-
PDF
- Split View
-
Views
-
Cite
Cite
Ana Serna, Elena Espinosa, Eva M Camacho, Josep Casadesús, Regulation of Bacterial Conjugation in Microaerobiosis by Host-Encoded Functions ArcAB and SdhABCD, Genetics, Volume 184, Issue 4, 1 April 2010, Pages 947–958, https://doi.org/10.1534/genetics.109.109918
Close - Share Icon Share
Abstract
The virulence plasmid of Salmonella enterica (pSLT) is an F-like conjugative plasmid. High rates of pSLT transfer occur in the mammalian gut, a microaerobic environment. In this study, we describe genetic screens for host-encoded activators and repressors of the transfer operon (tra) of pSLT. We show that the transcription factor ArcA is an activator of conjugation, especially under microaerobiosis. In turn, succinate dehydrogenase (SdhABCD) is a repressor of mating in aerobiosis. ArcA binds upstream of the main tra promoter (ptraY) and activates tra transcription, as previously described in F, R1, and R100. In the absence of ArcA, transfer of pSLT decreased 7-fold in aerobiosis and >100-fold in microaerobiosis. In aerobiosis, ArcA activates the traY promoter in an ArcB-independent manner, as described in other F-like plasmids. In microaerobiosis, however, the ArcB sensor is necessary for activation of ptraY. Lack of Sdh causes a >20-fold increase in pSLT transfer in aerobiosis, but has little effect under microaerobiosis. Sdh inhibits conjugal transfer by reducing traJ transcription, probably in an indirect manner. In turn, the sdhCDAB operon is repressed by the ArcAB system under microaerobiosis. Hence, the ArcAB two-component system of S. enterica stimulates pSLT transfer under microaerobiosis by two concerted actions: activation of the tra operon and repression of the sdhCDAB operon.
THE F-like plasmid family includes a large number of conjugative plasmids whose most conspicuous member is the F sex factor (Willetts and Skurray 1980). Plasmids harboring an F-like conjugation system fall into several incompatibility groups and determine a wide range of phenotypes including antibiotic resistance, colicin production, and synthesis of virulence factors such as enterotoxins and hemolysin (Willetts and Skurray 1980). Six decades of research on F-mediated conjugation have provided an exquisite picture of the mating process and a detailed knowledge of many of the gene products involved (Frostet al. 1994; Firthet al. 1996; Lawleyet al. 2003).
F relatives such as the antibiotic resistance plasmids R1 (Meynell and Datta 1966) and R100 (Nakayaet al. 1960) and the virulence plasmid of Salmonella enterica (Smithet al. 1973; Spratt and Rowbury 1973) have also played historic roles in the study of bacterial conjugation. The virulence plasmid of S. enterica (known as pSLT in serovar Typhimurium) is an F-like plasmid whose conjugation system is closely related to those of F and R100 (Rotger and Casadesus 1999). Transfer of pSLT occurs at low frequency in batch cultures (Ahmeret al. 1999; Camacho and Casadesus 2002) and becomes derepressed in the ileum of infected mice (Garcia-Quintanillaet al. 2008). High osmolarity and microaerobiosis, two reductionist conditions for imitation of the intestinal environment, derepress pSLT transfer in the laboratory (Garcia-Quintanillaet al. 2008).
Regulatory mechanisms that control expression of the conjugal gene cluster in the F episome and other F-like plasmids have been known since the 1970s (Willetts and Skurray 1980). Some such mechanisms rely on regulatory elements encoded on the plasmid itself. For instance, synthesis of TraJ, the main transcriptional activator of the tra operon, is controlled by the FinOP system of fertility inhibition (Frostet al. 1994; Firthet al. 1996). Regulatory feedback loops involving the TraM, TraJ, and TraY plasmid products also contribute to autogenous control of tra operon expression (Penfoldet al. 1996; Polzleitneret al. 1997; Stockwellet al. 2000).
The circuitry that governs mating involves also host-encoded functions, and the known controls involve both transcriptional and post-transcriptional regulation. Examples of transcriptional regulators are the transcription factors ArcA (Silvermanet al. 1991; Strohmaieret al. 1998), CRP (Starcicet al. 2003), and Lrp (Camacho and Casadesus 2002; Starcic-Erjavecet al. 2003; Camachoet al. 2005a) and the nucleoid protein H-NS (Willet al. 2004; Camachoet al. 2005b). The DNA binding capacity of some such factors is in turn controlled by the DNA methylation state of critical DNA regions (Camacho and Casadesus 2002, 2005). Post-transcriptional control of tra operon expression has been shown to involve the RNA chaperone Hfq (Will and Frost 2006), the GroEL heat-shock chaperone (Zahrlet al. 2007), and the extracytoplasmic stress CpxAR system (Gubbinset al. 2002; Zahrlet al. 2006; Lau-Wonget al. 2008). Some host-encoded regulators may control conjugal transfer in most (perhaps all) F-like plasmids while others may be plasmid specific. For instance, F transfer undergoes a drastic decrease in stationary phase (Frost and Manchak 1998), a behavior that is not observed in pSLT (Camachoet al. 2005b). Another example involves the leucine-responsive regulatory protein, which is an activator of traJ transcription in pSLT (Camacho and Casadesus 2002) but not in R100 (Starcic-Erjavecet al. 2003). Adaptation to the host lifestyle and adjustment of conjugal transfer to favorable circumstances can be postulated as tentative explanations for these differences and for others that may exist.
The identification of host-encoded regulators of plasmid transfer is amenable to classical genetics, as initially shown for the F sex factor (Silvermanet al. 1980, 1991) and later for pSLT (Torreblanca and Casadesus 1996; Camacho and Casadesus 2002; Camachoet al. 2005b). On the basis of these antecedents, below we describe genetic trials for host-encoded activators and host-encoded repressors of the pSLT tra operon. All screens involved visual scrutiny, distinguishing between Lac+ and Lac− colonies. The general layout of the screens was that mutations that decreased tra operon expression would identify activators, and mutations that increased tra expression would identify repressors. The trials were expected to reveal mutations that altered tra operon expression in aerobiosis and also in microaerobiosis, because the centers of Salmonella colonies become microaerobic during growth (Aliabadiet al. 1986; Wei and Miller 1999). We show that ArcAB, a two-component system that regulates gene expression in response to the availability of oxygen (Lynch and Lin 1996), is a key factor for the activation of pSLT transfer under microaerobiosis. Aerobic transfer of pSLT is also regulated by ArcA but in an ArcB-independent manner, as previously described in F (Beutin and Achtman 1979; Buxton and Drury 1984). We also show that the ArcAB system plays a second role in the activation of pSLT transfer under microaerobiosis: repression of the sdhCDAB operon, which encodes succinate dehydrogenase. Succinate dehydrogenase (SdhABCD) turns out to be an inhibitor of conjugation and represses traJ expression, probably in an indirect manner.
MATERIALS AND METHODS
Bacterial strains, plasmids, bacteriophages, and strain construction:
The strains of S. enterica used in this study (Table 1) belong to serovar Typhimurium and derive from strain LT2. For simplicity, S. enterica serovar Typhimurium is often abbreviated as S. enterica. The phagemid pBluescript II SK(+) and the Escherichia coli B derivative BL21 [F−dcm ompT hsdS(rB− mB−) gal [malB+]K-12(λS)] are products of Stratagene (La Jolla, CA). Transductional crosses using phage P22 HT 105/1 int201 (Schmieger 1972; G. Roberts, unpublished data) were used for strain construction operations involving chromosomal markers. The transduction protocol was described elsewhere (Garzonet al. 1995). To obtain phage-free isolates, transductants were purified by streaking on green plates. Phage sensitivity was tested by cross-streaking with the clear-plaque mutant P22 H5.
