-
PDF
- Split View
-
Views
-
Cite
Cite
Wilhelm Paulander, Dan I Andersson, Sophie Maisnier-Patin, Amplification of the Gene for Isoleucyl–tRNA Synthetase Facilitates Adaptation to the Fitness Cost of Mupirocin Resistance in Salmonella enterica, Genetics, Volume 185, Issue 1, 1 May 2010, Pages 305–312, https://doi.org/10.1534/genetics.109.113514
Close - Share Icon Share
Abstract
Mutations that cause resistance to antibiotics in bacteria often reduce growth rate by impairing some essential cellular function. This growth impairment is expected to counterselect resistant organisms from natural populations following discontinuation of antibiotic therapy. Unfortunately (for disease control) bacteria adapt and improve their growth rate, often without losing antibiotic resistance. This adaptation process was studied in mupirocin-resistant (MupR) strains of Salmonella enterica. Mupirocin (Mup) is an isoleucyl–adenylate analog that inhibits the essential enzyme, isoleucyl–tRNA synthetase (IleRS). Mutations causing MupR alter IleRS and reduce growth rate. Fitness is restored by any of 23 secondary IleRS amino acid substitutions, 60% of which leave resistance unaffected. Evidence that increased expression of the original mutant ileS gene (MupR) also improves fitness while maintaining resistance is presented. Expression can be increased by amplification of the ileS gene (more copies) or mutations that improve the ileS promoter (more transcription). Some adapted strains show both ileS amplification and an improved promoter. This suggests a process of adaptation initiated by common amplifications and followed by later acquisition of rare point mutations. Finally, a point mutation in one copy relaxes selection and allows loss of defective ileS copies. This sequence of events is demonstrated experimentally. A better understanding of adaptation can explain why antibiotic resistance persists in bacterial populations and may help identify drugs that are least subject to this problem.
DURING growth under nonselective conditions, spontaneous duplications form at rates (10−5−10−2/cell/division) that are several orders of magnitude higher than those for base changes (Anderson and Roth 1981; Andersson et al. 2009; Andersson and Hughes 2009; Reams et al. 2010). Further increases in gene copy number appear at about 10−2/cell/division. Copy-number changes are likely to be the first adaptive response when cell growth is limited by lack of some activity. For instance, copy number of the lacZYA, araBCD, and cat operons increases when growth is limited by ability to use lactose, arabinose, or benzote (Sonti and Roth 1989; Andersson et al. 1998; Reams and Neidle 2003). The frequent gene amplification and growth provides additional mutational targets and thereby enhances the likelihood of a rare point mutation that further improves gene function.
Mutations that provide resistance to an antibiotic usually reduce bacterial fitness (in the absence of antibiotic) due to alteration of an essential function (Andersson and Levin 1999). Compensatory mutations can improve fitness, often without reducing the level of resistance (Andersson 2003, 2006). This has been observed for several antibiotics, including the topical antibiotic mupirocin (pseudomonic acid A) (Hurdle et al. 2004; Paulander et al. 2007a).
Mupirocin is used mainly to inhibit methicillin-resistant Staphylococcus aureus or β-hemolytic streptococci during skin infections (impetigo) and to prevent presurgery nasal carriage of S. aureus (Cookson 1998; Edlich et al. 2005; Mori et al. 2005). The antibiotic that is an analog of isoleucyl–adenylate inhibits protein synthesis by binding to class I isoleucyl–tRNA synthetases (IleRS), preventing attachment of isoleucine to its cognate tRNA (Schimmel and Soll 1979; Yanagisawa et al. 1994; Pope et al. 1998; Silvian et al. 1999; Nakama et al. 2001). After clinical introduction of mupirocin in 1985, highly resistant strains of S. aureus were isolated [minimal inhibitory concentration (MIC) > 256 μg/ml]. This resistance was conferred by a plasmid that carried a gene (mupA) encoding a Mup-resistant isoleucyl–tRNA synthetase with 30% identity to the chromosomal IleRS enzyme (Hodgson et al. 1994). Point mutations in the chromosomally encoded ileS gene were shown to confer a lower level of resistance (MIC = 8–256 μg/ml) (Yanagisawa et al. 1994; Antonio et al. 2002; Hurdle et al. 2004). Both types of mupirocin-resistant S. aureus were isolated at equal frequency from patients in long-term care facilities (Yoo et al. 2006). Even the low-level resistance, thought initially to be less important because of the high mupirocin concentrations at the site of application, has been reported to cause therapeutic failures (Decousser et al. 2003).
