,
Yang, G., F. Zhang, C. N. Hancock and S. R. Wessler,
Yang, G., D. H. Nagel, C. Feshotte, C. N. Hancock and S. R. Wessler,
Yu, J., S. Hu, J. Wang, G. K.-S. Wong, S. Li et al.,