Strains of Salmonella enterica serovar Typhimurium
Strain . | Genotype . | Reference or source . |
|---|---|---|
| LT2 | Wild type | SGSCa |
| SV3003 | ΦtraB1∷MudJ | Torreblanca and Casadesus (1996) |
| SV3069 | dam-201∷Tn10dTc ΦtraB1∷MudJ | This study |
| SV3081 | pSLT− | Torreblancaet al. (1999) |
| SV3083 | pSLT−dam-201∷Tn10dTc | Torreblancaet al. (1999) |
| SV3109 | hisO1242 pdx-543 serC∷Tn10dTc | Mouslimet al. (2000) |
| SV4201 | hisI9960∷Mud1-8 spvA∷Tn5dKm | Camacho and Casadesus (2002) |
| SV4500 | arcA(G76A) sthE∷Tn10dCm ΦtraB1∷MudJ | This study |
| SV4508 | ΔfinO | Camacho and Casadesus (2002) |
| SV4509 | ΔfinO ΦtraB1∷MudJ | Camacho and Casadesus (2002) |
| SV4519 | ΔfinO dam-201∷Tn10dTc ΦtraB1∷MudJ | Camacho and Casadesus (2002) |
| SV4522 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO | Garcia-Quintanillaet al. (2008) |
| SV4761 | ΦtraJ∷lacZ | Camachoet al. (2005b) |
| SV4839 | ΦfinP∷lacZ | Camachoet al. (2005b) |
| SV4914 | ΦtraJ∷lacZ dam-201∷Tn10dTc | E. M. Camacho |
| SV5067 | arcA∷Cmr | This study |
| SV5068 | arcB∷Cmr | This study |
| SV5608 | sdhA∷Cmr | This study |
| SV5867 | ΦsdhB∷lacZ | R. Balbontín |
| SV5868 | arcA∷Cmr ΦsdhB∷lacZ | This study |
| SV5986 | sdhA∷Cmr ΦtraB1∷MudJ | This study |
| SV5987 | sdhA∷Cmr ΦtraB1∷MudJ ΔfinO | This study |
| SV6052 | ΦtraJ∷lacZ ΔfinO | This study |
| SV6053 | ΦtraJ∷lacZ ΔfinO ΔsdhA | This study |
| SV6054 | ΦtraJ∷lacZ ΔfinO ΔsdhA arcA∷Cmr | This study |
| SZ102 | arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ103 | arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ104 | arcA∷Cmr ΦtraB1∷MudJ dam-201∷Tn10dTc | This study |
| SZ105 | arcB∷Cmr ΦtraB1∷MudJ dam-201∷Tn10dTc | This study |
| SZ106 | ΔfinO arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ107 | ΔfinO arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ108 | dam-201∷Tn10dTc ΔfinO arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ109 | dam-201∷Tn10dTc ΔfinO arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ110 | hisI9960∷Mud1-8 spvA∷Tn5dKm arcA∷Cmr | This study |
| SZ111 | hisI9960∷Mud1-8 spvA∷Tn5dKm arcB∷Cmr | This study |
| SZ112 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO arcA∷Cmr | This study |
| SZ113 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO arcB∷Cmr | This study |
| SZ114 | ΦtraJ∷lacZ arcA∷Cmr | This study |
| SZ115 | ΦtraJ∷lacZ arcB∷Cmr | This study |
| SZ116 | ΦtraJ∷lacZ arcA∷Cmrdam-201∷Tn10dTc | This study |
| SZ117 | ΦtraJ∷lacZ arcB∷Cmrdam-201∷Tn10dTc | This study |
| SZ118 | ΦfinP∷lacZ arcA∷Cmr | This study |
| SZ119 | ΦfinP∷lacZ dam-201∷Tn10dTc | This study |
| SZ120 | ΦfinP∷lacZ arcA∷Cmrdam-201∷Tn10dTc | This study |
| SZ122 | hisI9960∷Mud1-8 spvA∷Tn5dKm sdhA∷Cmr | This study |
| SZ123 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO sdhA∷Cmr | This study |
Strain . | Genotype . | Reference or source . |
|---|---|---|
| LT2 | Wild type | SGSCa |
| SV3003 | ΦtraB1∷MudJ | Torreblanca and Casadesus (1996) |
| SV3069 | dam-201∷Tn10dTc ΦtraB1∷MudJ | This study |
| SV3081 | pSLT− | Torreblancaet al. (1999) |
| SV3083 | pSLT−dam-201∷Tn10dTc | Torreblancaet al. (1999) |
| SV3109 | hisO1242 pdx-543 serC∷Tn10dTc | Mouslimet al. (2000) |
| SV4201 | hisI9960∷Mud1-8 spvA∷Tn5dKm | Camacho and Casadesus (2002) |
| SV4500 | arcA(G76A) sthE∷Tn10dCm ΦtraB1∷MudJ | This study |
| SV4508 | ΔfinO | Camacho and Casadesus (2002) |
| SV4509 | ΔfinO ΦtraB1∷MudJ | Camacho and Casadesus (2002) |
| SV4519 | ΔfinO dam-201∷Tn10dTc ΦtraB1∷MudJ | Camacho and Casadesus (2002) |
| SV4522 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO | Garcia-Quintanillaet al. (2008) |
| SV4761 | ΦtraJ∷lacZ | Camachoet al. (2005b) |
| SV4839 | ΦfinP∷lacZ | Camachoet al. (2005b) |
| SV4914 | ΦtraJ∷lacZ dam-201∷Tn10dTc | E. M. Camacho |
| SV5067 | arcA∷Cmr | This study |
| SV5068 | arcB∷Cmr | This study |
| SV5608 | sdhA∷Cmr | This study |
| SV5867 | ΦsdhB∷lacZ | R. Balbontín |
| SV5868 | arcA∷Cmr ΦsdhB∷lacZ | This study |
| SV5986 | sdhA∷Cmr ΦtraB1∷MudJ | This study |
| SV5987 | sdhA∷Cmr ΦtraB1∷MudJ ΔfinO | This study |
| SV6052 | ΦtraJ∷lacZ ΔfinO | This study |
| SV6053 | ΦtraJ∷lacZ ΔfinO ΔsdhA | This study |
| SV6054 | ΦtraJ∷lacZ ΔfinO ΔsdhA arcA∷Cmr | This study |
| SZ102 | arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ103 | arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ104 | arcA∷Cmr ΦtraB1∷MudJ dam-201∷Tn10dTc | This study |
| SZ105 | arcB∷Cmr ΦtraB1∷MudJ dam-201∷Tn10dTc | This study |
| SZ106 | ΔfinO arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ107 | ΔfinO arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ108 | dam-201∷Tn10dTc ΔfinO arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ109 | dam-201∷Tn10dTc ΔfinO arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ110 | hisI9960∷Mud1-8 spvA∷Tn5dKm arcA∷Cmr | This study |
| SZ111 | hisI9960∷Mud1-8 spvA∷Tn5dKm arcB∷Cmr | This study |
| SZ112 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO arcA∷Cmr | This study |
| SZ113 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO arcB∷Cmr | This study |
| SZ114 | ΦtraJ∷lacZ arcA∷Cmr | This study |
| SZ115 | ΦtraJ∷lacZ arcB∷Cmr | This study |
| SZ116 | ΦtraJ∷lacZ arcA∷Cmrdam-201∷Tn10dTc | This study |
| SZ117 | ΦtraJ∷lacZ arcB∷Cmrdam-201∷Tn10dTc | This study |
| SZ118 | ΦfinP∷lacZ arcA∷Cmr | This study |
| SZ119 | ΦfinP∷lacZ dam-201∷Tn10dTc | This study |
| SZ120 | ΦfinP∷lacZ arcA∷Cmrdam-201∷Tn10dTc | This study |
| SZ122 | hisI9960∷Mud1-8 spvA∷Tn5dKm sdhA∷Cmr | This study |
| SZ123 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO sdhA∷Cmr | This study |
Salmonella Genetic Stock Centre, University of Calgary, Calgary, Alberta, Canada.