Mupirocin-resistance mutations in the chromosomal ileS gene cause a severe reduction in fitness due the impairment of the IleRS enzyme (Hurdle et al. 2004; Paulander et al. 2007a). Full activity can be restored to the mutant enzyme (MupR) by secondary mutations in the ileS gene. This was determined by allowing MupR strains of Salmonella typhimurium to improve during long-term growth in the absence or presence of antibiotic (Paulander et al. 2007a). Sixty percent of the faster-growing lineages remained highly resistant to the antibiotic. Some lineages that grew faster but retained MupR had a unique set of IleRS amino acid substitutions. However, other improved MupR lineages showed no change in the ileS coding sequence. These strains (analyzed here) owed their growth improvement to increased expression of the original mutant IleRS protein.
Here we describe mechanisms by which IleRS expression increased during adaptation. In some lineages, fitness improvement resulted from amplification of the ileS gene (more gene copies) and in others from changes in the ileS promoter (more transcription). A few lineages had increases both in copy number and transcription, suggesting adaptation by a multistep process. This process was demonstrated by the observation that strains with a simple amplification can gain fitness by acquiring a IleRS substitution and then losing the amplification. Results suggest that all MupR mutants may have improved their fitness by a process initiated by amplification. The added ileS copies (provided by amplification and slow growth) increased the likelihood of subsequent rare point mutations (promoter or amino acid substitutions).
MATERIAL AND METHODS
Strains, media and genetic methods:
Strains used are derivatives of Salmonella enterica var. Typhimurium (referred to as S. typhimurium) LT2 (supporting information, Table S1). All strains were grown at 37° in Luria–Bertani broth (LB) with or without 100 μg/ml mupirocin (GlaxoSmithKline). MICs were determined by using E-test strips (Biodisk) according to the manufacturer's instructions.
Quantitating DNA and RNA by qPCR:
Genomic DNA was prepared from stationary phase cells using the Qiagen kit for total genomic DNA preparation. RNA was isolated using the SV total RNA isolation system from Promega and bacteria grown to midexponential phase (OD600 nm = 0.4). RNA was converted to cDNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems) with an RNase inhibitor. Genomic DNA or cDNA were the templates for real-time quantitative PCR reactions using the primers listed in Table S2 and the power SYBR green kit. Products of PCR were detected using the ABI PRISM 7900 Sequence Detection System (Applied Biosystems) and results were analyzed with the RQ manager 1.2 program (Applied Biosystems).
Size of amplicons and sequence of joint points:
To characterize amplified DNA arrays, gene dosage was first estimated semiquantitatively by Southern blot. Genomic DNA purified with the Qiagen kit was digested with AatII and BglII and the resulting fragments were separated by electrophoresis on 1.2% gels, transferred to a HyBondP membrane and hybridized with several 500-bp probes that correspond to DNA loci on either side of ileS. The probes that were made by PCR using primers listed in Table S2 were individually labeled using the ECL random-prime labeling system (Amersham Biosciences), pooled, and used for hybridization against the membrane; detection of hybridizing fragments was performed according to the manufacturer's instructions (Amersham Biosciences). For determining the sequence of amplification join points, PCRs were performed with mixes of primers (Table S2), whose replication tracks diverged from the ileS gene sequence. Fragments could be obtained only if two replication strands converged across a duplication junction. PCR products were purified with the GFX purification kit (Amersham Biosystems) and sequenced by reactions performed with the Big dye terminator sequencing kit V1.1. (Applied Biosystems). Sequences were read with an ABI prism 3100 genetic analyzer.
Strain evolution and fitness measurements:
The evolved strain C12 that carried several copies of ileS was further serially passaged to improve fitness. Lineages were grown overnight in liquid medium to a density of 3 × 109 bacteria/ml and then diluted 1000-fold into fresh medium, 1 ml LB cultures either with or without mupirocin (100 μg/ml). This procedure was repeated for up to 40 passages (corresponding to approximately 400 generations of growth). At regular time points, the cultures were diluted and plated on LA agar plates to check for changes in colony size and estimate the proportion of cells that showed improved fitness. When the cultures contained mainly cells with improved fitness, i.e., >50% cells showed a large colony size, one large colony was randomly picked and saved at −70°. To determine growth rate, cells were grown in LB medium at 37° and optical density (600 nm) was measured over time by using a BioscreenC reader (Labsystems). The relative fitness of the strains was calculated as the ratio of their generation times: tgen(wt)/tgen(mutant).
RESULTS
Increased expression of ileS restores fitness to MupR mutants:
Previously we isolated four different ileS mutants (MupR) of S. typhimurium, all of which showed reduced fitness during growth (without antibiotic) in vitro and in animal models (Paulander et al. 2007a,b). The resistant strains evolved higher fitness within 80–360 generations without mupirocin or 260–520 generations with mupirocin. Of the 50 evolved lineages, 34 had each acquired an additional point mutation in IleRS that fully or partly restored the enzyme function to the level of the sensitive strain with a wild-type ileS (Paulander et al. 2007a).
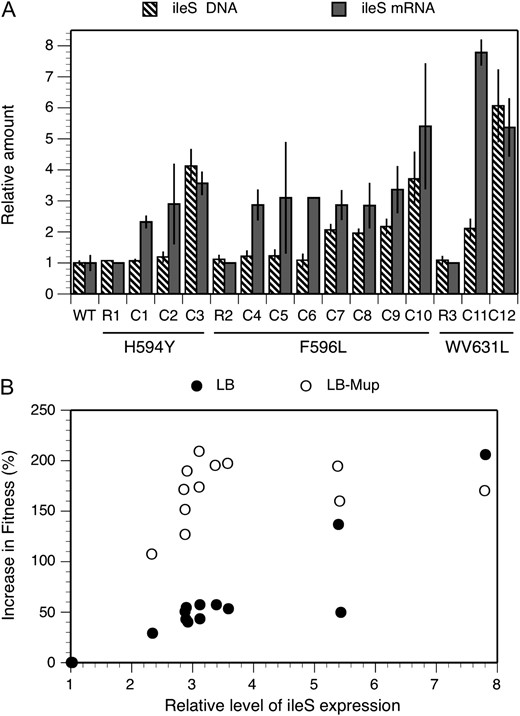
Here we describe the compensation mechanisms that allowed the remaining 16 lineages to improve fitness without a change in the ileS coding sequence. To look for increases in ileS expression (increased transcription or gene amplification) we used real-time PCR to examine the levels of ileS mRNA and DNA relative to those of an internal control, the recA gene (the primers used are listed in Table S2). Of the 16 lineages, 12 (C1–C12) showed a two to eightfold higher level of ileS mRNA than that of wild type or the parent resistant mutant (Figure 1A and Table 1). All these lineages except for C1 evolved in presence of mupirocin. Generally there was a correlation between level of mRNA and fitness (growth rate) whether fitness was tested with or without mupirocin, but this correlation was stronger without drug (Figure 1B). The remaining four mutants (4/50) showed neither a mutation within the ileS gene nor a change in ileS expression; the mechanism of compensation in these mutants remains unknown, but several possibilities are discussed below.
(A) Copy number of ileS gene and levels of mRNA ileS in wild-type (WT), mupirocin-resistant (R1, R2, and R3), and compensated strains (C1–C12). The amino acid substitutions in IleRS that confer mupirocin resistance are indicated under the x-axis (H594Y, F596L, and WV631L). Wild-type level was set to 1.0. (B) Improvement of fitness as a function of the relative level of ileS expression. Fitness corresponds to the relative growth rate measured in LB (solid circles) and LB containing 100 μg/ml of mupirocin (open circles). Wild-type fitness in LB was set to 1.0 and the relative fitness of the strains was calculated as the ratio of tgen(wt)/tgen(mutant).