Strains of Salmonella enterica serovar Typhimurium
Strain . | Genotype . | Reference or source . |
|---|---|---|
| LT2 | Wild type | SGSCa |
| SV3003 | ΦtraB1∷MudJ | Torreblanca and Casadesus (1996) |
| SV3069 | dam-201∷Tn10dTc ΦtraB1∷MudJ | This study |
| SV3081 | pSLT− | Torreblancaet al. (1999) |
| SV3083 | pSLT−dam-201∷Tn10dTc | Torreblancaet al. (1999) |
| SV3109 | hisO1242 pdx-543 serC∷Tn10dTc | Mouslimet al. (2000) |
| SV4201 | hisI9960∷Mud1-8 spvA∷Tn5dKm | Camacho and Casadesus (2002) |
| SV4500 | arcA(G76A) sthE∷Tn10dCm ΦtraB1∷MudJ | This study |
| SV4508 | ΔfinO | Camacho and Casadesus (2002) |
| SV4509 | ΔfinO ΦtraB1∷MudJ | Camacho and Casadesus (2002) |
| SV4519 | ΔfinO dam-201∷Tn10dTc ΦtraB1∷MudJ | Camacho and Casadesus (2002) |
| SV4522 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO | Garcia-Quintanillaet al. (2008) |
| SV4761 | ΦtraJ∷lacZ | Camachoet al. (2005b) |
| SV4839 | ΦfinP∷lacZ | Camachoet al. (2005b) |
| SV4914 | ΦtraJ∷lacZ dam-201∷Tn10dTc | E. M. Camacho |
| SV5067 | arcA∷Cmr | This study |
| SV5068 | arcB∷Cmr | This study |
| SV5608 | sdhA∷Cmr | This study |
| SV5867 | ΦsdhB∷lacZ | R. Balbontín |
| SV5868 | arcA∷Cmr ΦsdhB∷lacZ | This study |
| SV5986 | sdhA∷Cmr ΦtraB1∷MudJ | This study |
| SV5987 | sdhA∷Cmr ΦtraB1∷MudJ ΔfinO | This study |
| SV6052 | ΦtraJ∷lacZ ΔfinO | This study |
| SV6053 | ΦtraJ∷lacZ ΔfinO ΔsdhA | This study |
| SV6054 | ΦtraJ∷lacZ ΔfinO ΔsdhA arcA∷Cmr | This study |
| SZ102 | arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ103 | arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ104 | arcA∷Cmr ΦtraB1∷MudJ dam-201∷Tn10dTc | This study |
| SZ105 | arcB∷Cmr ΦtraB1∷MudJ dam-201∷Tn10dTc | This study |
| SZ106 | ΔfinO arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ107 | ΔfinO arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ108 | dam-201∷Tn10dTc ΔfinO arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ109 | dam-201∷Tn10dTc ΔfinO arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ110 | hisI9960∷Mud1-8 spvA∷Tn5dKm arcA∷Cmr | This study |
| SZ111 | hisI9960∷Mud1-8 spvA∷Tn5dKm arcB∷Cmr | This study |
| SZ112 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO arcA∷Cmr | This study |
| SZ113 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO arcB∷Cmr | This study |
| SZ114 | ΦtraJ∷lacZ arcA∷Cmr | This study |
| SZ115 | ΦtraJ∷lacZ arcB∷Cmr | This study |
| SZ116 | ΦtraJ∷lacZ arcA∷Cmrdam-201∷Tn10dTc | This study |
| SZ117 | ΦtraJ∷lacZ arcB∷Cmrdam-201∷Tn10dTc | This study |
| SZ118 | ΦfinP∷lacZ arcA∷Cmr | This study |
| SZ119 | ΦfinP∷lacZ dam-201∷Tn10dTc | This study |
| SZ120 | ΦfinP∷lacZ arcA∷Cmrdam-201∷Tn10dTc | This study |
| SZ122 | hisI9960∷Mud1-8 spvA∷Tn5dKm sdhA∷Cmr | This study |
| SZ123 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO sdhA∷Cmr | This study |
Strain . | Genotype . | Reference or source . |
|---|---|---|
| LT2 | Wild type | SGSCa |
| SV3003 | ΦtraB1∷MudJ | Torreblanca and Casadesus (1996) |
| SV3069 | dam-201∷Tn10dTc ΦtraB1∷MudJ | This study |
| SV3081 | pSLT− | Torreblancaet al. (1999) |
| SV3083 | pSLT−dam-201∷Tn10dTc | Torreblancaet al. (1999) |
| SV3109 | hisO1242 pdx-543 serC∷Tn10dTc | Mouslimet al. (2000) |
| SV4201 | hisI9960∷Mud1-8 spvA∷Tn5dKm | Camacho and Casadesus (2002) |
| SV4500 | arcA(G76A) sthE∷Tn10dCm ΦtraB1∷MudJ | This study |
| SV4508 | ΔfinO | Camacho and Casadesus (2002) |
| SV4509 | ΔfinO ΦtraB1∷MudJ | Camacho and Casadesus (2002) |
| SV4519 | ΔfinO dam-201∷Tn10dTc ΦtraB1∷MudJ | Camacho and Casadesus (2002) |
| SV4522 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO | Garcia-Quintanillaet al. (2008) |
| SV4761 | ΦtraJ∷lacZ | Camachoet al. (2005b) |
| SV4839 | ΦfinP∷lacZ | Camachoet al. (2005b) |
| SV4914 | ΦtraJ∷lacZ dam-201∷Tn10dTc | E. M. Camacho |
| SV5067 | arcA∷Cmr | This study |
| SV5068 | arcB∷Cmr | This study |
| SV5608 | sdhA∷Cmr | This study |
| SV5867 | ΦsdhB∷lacZ | R. Balbontín |
| SV5868 | arcA∷Cmr ΦsdhB∷lacZ | This study |
| SV5986 | sdhA∷Cmr ΦtraB1∷MudJ | This study |
| SV5987 | sdhA∷Cmr ΦtraB1∷MudJ ΔfinO | This study |
| SV6052 | ΦtraJ∷lacZ ΔfinO | This study |
| SV6053 | ΦtraJ∷lacZ ΔfinO ΔsdhA | This study |
| SV6054 | ΦtraJ∷lacZ ΔfinO ΔsdhA arcA∷Cmr | This study |
| SZ102 | arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ103 | arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ104 | arcA∷Cmr ΦtraB1∷MudJ dam-201∷Tn10dTc | This study |
| SZ105 | arcB∷Cmr ΦtraB1∷MudJ dam-201∷Tn10dTc | This study |
| SZ106 | ΔfinO arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ107 | ΔfinO arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ108 | dam-201∷Tn10dTc ΔfinO arcA∷Cmr ΦtraB1∷MudJ | This study |
| SZ109 | dam-201∷Tn10dTc ΔfinO arcB∷Cmr ΦtraB1∷MudJ | This study |
| SZ110 | hisI9960∷Mud1-8 spvA∷Tn5dKm arcA∷Cmr | This study |
| SZ111 | hisI9960∷Mud1-8 spvA∷Tn5dKm arcB∷Cmr | This study |
| SZ112 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO arcA∷Cmr | This study |
| SZ113 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO arcB∷Cmr | This study |
| SZ114 | ΦtraJ∷lacZ arcA∷Cmr | This study |
| SZ115 | ΦtraJ∷lacZ arcB∷Cmr | This study |
| SZ116 | ΦtraJ∷lacZ arcA∷Cmrdam-201∷Tn10dTc | This study |
| SZ117 | ΦtraJ∷lacZ arcB∷Cmrdam-201∷Tn10dTc | This study |
| SZ118 | ΦfinP∷lacZ arcA∷Cmr | This study |
| SZ119 | ΦfinP∷lacZ dam-201∷Tn10dTc | This study |
| SZ120 | ΦfinP∷lacZ arcA∷Cmrdam-201∷Tn10dTc | This study |
| SZ122 | hisI9960∷Mud1-8 spvA∷Tn5dKm sdhA∷Cmr | This study |
| SZ123 | hisI9960∷Mud1-8 spvA∷Tn5dKm ΔfinO sdhA∷Cmr | This study |
Salmonella Genetic Stock Centre, University of Calgary, Calgary, Alberta, Canada.