Type of compensatory mechanisms that restores fitness of mupirocin-resistant strains of S. typhimurium LT2 carrying the W443R, H594Y, F596L, or WV631L amino acid substitutions in IleRS
. | Adaptation to fitness cost . | |
|---|---|---|
| Mechanism of compensation . | Evolved in absence of antibiotic . | Evolved in presence of antibiotic . |
| Intragenic mutation | 22 | 12 |
| Increased gene expression | ||
| Gene amplification | 0 | 3 |
| Gene amplification and promoter mutation | 0 | 4 |
| Promoter mutation | 1 | 4 |
| Unknown | 2 | 2 |
| Total | 25 | 25 |
. | Adaptation to fitness cost . | |
|---|---|---|
| Mechanism of compensation . | Evolved in absence of antibiotic . | Evolved in presence of antibiotic . |
| Intragenic mutation | 22 | 12 |
| Increased gene expression | ||
| Gene amplification | 0 | 3 |
| Gene amplification and promoter mutation | 0 | 4 |
| Promoter mutation | 1 | 4 |
| Unknown | 2 | 2 |
| Total | 25 | 25 |
Type of compensatory mechanisms that restores fitness of mupirocin-resistant strains of S. typhimurium LT2 carrying the W443R, H594Y, F596L, or WV631L amino acid substitutions in IleRS
. | Adaptation to fitness cost . | |
|---|---|---|
| Mechanism of compensation . | Evolved in absence of antibiotic . | Evolved in presence of antibiotic . |
| Intragenic mutation | 22 | 12 |
| Increased gene expression | ||
| Gene amplification | 0 | 3 |
| Gene amplification and promoter mutation | 0 | 4 |
| Promoter mutation | 1 | 4 |
| Unknown | 2 | 2 |
| Total | 25 | 25 |
. | Adaptation to fitness cost . | |
|---|---|---|
| Mechanism of compensation . | Evolved in absence of antibiotic . | Evolved in presence of antibiotic . |
| Intragenic mutation | 22 | 12 |
| Increased gene expression | ||
| Gene amplification | 0 | 3 |
| Gene amplification and promoter mutation | 0 | 4 |
| Promoter mutation | 1 | 4 |
| Unknown | 2 | 2 |
| Total | 25 | 25 |
Gene copy-number increase or promoter improvement augments ileS expression:
The 12 strains with increased ileS expression fell into three classes. In 3 of 12 lineages (C3, C10, and C12), the increased mRNA level was directly proportional to an increased ileS gene copy number, varying from two- to sixfold for the three mutants (Figure 1A). For these mutants, it seems likely that the amplified chromosomal region includes the entire ileS. In 5 of the 12 lineages (C1, C2, C4, C5, and C6) the levels of mRNA were increased without any associated increase in ileS gene copy number. For the remaining 4 lineages (C7, C8, C9, and C11) both mRNA and DNA copy number increased but the mRNA increase exceeded the copy-number increase. To test for evidence of improved transcription in the 9 lineages, the DNA region upstream of the ileS coding sequence was analyzed in hopes of seeing changes in the promoter sequence. The ileS gene is part of an operon that consists of five genes (ribF–ileS–lspA–slpA–ispH) and is transcribed from a promoter located either upstream of or within ribF gene (Miller et al. 1987).
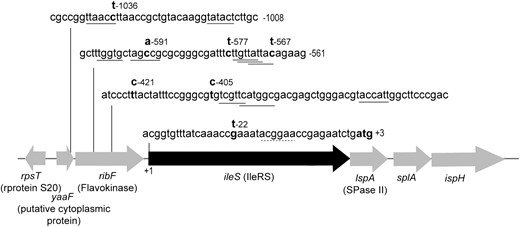
For clones with two copies of ileS, the upstream region of ileS was amplified by PCR and cloned into a cloning vector. At least four independent clones from each lineage were sequenced to assure that potential point mutations that appeared in only one of the gene copies were likely to be detected. A total of seven point mutations were found in the promoter region of the nine clones sequenced (Figure 2). All mutations except G → T (-22) were located in or near one of the three promoter regions previously identified in Escherichia coli by mRNA 5′-end mapping (Miller et al. 1987). The G → T (-22) mutation was located just upstream of a predicted Shine–Dalgarno sequence and seems likely to affect either access to the ribosomal binding site or mRNA stability. The stimulatory effect of these mutations on ileS expression was confirmed by PCR amplifying a 1.4-kb genomic region that includes the sequence upstream of ileS and 95 bp downstream of the ileS gene start codon (ATG). This fragment was cloned in-frame with the lacZ coding region of plasmid pRS552 (Simons et al. 1987). The level of the fused IleRS–β-galactosidase (LacZ) protein was then determined by measuring the activity of β-galactosidase. Clones with the strongest promoter-improving mutations (e.g., C4, C → T-577) showed a measurable 1.5- to 2-fold increase in lacZ gene expression (data not shown). The strains (C1, C5–9) that showed no increase in LacZ expression in this test may have promoter mutations whose effect on ileS was significant when the whole region was amplified (promoter and ileS), but hard to detect using these single-copy fusions.
Map of the S. typhimurium LT2 chromosome near ileS. Mutations in the evolved lineages C1 (C-591 → A), C2 (C-1036 → T), C4 (C-577 → T), C5 (G-22 → T), C6 (C-1036 → T), C7 (T-421 → C), C8 (T-405 → C), C9 (T-405 → C), and C11 (C-567 → T) found upstream of the ileS gene are indicated in boldface type above the sequence; the promoter sequences previously characterized by Miller et al. (1987) are underlined. The RBS region is indicated by a dotted line.
Amplicon structure:
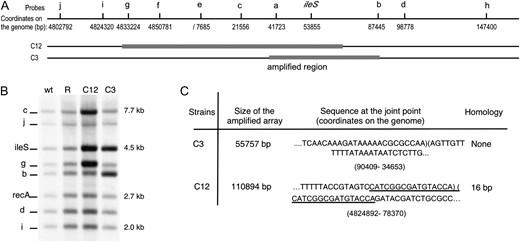
For a few lineages, we determined the size and endpoints of the amplified region. The degree of ileS DNA amplification was first determined by Southern hybridizations (Figure 3A). The DNA level for several chromosomal sites on either side of the ileS gene (probe a–j, Figure 3 and Table S2) was compared to the level of a distant control sequence (recA). Figure 3B shows an example of blots in which intense bands correspond to amplified regions. There it can be seen that lineage C12 includes ileS and the two nearest tested sites on its left (sequences c and g). Lineage C3 shows amplification of ileS and nearest marker to its right (sequence b).
(A) Localization of the markers a–j used as probes for Southern blot. A gray rectangle indicates the amplified regions determined from Southern blot done with several set of probes. (B) Marker frequency hybridization with a set of the probes (ileS, j, g, b, d, i, and recA used as standard) and targets chosen so that each probe lights up one band of distinct size (see materials and methods for details). (C) Size of the amplified arrays and sequence at the joint points in the lineages C3 and C12.
The join points of the amplification were then mapped by PCR using multiple primers combinations that direct replication away from ileS on either end of the amplified region. A PCR fragment can be produced when two primers direct divergent replication tracks across a duplication join point. DNA sequencing of the PCR products revealed the structure of the junction and any sequence element that might have been involved in the exchange that generated the underlying duplication. From the positions of the junction sequences in the wild-type genome sequence, one can infer the size of the amplified unit. Lineage C12 showed a 16-bp sequence at the duplication junction that occurs on both sides of ileS about 110 kbp apart in the wild-type sequence, suggesting that the amplified region is 110-kbp in size (Figure 3C). Lineage C3 had no small sequence repeat at the join point but the position of the junction sequences suggested for the amplified region was 55 kbp. In view of the short (16 or 0 bp) junction repeats, it seems unlikely that RecA contributed to formation of the ultimate duplication junction. Recombination between short sequence homologies seems to be frequent (Edlund and Normark 1981; Whoriskey et al. 1987; Reams and Neidle 2004). However, RecA could have contributed to formation of an initial larger duplication that was later remodeled by deletion and then could contribute to amplification of the duplicated region (E. Kofoid and J. R. Roth, personal communication).
A model for genetic adaptation of cells to the growth limitation caused by their MupR ileS mutation:
Four types of compensated mutants were characterized among 50 different lineages that evolved independently from a common MupR parent strain. Type 1 shows a simple increase in ileS gene copy number (3/50). Type 2 shows both increased ileS gene copy number and a promoter point mutation in one copy (4/50). Type 3 has only a promoter point mutation (5/50). Type 4, described previously, has only a secondary IleRS amino acid substitution that improves fitness often without compromising antibiotic resistance (34/50) (Paulander et al. 2007a). We suggest that all of these types may arise by a shared stepwise process and the types have simply appeared at different points in this process. That is, duplication and amplification of ileS are likely to be the most frequent events and may improve fitness by providing more copies of the compromised ileS allele that provides Mup resistance. This is exemplified by type 1 lineages. The small growth improvement provided by amplification of the mutant ileS gene also adds copies of ileS gene in which mutation can arise. The increased number of ileS target sequences (more copies per cell and more cells in the culture) increases the likelihood of a rare point mutation arising in some copy of the ileS locus—either a promoter up mutation or a secondary IleRS amino acid substitution that improves isoleucine–tRNA synthetase activity. This is exemplified by type 2 lineages with both amplification and one mutant allele. Either sort of point mutation might improve growth sufficiently to relax selection for amplification and allow the array to lose unimproved copies of the ileS gene. This can give rise to the type 3 and 4 lineages in which a rare mutant type (made possible by prior amplification) has taken over the culture and the enabling copy-number increase is lost. Not surprisingly, type 4 mutants are more common than type 3, consistent with a large number of sites at which they can occur compared to critical positions in a promoter.