Media, chemicals, and growth conditions:
E medium (Vogel and Bonner 1956) was used as minimal medium for S. enterica. The rich medium was Luria–Bertani (LB). Solid media contained agar at 1.5% final concentration. Green plates were prepared according to the original recipe (Chanet al. 1972), except that methyl blue (Sigma-Aldrich, St. Louis) substituted for aniline blue. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) was also from Sigma-Aldrich. Antibiotics were used at the final concentrations described elsewhere (Garzónet al. 1996). YT liquid medium, used for production of recombinant GST-ArcA protein, contained tryptone (16 g/liter), yeast extract (10 g/liter), glucose (5 g/liter), and ampicillin. Microaerobic conditions for culture on solid media were created using GasPak incubation jars (Becton Dickinson Biosciences, San Agustín de Guadalix, Spain). For liquid cultures, microaerobiosis was achieved by incubation without shaking. Neither GasPak jars nor static incubation produce strict anaerobiosis; for this reason, the term “microaerobiosis” is used.
Diethyl sulfate mutagenesis:
Fifty microliters of diethyl sulfate (DES) were dissolved in 5 ml of liquid E medium without carbon source and kept at 37° during 10 min in a screw-capped tube. One hundred microliters of a bacterial suspension (∼108 bacterial cells) was then added. The treatment was allowed to proceed at 37° during 30 min, without shaking. Two hundred microliters of DES-treated bacterial suspension was then used to start a liquid culture in LB medium. When the culture reached saturation, aliquots were spread on LB medium supplemented with X-gal. Use of the traB1∷lac fusion permits the detection of mutations affecting the expression of tra, traJ, or finP (Camacho and Casadesus 2002; Camachoet al. 2005b). In fact, amplification down the regulatory cascade facilitates the discrimination of changes, even if subtle, in the expression of either finP or traJ (Camachoet al. 2005b). DES-induced mutations were transferred from strain to strain using a cotransducible Tn10dCm element, as previously described (Camachoet al. 2005b). Whenever a Tn10dCm insertion was linked (>60%) to the point mutation, the boundaries of the Tn10dCm element were sequenced (Torreblancaet al. 1999). Primers for chromosome walking and serial DNA sequencing were designed on the basis of such sequences. The identification of point mutations was achieved by DNA sequence alignment, using the LT2 genome database (McClellandet al. 2001).
Tn10dCm mutagenesis:
The pSLT-cured strain SV3081 was mutagenized with Tn10dCm as previously described (Torreblanca and Casadesus 1996). Pools of 5000 colonies, each carrying an independent Tn10dCm insert, were then prepared and lysed with phage P22 HT. The lysates were used to transduce either SV3003 or SV3069, selecting chloramphenicol-resistant transductants on LB plates supplemented with X-gal. Candidates were made phage free and reconstructed by P22 HT transduction (Torreblanca and Casadesus 1996).
Construction of S. enterica arcA, arcB, and sdhA mutants by gene targeting:
Targeted disruption of genes in the S. enterica chromosome was achieved by adapting to S. enterica a method previously described in E. coli (Datsenko and Wanner 2000). Primers designed to eliminate specific DNA stretches were based on the LT2 nucleotide sequence (McClellandet al. 2001). When necessary, the kanamycin resistance cassette introduced by the gene targeting procedure was eliminated by recombination with plasmid pCP20 (Datsenko and Wanner 2000). Pairs of additional, external PCR primers were used to verify the predicted gene deletions. Gene-specific primers were designed using PRIMER3 software (http://primer3.sourceforge.net). Disruption of arcA was achieved with primers 5′ TAA CTT ACC GGC TGT TTT TAC AGT TTG GCG CCT GGG CCG AGT GTA GGC TGG AGC TGC C 3′ and 5′ TTG TAC TTC CTG TTT CGA TTT AGT TGG CAA TTT AGG TAG CCA TAT GAA TAT CCT CCT TAG 3′. Verification was performed with primers 5′ CGC AAG CTG AGA TAA ACA GC 3′ and 5′ GTC ATG TT CGC CGA TCA TG 3′. Primers for arcB disruption were 5′ TGG TGT TGG CGC AGT ATT CGC GCA CCC CGG TCA AAC CGG GGT GTA GGC TGG AGC TGC C 3′ and 5′ TAA TTG GGT ATT ATG TGC GAA GTT GTG GTG AAG GAA TCC TCA TAT GAA TAT CCT CCT TAG 3′. Primers for verification of arcB disruption were 5′ ACT GCG CCT TTG ACA TCA TC 3′ and 5′ CTG TAG CGT AGC GTG ATG AG 3′. Primers 5′ TGT AAC CGA AGT CTT AAG GGA ATA ATA AGA ACA GCATGT GGT GTA GGC TGG AGC TGC TTC 3′ and 5′ AGA CTG TAC GTC GCC ATC CGG CAA CCA CTA CAA CTA CTT ACA TAT GAA TAT CCT CCT TAG 3′ were used for sdhA disruption. Strain SV5608 (SdhA−) was verified with primers 5′ TGG CTA CAG GTA GAT TCA CC 3′ and 5′ CAC TTC TAT TGC CTG ATG GC 3′.
β-Galactosidase assays:
Levels of β-galactosidase activity were assayed using the CHCl3-sodium dodecyl sulfate permeabilization procedure (Miller 1972). To measure β-galactosidase activities below 10 Miller units, bacterial cell lysis was employed instead of permeabilization.
Construction and purification of a GST-ArcA fusion protein:
The S. enterica arcA gene was PCR amplified using primers 5′ TTT GGA TCC TAT TAG GTG TCC GGT ACG TC 3′ and 5′ CCG GAA TTC CGC AAG CTG AGA TAA ACA GC 3′. The resulting fragment was purified with the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI). After digestion with BamHI and EcoRI the amplified fragment was cloned onto pGEX4T-1 (GE Healthcare, Little Chalfont, UK) to obtain a fusion protein containing glutathione-S-transferase (GST) at the N terminus (Smith and Johnson 1988) and ArcA at the C terminus. The ligation mixture was used to transform E. coli BL21, selecting Apr. Candidate clones were analyzed by restriction analysis. An Escherichia coli BL21 derivative carrying a plasmid-borne GST-arcA gene construct was thus obtained. Expression of the GST-ArcA recombinant protein was induced with 1 mm IPTG. GST-ArcA was purified from cultures grown in YT, at an OD600 = 1. The culture was centrifuged at 8000 rpm for 10 min, and the pellet was resuspended in 1 ml of lysis buffer (10 mm Tris–HCl, pH 7.4, 150 mm NaCl, 10% glycerol, 1% NP40, 1 mm EDTA, 1 mm dithiothreitol, 1 mm PSMF, and 1 μg/ml commercial protein inhibitors). The mixture was sonicated for 3 min using a Branson Sonifier 2005 (Biogen Cientifica, Madrid), and the resulting lysate was centrifuged at 10,000 rpm at 4° during 30 min. The supernatant and the pellet (resuspendend in lysis buffer) were both immersed in liquid nitrogen. To identify the fraction that contained the GST-ArcA protein, 10-μl aliquots from the supernatant and the pellet were heated at 95° during 5 min and subjected to SDS–PAGE. Electrophoresis was carried out at 175 V for 45–60 min. After drying, gels were stained with Coomassie blue. Because the GST-ArcA protein was found in the supernatant, large-scale purification was carried out on this fraction. Elution from glutathione–agarose was achieved with a solution of 10 mm glutathione, prepared in 50 mm Tris–HCl, pH 8.0. Further work was carried out with a GST-ArcA preparation judged to be ≥95% pure by SDS–PAGE and Coomassie blue staining.