Testing the model for genetic adaptation:
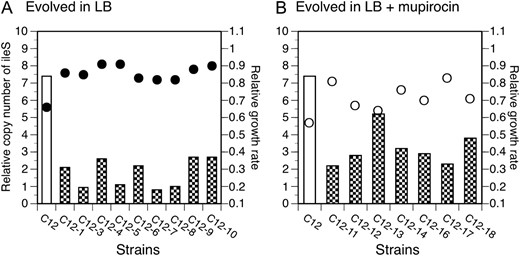
To examine the model outlined above, we used one type 1 lineage (C12) with six copies of the ileS gene and allowed it to evolve further. We evolved 20 sublineages from strain C12 in LB medium either with mupirocin (10 cultures) or without (10 cultures). The 20 lineages were passaged serially until more than 50% of the cells in the population showed an improved fitness, which was monitored by visual examination of colony size on solid agar medium (Maisnier-Patin et al. 2002). As seen in Figure 4, 9 of 10 lineages evolved in the absence of drug and 7 of 10 evolved in presence of drug showed improved fitness after 200–400 generations of growth. Fitness relative to wild type increased from 0.66 to up to 0.91 in LB (Figure 4A) and from 0.57 to 0.83 in LB plus mupirocin (Figure 4B).
Fitness and copy number of ileS in lineages of C12 evolved in LB (A) or LB containing 100 μg/ml of mupirocin (B). Wild-type fitness in LB was set to 1.0 and the relative fitness of the strains was calculated as the ratio of tgen(wt)/tgen(mutant). Bars represent relative ileS DNA levels; circles are relative growth rates in LB (solid) or LB containing 100 μg/ml of mupirocin (open).
As predicted by the model, all of the improved lineages had lower ileS copy number (one to five copies) than the parent strain (six copies) (Figure 4). Copy number remained generally higher for lineages evolved in the presence of mupirocin (two to five copies) than for those evolved in the absence of drug (one to two copies). This result was expected since the number of potential compensatory mutations that can restore fitness in the absence of drug is higher than in the presence of drug (Paulander et al. 2007a).
The drop in ileS gene copy number from six to one suggested that the array might have been lost following acquisition of a stable compensatory mutation that provided increased ileS gene expression or a qualitatively improved IleRS specific activity. The four of nine lineages evolved without muporicin (C12–3, C12–5, C12–7, and C12–8) that had one ileS copy showed a level of ileS mRNA like that of the MupR parent strain C12 (data not shown). But all four lineages had acquired a second-site mutation in the ileS coding sequence—either amino acid substitution Q420H or P184T in IleRS (Table 2). The substitution Q420H had previously been isolated as a mutation that restores fitness (Paulander et al. 2007a) whereas P184T was a novel compensatory mutation. Thus, as predicted by the model, a lineage with a simple amplification (C12) had acquired compensatory mutations and lost their amplified array. Note that only two substitutions were found and one recurred three times—in two different ways. This suggests that selection detected extremely rare events, whose enhanced frequency might have been provided by prior amplification of the ileS locus.
Compensatory mutations that restore fitness to the mupirocin-resistant strain of S. typhimurium LT2 carrying the IleRS amino acid substitution WV631L and an amplified array with 6 copies of the resistant ileS (C12, see Figure 1)
Strains . | Resistance mutation . | Presence of mupirocin in LB . | Compensatory amino acid change (base substitution) . |
|---|---|---|---|
| C12–3 | WV631L | — | Q420H (G1259 → C) |
| C12–5 | WV631L | — | Q420H (G1260 → C) |
| C12–7 | WV631L | — | Q420H (G1259 → C) |
| C12–8 | WV631L | — | P184T (C550 → A) |
Strains . | Resistance mutation . | Presence of mupirocin in LB . | Compensatory amino acid change (base substitution) . |
|---|---|---|---|
| C12–3 | WV631L | — | Q420H (G1259 → C) |
| C12–5 | WV631L | — | Q420H (G1260 → C) |
| C12–7 | WV631L | — | Q420H (G1259 → C) |
| C12–8 | WV631L | — | P184T (C550 → A) |
Compensatory mutations that restore fitness to the mupirocin-resistant strain of S. typhimurium LT2 carrying the IleRS amino acid substitution WV631L and an amplified array with 6 copies of the resistant ileS (C12, see Figure 1)
Strains . | Resistance mutation . | Presence of mupirocin in LB . | Compensatory amino acid change (base substitution) . |
|---|---|---|---|
| C12–3 | WV631L | — | Q420H (G1259 → C) |
| C12–5 | WV631L | — | Q420H (G1260 → C) |
| C12–7 | WV631L | — | Q420H (G1259 → C) |
| C12–8 | WV631L | — | P184T (C550 → A) |
Strains . | Resistance mutation . | Presence of mupirocin in LB . | Compensatory amino acid change (base substitution) . |
|---|---|---|---|
| C12–3 | WV631L | — | Q420H (G1259 → C) |
| C12–5 | WV631L | — | Q420H (G1260 → C) |
| C12–7 | WV631L | — | Q420H (G1259 → C) |
| C12–8 | WV631L | — | P184T (C550 → A) |
DISCUSSION
Adaptive evolution in response to a common selective pressure can follow different trajectories, resulting in different genetic endpoints (Nagaev et al. 2001; Maisnier-Patin et al. 2002, 2007; Lunzer et al. 2005; Poelwijk et al. 2007). In the experimental system studied here, 50 independently evolved lineages of low-fitness mupirocin-resistant mutants accumulated at least five classes of compensatory mutations. The majority of lineages (34/50) accumulated 1 of 23 different secondary amino acid substitutions in the mutant IleRS protein that provided the initial drug resistance (Paulander et al. 2007a). Twelve of the remaining 16 mutants showed increased expression of the mutant ileS gene either by (i) increased ileS gene copy number (3/50), (ii) increased ileS gene copy number combined with a promoter point mutation (4/50), or (iii) a promoter point mutation (5/50). These different lineages may all have arisen by a shared pathway—amplification, point mutation in one copy, and loss of unimproved copies—that ended with a haploid strain carrying a single rare base-substitution mutation.
Several predictions of this model were tested by further evolution of a strain carrying a simple increase in ileS gene copy number. In the course of further evolution almost all lineages improved their fitness and lost copies of their ileS amplification. The four lineages whose copy number returned to 1 were tested and proved to carry a stable amino acid substitution mutation in the IleRS. Thus, the present findings show that gene amplification can serve as an intermediate step that allows subsequent adaptation by stable mutational changes. We anticipate that other derived lineages are in the process of approaching this endpoint. Previous modeling of the effect of gene amplification on the rate of adaptation under several different conditions with variable duplication rates, mutation rates, and population sizes predicted that gene amplification will, especially under conditions when the mutation supply rate is low (small populations and/or mutation rates), provide a significant force to facilitate adaptation (Sun et al. 2009).
Why does an increased level of mutated IleRS improve fitness? The altered structure of resistant enzyme typically leads to both lower affinity for the antibiotic and for the acylated amino acid (Paulander et al. 2007a). Thus, by increasing gene expression of isoleucyl–tRNA synthetase (promoter mutation or gene amplification) the elevated level of defective enzyme may improve the rate of aminoacylation of tRNAile and thereby improve fitness. Furthermore in the presence of mupirocin, an increase of the intracellular level of isoleucyl–tRNA synthetase could also act to titrate out the drug and hence increase fitness by increasing the level of resistance. Increasing the concentration of defective aminoacyl–tRNA synthetases has previously been reported as a mechanism for restoring aminoacylation rates. Thus, some glycyl– or phenyl–tRNA synthetase mutants with reduced affinity for their corresponding amino acid (Km 20 times higher than that of the wild-type strain) could revert to Gly+ or Phe+ phenotype with high frequencies (Folk and Berg 1971; Grull et al. 1979). The Gly+ or Phe+ phenotypes were rapidly lost after growing the cells in absence of selection and prevented by the presence of RecA, implying that duplication and amplification of the respective mutant allele caused suppression. Furthermore, mutations that give resistance to borrelidin, an antibiotic interfering with the activity of threonyl–tRNA synthetase (Poralla 1975), and that lead to overproduction of the wild-type threonyl–tRNA synthetase map in the vicinity of the thrS gene suggesting a mutation in a promoter–operator-like regulatory element (Frohler et al. 1980).
Four of the 50 lineages improved their fitness and remained MupR without any change in either the level of ileS expression or the quality of the IleRS protein. We suggest that these strains may prove to have higher levels of the isoleucine–tRNA or may produce higher levels of the amino acid isoleucine. Either change might increase the rate of producing isoleucyl–tRNA and thereby improve growth of the drug-resistant mutant strain.