Gel retardation assays with GST-ArcA protein:
For gel retardation analysis, a 618-bp DNA fragment encompassing the traY upstream activating sequence (UAS), the traY promoter, and part of the traY coding sequence was end labeled with Klenow DNA polymerase in the presence of [γ-32P]dATP. DNA-binding reactions were prepared to obtain a final volume of 20 μl, as described elsewhere (Camacho and Casadesus 2002). Each binding reaction contained 0.4 pmol of labeled DNA, 4 μl of GST-ArcA protein diluted in binding buffer, and 0.5 μg of competitor DNA [poly(dI-dC)]. The final composition of the binding buffer was 20 mm Tris-HCl, pH 8.0, 75 mm NaCl, 5 mm MgCl2, 1 mm dithiothreithol, 12.5% glycerol, 0.1 mg/ml bovine serum albumin, and 25 μg/ml poly(dI-dC). For a specific competitor, the same DNA fragment that was used as probe (unlabeled) was added in excess. Binding was allowed to proceed for 20 min at room temperature. Five microliters of loading buffer was then added. Samples were subjected to electrophoretic separation in a nondenaturing 5% polyacrylamide gel prepared in 1× TBE. Electrophoresis was carried out at 200 V for 2–3 hr. After drying, gels were analyzed with a Fujifilm FLA-3000 betascope.
Quantitative reverse transcriptase PCR and calculation of relative expression levels:
Salmonella RNA was extracted from stationary phase cultures using the SV total RNA isolation system (Promega). The quantity and quality of the extracted RNA were determined using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). To diminish genomic DNA contamination, the preparation was treated with DNase I (Turbo DNA free; Applied Biosystems/Ambion, Austin, TX) as previously described (Beuzonet al. 1999). An aliquot of 0.5 μg of DNase I-treated RNA was used for cDNA synthesis, using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Real-time PCR reactions were performed in an Applied Biosystems 7500 Fast Real-Time PCR System. Each reaction was carried out in a total volume of 15 μl on a 96-well optical reaction plate (Applied Biosystems) containing 7.5 μl Power SYBR Green PCR Master Mix (Applied Biosystems), 6.9 μl cDNA (1/10 dilution), and two gene-specific primers at a final concentration of 0.2 μm each. Real-time cycling conditions were as follows: (i) 95° for 10 min and (ii) 40 cycles at 95° for 15 sec and 60° for 1 min. No-template and no reverse-transcriptase controls were included for each primer set and template. Melting curve analysis verified that each reaction contained a single PCR product. Reported gene expression levels were normalized to transcripts of ompA, a housekeeping gene that served as an internal control. Gene-specific primers, designed with PRIMER3 software (http://primer3.sourceforge.net), were as follows: for traJ, 5′ TCA GCC TCT TTC GGG AGA TAG T 3′ and 5′ AGC GAC TGA CAT TCA AGT TCC A 3′; for traY, 5′ GAG GGA TCA TCT GAA ACG ATA TCC 3′ and 5′ AAT GTG GAC TCT GTT TCT TCA ATT ACC T 3′; for finP, 5′ TTC TCA CGA TGC GTC GGA CAC AT 3′ and 5′ TAA ATC GCC GAT ACA GGG AG 3′; for sdhA, 5′ TGG CTA CAG GTA GAT TCA CC 3′ and 5′ CAC TTC TAT TGC CTG ATG GC 3′; and for ompA, 5′ TGT AAG CGT CAG AAC CGA TAC G 3′, and 5′ GAG CAA CCT GGA TCC GAA AG 3′.
Cloning and molecular characterization of Tn10dCm inserts:
Genomic DNA from each Tn10dCm-carrying isolate was digested with SmaI and PstI and cloned onto pBluescript II SK(+). Plasmid inserts were sequenced at the facilities of Sistemas Genómicos SL, Parque Tecnológico de Valencia, Paterna, Valencia, Spain, using the M13L and M13R universal primers.
Bacterial matings:
Overnight cultures of the donor and the recipient were prepared in LB medium. Aliquots of 500 μl were mixed to obtain a donor/recipient ratio of 1:1. Each mixture was centrifuged 2 min at 13,000 rpm, and the supernatant was discarded. The pellet was resuspended in 50 μl of LB broth. Mating mixtures were incubated at 37° during 4 h. Diluted and undiluted aliquots were then spread on selective plates. Microaerobic conditions were obtained using GasPak microaerobic jars as previously described (Garcia-Quintanillaet al. 2008). Irrespective of the conditions in which mating was carried out, all crosses had the same design: the donor strain was a histidine auxotroph whose pSLT plasmid carried a kanamycin resistance tag in the spv locus, which is dispensable for conjugation (Garcia-Quintanillaet al. 2008), and the recipient was a pSLT− prototroph. This combination permitted selection of transconjugants on E plates supplemented with kanamycin.
RESULTS
Genetic trials for chromosomal regulators of the pSLT tra operon: Tn10dCm mutagenesis:
Screens performed in this study made use of the traB1∷lac transcriptional fusion (Torreblanca and Casadesus 1996) and involved discrimination of Lac+ and Lac− colonies on indicator plates (Camacho and Casadesus 2002; Camachoet al. 2005b). All trials were carried out in aerobiosis. However, because the centers of Salmonella colonies become microaerobic or even anaerobic (especially when the colony diameter is >1 mm), colony trials can also detect changes in gene expression in response to oxygen availability (Aliabadiet al. 1986; Wei and Miller 1999). Two types of screens were carried out:
Searches for tra operon repressors, seeking Tn10dCm insertions that derepressed the traB1∷lac fusion: Tn10dCm pools were used to transduce SV3003, selecting chloramphenicol resistance in the presence of X-gal. Candidates were detected by the formation of Lac+ (blue) colonies. The Tn10dCm pools used in this screen had been prepared in SV3083 (pSLT− Dam−). Use of a pSLT− strain prevented the isolation of pSLT-borne insertions (e.g., in finO). In turn, the fact that the strain was Dam− prevented the isolation of insertions in dam, which are a common class of mutations that derepress tra (Camacho and Casadesus 2002).
Trials for tra operon activators, seeking Tn10dCm insertions that prevented pSLT tra operon expression: Genetic screens for mutations that disrupt chromosomal tra activators cannot be performed in the wild type, because the tra operon is tightly repressed in pSLT (Camacho and Casadesus 2002), as in other FinOP+ F-like plasmids (Yoshiokaet al. 1987; Koraimannet al. 1996). However, tra derepression occurs in S. enterica mutants lacking Dam methylase (Torreblanca and Casadesus 1996; Camacho and Casadesus 2002). As a consequence, S. enterica strains carrying the traB1∷lac fusion are Lac+ in a Dam− background (Torreblanca and Casadesus 1996; Camacho and Casadesus 2002). Isolates carrying Tn10dCm insertions that prevented pSLT tra operon expression were thus sought in a Dam− host (SV3069), and candidates were detected by the formation of Lac− (white) colonies. A variant of this trial involved diethyl sulfate mutagenesis instead of Tn10dCm mutagenesis (see below).
Characterization of mutants lacking putative tra repressors:
Analysis of 45,000 Tn10dCm-containing isolates yielded 22 independent Lac+ transductants whose Lac+ phenotype was 100% linked to the Tn10dCm insertion. Nearby DNA sequencing with a Tn10 primer (Way and Kleckner 1984) indicated that more than half of the insertions (14/22) were in the sdh operon: 3 in sdhA, 6 in sdhC, and 5 in sdhD. The remaining insertions were in flhC (3 insertions), flhD (3 insertions), gcvA (1 insertion), and fruR (1 insertion).
Characterization of mutants lacking putative tra activators:
Analysis of 38,000 Tn10dCm-containing isolates yielded 30 independent Lac− transductants whose Lac− phenotype was 100% linked to the Tn10dCm insertion. Eight isolates of this class were Tcs, indicating that the Tn10dCm element was linked to dam and thus suggesting that their Lac− phenotype was due to cotransduction of the wild-type dam allele. DNA from 3 Tcr isolates was sequenced using a Tn10 primer (Way and Kleckner 1984), as above. All three insertions were in lrp. Genetic mapping of additional isolates was performed in transductional crosses using SV3109 as donor. Appearance of Tcr Cms transductants indicated that all Tn10dCm insertions might be in lrp (cotransducible with serC). Tn10 insertion is known to have preferential DNA targets (Kleckneret al. 1979), even if the ATS transposase is used to generate the pool of inserts (Kleckneret al. 1991). The failure of the screen to provide knockouts in tra operon activators other than Lrp, a well known traJ activator (Camacho and Casadesus 2002), led us to use chemical mutagenesis by DES.
DES mutagenesis was carried out on strain SV3069 and provided us with ∼60 independent Lac− isolates that were not in lrp or linked to dam. Around one-third of the isolates analyzed (14/38) were ascribed to a single phenotypic class on the basis of their tra expression pattern, which showed a mild decrease in aerobiosis and a stronger decrease under microaerobiosis (see below). One such isolate was propagated as strain SZ101. The point mutation carried by this isolate was identified as follows:
The isolate was transduced with a pool of Tn10dCm insertions, prepared in a pSLT− strain (SV3081). Transductants were selected on plates containing chloramphenicol and X-gal.
Several Lac+ transductants were purified, lysed with P22, and used to transduce the original isolate. Transductants were selected on LB plates containing chloramphenicol and X-gal. Occurrence of both Lac+ and Lac− transductants provided evidence for linkage between the Tn10dCm element and the chromosomal locus containing the mutation that affected tra expression.
A Tn10dCm insertion 66% linked to the chromosomal mutation was chosen for further study. A 66% linkage upon P22 transduction can be expected to be indicative of an ∼5- to 6-kb distance (Wu 1966). Genomic DNA from this isolate (propagated as strain SV4500) was extracted, digested with PstI, and cloned on pBluescript SKII(+). DNA sequencing with T1 and T7 primers indicated that the Tn10dCm element was inserted in the sthE gene (McClellandet al. 2001). Additional sequencing reactions using primers ad hoc revealed a GC → AT transition in the coding sequence of the arcA gene. This change is predicted to cause an amino acid substitution (Glu → Lys). Six additional isolates of the same phenotypic class as SZ101 carried point mutations 65–67% linked to sthE, suggesting the occurrence of arcA mutations.
Altogether, these experiments suggested that ArcA might be an activator of the tra operon in the Salmonella virulence plasmid, as previously described for F, R100, and R1 (Silvermanet al. 1991; Strohmaieret al. 1998; Takiet al. 1998).
Activation of tra in the Salmonella virulence plasmid requires both ArcA and ArcB:
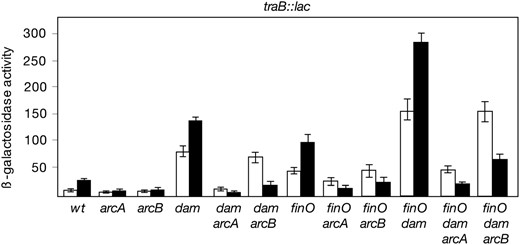
In the absence of proof that the GC → AT transition of strain SV4500 (and the additional alleles of the same class) caused loss of arcA function, we constructed a null arcA allele in S. enterica. Targeted disruption of the S. enterica arcA gene was achieved by the λ Red recombination method (Datsenko and Wanner 2000) to generate strain SV5067. Even though ArcB is not involved in regulation of the tra operon in the F sex factor (Silvermanet al. 1991), an arcB null allele was also constructed (strain SV5068). The effect of arcA and arcB mutations on pSLT tra operon expression was then tested in the wild type and in strains carrying mutations that derepress the pSLT tra operon (finO and dam, alone and combined). The activity of the traB1∷lac fusion was measured in shaken (aerobic) LB cultures and static (microaerobic) LB cultures. A summary of these experiments, shown in Figure 1, is as follows:
Lack of ArcA caused a decrease in tra operon expression, which was best observed under microaerobiosis, probably because expression of the tra operon is higher under such conditions. When tra expression was derepressed by dam and finO mutations, alone or combined, the arcA mutation was epistatic over both dam and finO (Figure 1). These observations provided evidence that ArcA is a tra activator.
Lack of ArcB had little or no effect in aerobiosis but caused a significant reduction of tra operon expression in microaerobiosis (Figure 1), suggesting that both ArcA and ArcB are necessary to activate the pSLT tra operon under microaerobiosis. The latter conclusion is consistent with the physiological activity of the ArcAB system in response to the redox state of the bacterial cell (Lynch and Lin 1996).
When both arcA and arcB mutations were present, the β-galactosidase activity of the traB1∷lac fusion was similar to that detected in the ArcA− background (data not shown), indicating that the arcA mutation was epistatic over arcB. The latter observation is consistent with the known workings of the ArcAB signal transduction system (Iuchi and Lin 1991).
β-Galactosidase activity of the traB1∷lac transcriptional fusion in different genetic backgrounds, monitored in aerobic cultures (open histograms) and microaerobic cultures (solid histograms). Strains and relevant genotypes were as follows (from left to right): SV3003 (wild type), SZ102 (ArcA−), SZ103 (ArcB−), SV3069 (Dam−), SZ104 (Dam− ArcA−), SZ105 (Dam− ArcB−), SV4509 (FinO−), SZ106 (FinO− ArcA−), SZ107 (FinO− ArcB−), SV4519 (FinO− Dam−), SZ108 (FinO− Dam− ArcA−), and SZ109 (FinO− Dam− ArcB−). Enzymatic activities are averages and standard deviations from four to six independent experiments.
Effects of arcA and arcB mutations on conjugal transfer of pSLT:
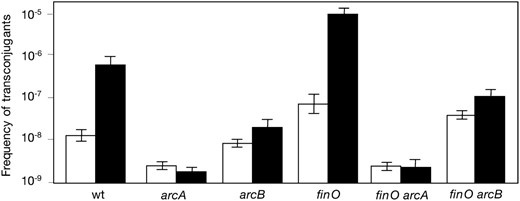
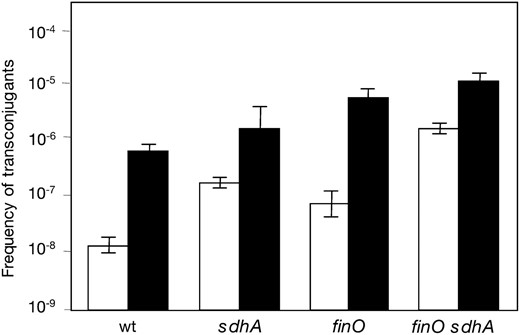
S. enterica strains carrying arcA or arcB mutations were used as donors in mating experiments carried out under either aerobiosis or microaerobiosis. Mating conditions were as previously described (Garcia-Quintanillaet al. 2008). Because the effect of arcA and arcB mutations on tra operon expression is better observed in strains derepressed for conjugation (Figure 1), a pair of isogenic FinO− and FinO− ArcA− donors was included in the study (SV4522 and SZ112, respectively). Results from these experiments can be summarized as follows:
Under aerobiosis, lack of ArcA caused a significant reduction in the frequency of transconjugants: 7-fold when the donor was FinO+ and 50-fold when the donor was FinO− (Figure 2). In contrast, lack of ArcB did not alter the frequency of conjugal transfer upon aerobic mating (Figure 2). These results are strongly reminiscent of classical studies of the F sex factor, where ArcA regulates ptraY in an ArcB-independent manner (Silvermanet al. 1991).
Both arcA and arcB mutations drastically reduced the frequencies of transconjugants under microaerobiosis: in the presence of an arcA mutation, pSLT transfer decreased two orders of magnitude in a FinO+ background and nearly three orders of magnitude in a FinO− background. The reduction in the frequency of transconjugants caused by a null arcB mutation under microaerobiosis was ∼50-fold (Figure 2). Hence, a functional ArcAB signal transduction system is necessary to activate microaerobic transfer of pSLT.