In conclusion, these results demonstrate how the fitness reduction associated with deleterious antibiotic resistance mutations can be compensated by mutational mechanisms that alter either the quality (34/50 mutants) or the quantity (12/50 mutants) of the impaired IleRS function. We propose that this occurs by a pathway of events in which early amplification provides some improvement while also increasing the likelihood of a secondary mutational change. These results are discouraging for those hoping that pathogens might lose costly antibiotic resistance during growth following exposure to the antibiotic. Even though the ileS resistance mutations reduce fitness considerably, bacteria adapt rapidly and circumvent this cost often without loss of resistance, implying that resistance is likely to persist in natural bacterial populations.
The rapidity of adaptation may reflect use of adaptive pathways in which extremely frequent copy-number changes improve growth and provide more targets for rare mutations that provide stable improvement. In this case, it seems likely that rare improvements of a promoter are made possible by prior target amplification and growth.
Footnotes
Supporting information available online at http://www.genetics.org/cgi/content/full/genetics.109.113514/DC1.
Footnotes
Communicating editor: S. Gottesman
Acknowledgements
We thank Eric Kofoid for designing the primers for mapping of join points and John R. Roth for discussions and many useful suggestions. This work was supported by grants from the Carl Tryggers foundation (to S.M.P.), from National Institutes of Health (GM270680) to John R. Roth, in whose lab some of this work was done, and from the Swedish Research Council and European Union 6th and 7th framework programs (to D.I.A.).
References
Anderson, P., and J. Roth,
Andersson, D. I.,
Andersson, D. I.,
Andersson, D. I., and D. Hughes,
Andersson, D. I., and B. R. Levin,
Andersson, D. I., E. S. Slechta and J. R. Roth,
Andersson, D. I., D. Hughes and J. R. Roth,
Antonio, M., N. McFerran and M. J. Pallen,
Cookson, B. D.,
Decousser, J. W., P. Pina, J. C. Ghnassia, J. P. Bedos and P. Y. Allouch,
Edlich, R. F., K. L. Winters, L. D. Britt and W. B. Long, 3rd,
Edlund, T., and S. Normark,
Folk, W. R., and P. Berg,
Frohler, J., A. Rechenmacher, J. Thomale, G. Nass and A. Bock,
Grull, J. M., H. Hennecke, J. Frohler, J. Thomale, G. Nass et al.,
Hodgson, J. E., S. P. Curnock, K. G. Dyke, R. Morris, D. R. Sylvester et al.,
Hurdle, J. G., A. J. O'Neill, E. Ingham, C. Fishwick and I. Chopra,
Lunzer, M., S. P. Miller, R. Felsheim and A. M. Dean,
Maisnier-Patin, S., O. G. Berg, L. Liljas and D. I. Andersson,
Maisnier-Patin, S., W. Paulander, A. Pennhag and D. I. Andersson,
Miller, K. W., J. Bouvier, P. Stragier and H. C. Wu,
Mori, N., S. Hitomi, J. Nakajima, K. Okuzumi, A. Murakami et al.,
Nagaev, I., J. Björkman, D. I. Andersson and D. Hughes,
Nakama, T., O. Nureki and S. Yokoyama,
Paulander, W., S. Maisnier-Patin and D. I. Andersson,
Paulander, W., A. Pennhag, D. I. Andersson and S. Maisnier-Patin,
Poelwijk, F. J., D. J. Kiviet, D. M. Weinreich and S. J. Tans,
Pope, A. J., K. J. Moore, M. McVey, L. Mensah, N. Benson et al.,
Poralla, K.,
Reams, A. B., and E. L. Neidle,
Reams, A. B., and E. L. Neidle,
Reams, A. B., E. Kofoid, M. Savageau and J. R. Roth,
Schimmel, P. R., and D. Soll,
Silvian, L. F., J. Wang and T. A. Steitz,
Simons, R. W., F. Houman and N. Kleckner,
Sonti, R. V., and J. R. Roth,
Sun, S., O. G. Berg, J. R. Roth and D. I. Andersson,
Whoriskey, S. K., V. H. Nghiem, P. M. Leong, J. M. Masson and J. H. Miller,
Yanagisawa, T., J. T. Lee, H. C. Wu and M. Kawakami,
Yoo, J. I., E. S. Shin, J. O. Cha, J. K. Lee, Y. H. Jung et al.,