Effect of arcA and arcB mutations on conjugal transfer of pSLT in aerobiosis (open histograms) and in microaerobiosis (solid histograms). The recipient was SV3081 in all matings. Donors were SV4201 (relevant genotype: wild type), SZ110 (ArcA−), SZ111 (ArcB−), SV4522 (FinO−), SZ112 (FinO− ArcA−), and SZ113 (FinO− ArcB−). Frequencies are averages and standard deviations from six independent matings.
Identification of the pSLT promoter under ArcAB control: experiments using gene fusions:
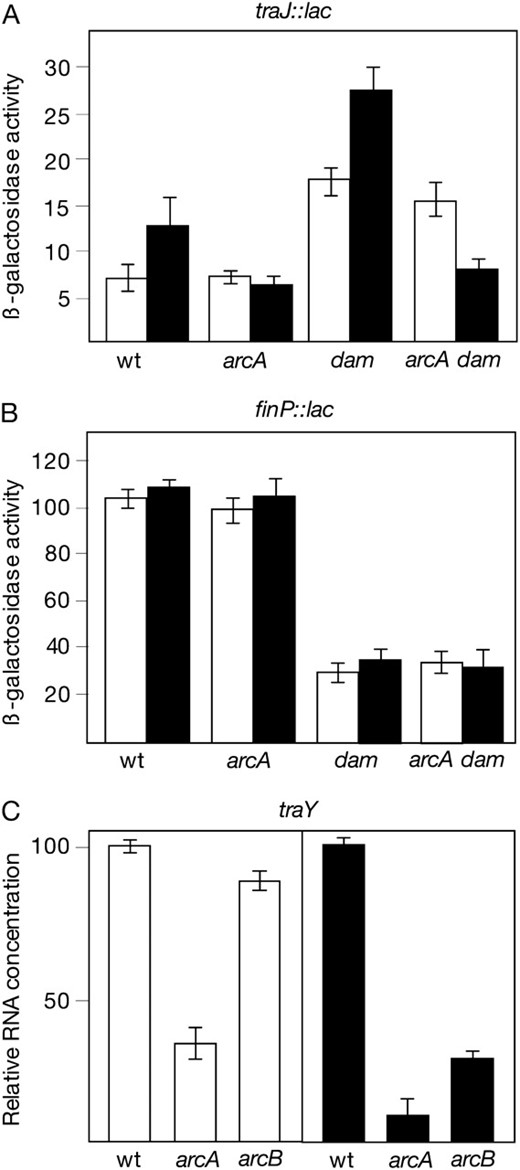
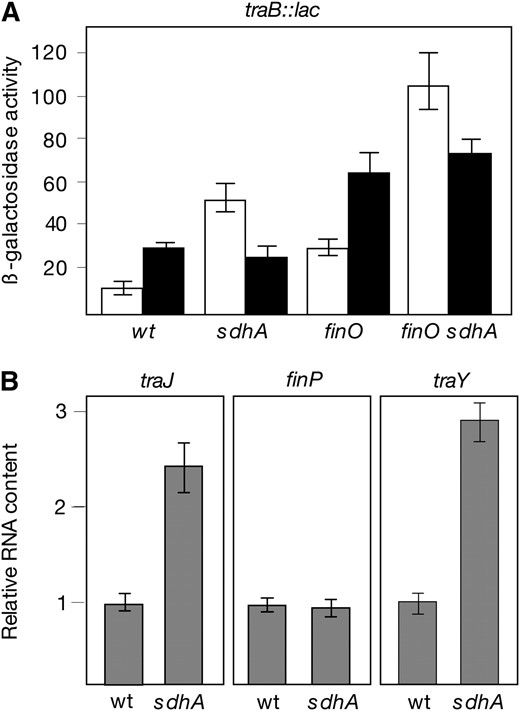
The effect of arcA and arcB mutations on the expression of the traJ and finP genes of pSLT was examined using traJ∷lac and finP∷lac transcriptional fusions (Camacho and Casadesus 2005; Camachoet al. 2005b). β-Galactosidase activities were measured in LB cultures grown under aerobiosis and under microaerobiosis. The main observations in these experiments were as follows:
In a repressed background, an arcA mutation did not alter traJ expression when the culture was grown under aerobiosis but caused a one-half reduction in traJ expression under microaerobiosis (Figure 3). The sensitivity of this assay was judged to be limited, given the low level of traJ expression in a FinOP+ strain (Camacho and Casadesus 2002). When the same experiments were carried out in a derepressed (Dam−) background, no difference in traJ expression was observed between ArcA+ and ArcA− hosts grown under aerobiosis (Figure 3). In contrast, the activity of the traJ∷lac fusion decreased under microaerobiosis. Albeit modest, this reduction was surprising, since ArcA is not known regulate traJ in other F-like plasmids. However, regulation of traJ by ArcA appears to be an indirect effect (see below).
A finP∷lac transcriptional fusion was expressed at similar levels in ArcA+ and ArcA− hosts, under both aerobiosis and microaerobiosis (Figure 3). A dam mutation decreased finP∷lac activity as previously described (Torreblancaet al. 1999), but similar expression levels were detected in Dam− ArcA+ and Dam−ArcA− isogenic hosts (Figure 3).
(A) Activity of a traJ∷lac transcriptional fusion in a wild-type background (strain SV4761) and in ArcA−, Dam−, and ArcA− Dam− mutants (SZ114, SV4914, and SZ116, respectively). β-Galactosidase activities are averages from five independent experiments. (B) Activity of a finP∷lac transcriptional fusion in a wild-type background (strain SV4839) and in ArcA−, Dam−, and ArcA− Dam− strains (SZ118, SZ119, and SZ120, respectively). β-Galactosidase activities are averages and standard deviations from four to six independent experiments. (C) Relative amounts of traY mRNA, normalized to ompA mRNA, in the wild type (LT2) and in ArcA− and ArcB− mutant derivatives (SV5067 and SV5068, respectively). Data are averages from three independent experiments. In A–C, histograms are as follows: open, aerobiosis; solid, microaerobiosis.
Identification of the pSLT promoter under ArcAB control: quantitative reverse transcriptase–PCR analysis of traY mRNA:
The product of the first gene of the tra operon (traY) has been shown to undergo autogenous control of tra operon transcription in the F sex factor (Silverman and Sholl 1996; Lumet al. 2002) and in R100 (Stockwellet al. 2000). Evidence that TraY is an autogenous activator of the tra operon also exists in pSLT (unpublished data from our laboratory). For this reason, we avoided the use of traY∷lac fusions and employed quantitative reverse transcriptase (RT)–PCR to monitor the effect of arcA and arcB mutations on traY expression. The results were clear cut: in aerobiosis, an arcA mutation caused a 3-fold decrease in traY mRNA content, while an arcB mutation had little or no effect (Figure 3). Microaerobic conditions amplified up to 10-fold the difference in traY mRNA content between ArcA+ and ArcA− hosts and revealed a 3-fold difference between ArcB+ and ArcB− hosts (Figure 3). Together with results described in the former section, these observations suggest that the promoter under ArcA control is mainly ptraY. However, ArcA appears to regulate ptraY in two distinct ways: (i) in an ArcB-independent manner under aerobiosis, as in the F sex factor (Silvermanet al. 1991), and (ii) in an ArcB-dependent manner under microaerobiosis. ArcA function is modulated by phosphorylation mediated by ArcB (Iuchi and Lin 1991). Hence, the ArcAB system may activate the pSLT ptraY promoter in response to low oxygen concentration, thus explaining the high rates of pSLT transfer detected in microaerobiosis (Garcia-Quintanillaet al. 2008).
Binding of ArcA to the upstream activating sequence of the pSLT tra operon:
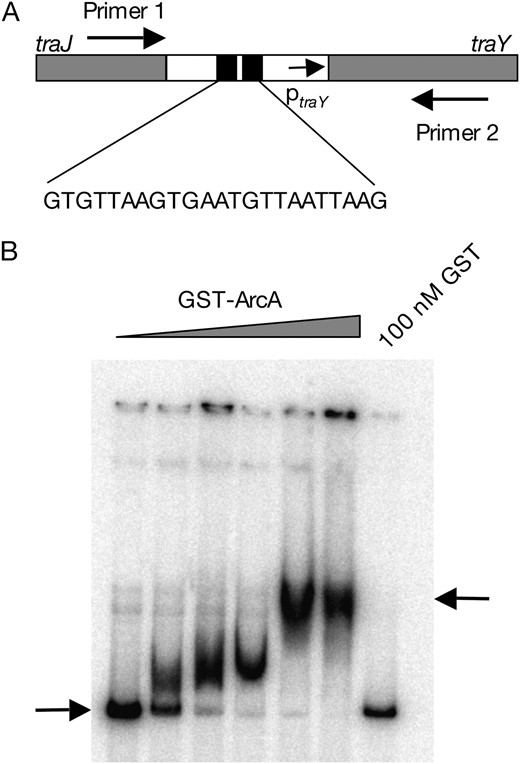
Computer analysis of DNA sequences upstream of the traY promoter of plasmid pSLT was performed using Clustal W, in a search for regions homologous to the consensus sequence for ArcA binding found in other F-like plasmids. The database DNA sequences used were NC002483 (F), NC00234 (R100), M19710 (R1), and NC003277 (pSLT). A region containing two overlapping ArcA binding motifs was found (Figure 4A), as in other F-like plasmids (Silvermanet al. 1991; Strohmaieret al. 1998). To investigate whether the ArcA protein was able to bind this DNA region, gel retardation assays were carried out. A 0.6-kb fragment of pSLT containing the traY promoter and the traY UAS was PCR amplified, purified, and end labeled with [γ-32P]dATP. This labeled DNA was mixed with aliquots containing increasing concentrations of GST-ArcA protein. Binding reactions were allowed to proceed for 20 min at room temperature. Electrophoretic separation was then carried out in an 8% polyacrylamide gel. A representative experiment is shown in Figure 4B. Retardation of the DNA fragment is clearly observed as the GST-ArcA protein concentration increases. As a control, GST alone did not cause DNA retardation (Figure 4B). Excess nonspecific competitor DNA [poly(dI-dC)] did not alter retardation. In contrast, addition of unlabeled traY UAS caused a decrease in the amount of retarded DNA (data not shown). These observations indicate that ArcA specifically binds the traY UAS in the Salmonella virulence plasmid and suggest that ArcA may activate transcription of the pSLT tra operon by a mechanism similar to those described in F (Silvermanet al. 1991) and R1 (Strohmaieret al. 1998).
(A) Diagram of the traJ-traY border in the pSLT plasmid, indicating the position of ptraY, the region homologous to ArcA binding sites of other F-like plasmids, and the locations of the primers used for PCR amplification. (B) Gel retardation analysis of GST-ArcA binding to the traY UAS. GST-ArcA concentrations were, from left to right, 0, 5, 20, 40, 80, and 160 nM.
Effect of sdh mutations on the expression of the pSLT tra operon:
Tn10dCm insertions in sdhA, sdhC, and sdhD caused a 4- to 5-fold increase in the expression of the traB1∷lac fusion under aerobiosis but had little effect on the expression of the fusion under microaerobiosis (data not shown). An sdhA deletion constructed by gene targeting conferred an identical phenotype (Figure 5A). These observations provided evidence that loss of function of the sdhCDAB operon derepressed tra operon expression under aerobiosis. In E. coli, sdhCDAB encodes succinate dehydrogenase, a membrane-bound enzymatic complex composed of four subunits (Cecchiniet al. 2002).
(A) Activity of the traB1∷lac transcriptional fusion in SdhA+ and SdhA− hosts. Experiments were carried out in FinO+ and FinO− backgrounds. Strains were SV3003 (wild-type background), SV5986 (SdhA−), SV4509 (FinO−), and SV5987 (FinO− SdhA−). Histograms represent averages and standard deviations from three experiments. Open histograms correspond to β-galactosidase activities under aerobiosis. Solid histograms correspond to β-galactosidase activities under microaerobiosis. (B) Pairwise comparisons of the relative amounts of traJ mRNA, FinP RNA, and traY mRNA in SdhA+ and SdhA− strains (LT2 and SV5608, respectively). Data for each transcript were normalized to ompA mRNA. To avoid disparate histogram sizes, the absolute amount of each individual transcript in the wild type is represented as “1”. Data are averages and standard deviations from four experiments.
To identify the pSLT promoter under SdhABCD control, levels of the traJ, finP, and traY transcripts were compared in the wild type and in an SdhA− mutant (SV5608), using quantitative RT–PCR. A housekeeping transcript, ompA, was used as a loading control in all cases. In the results shown in Figure 5B, the absolute mRNA content found in the wild type for each individual promoter has been normalized to “1”. Normalization makes the figure simpler given the disparate levels of finP and traJ mRNAs typically found in S. enterica (finP mRNA is >50-fold more abundant than traJ mRNA; data not shown). Data in Figure 5 rule out the possibility that SdhABCD might control finP transcription. In contrast, higher amounts of both traJ and traY mRNAs were found in the SdhA− mutant. These experiments indicate that Sdh represses traJ transcription and leave open the possibility that repression might also occur at the PtraY promoter. However, because TraJ is required to activate ptraY, repression of traJ transcription seems a priori sufficient to explain Sdh-mediated inhibition of tra operon expression.
Effects of sdh mutations on conjugal transfer of pSLT:
Sdh+ FinO+, Sdh+ FinO−, Sdh− FinO+, and Sdh− FinO− isogenic strains were used as donors in matings with a pSLT− recipient. Matings were carried out under both aerobiosis and microaerobiosis, and their results are summarized in Figure 6. Under aerobiosis, lack of SdhA increased pSLT transfer more than one order of magnitude both from FinO+ and from FinO− donors. Under microaerobiosis, lack of SdhA had a much smaller effect, with differences near the limit of significance (Figure 6). We thus concluded that succinate dehydrogenase is a repressor of pSLT transfer, mainly (perhaps only) in aerobiosis.
Effect of Sdh absence on conjugal transfer of pSLT under aerobiosis (open histograms) and under microaerobiosis (solid histograms). Donors were SV4201 (wild-type background), SZ122 (SdhA−), SV4522 (FinO−), and SZ123 (FinO− SdhA−). The recipient was SV3081 in all cases. Frequencies are averages and standard deviations from eight independent matings.
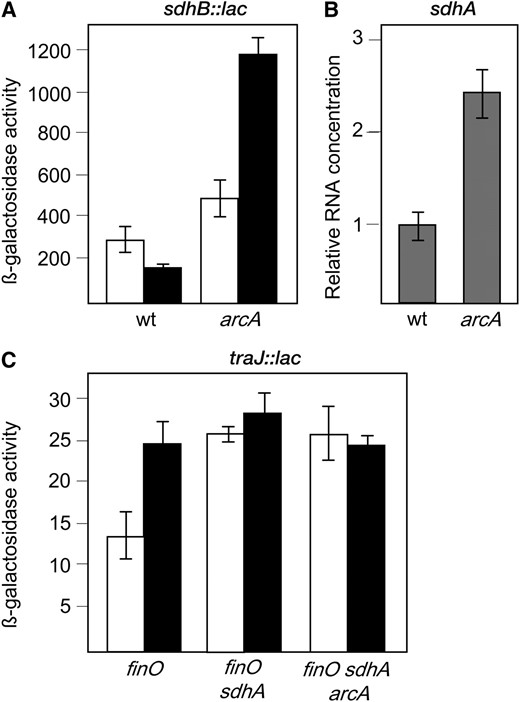
Regulation of sdhABCD by the ArcAB system:
Studies in E. coli have shown that ArcA is a repressor of the sdhCDAB operon (Parket al. 1995). To investigate whether ArcA plays an analogous role in S. enterica, we examined the effect of an arcA mutation on sdh expression. Expression of sdh in ArcA+ and ArcA− strains of S. enterica was monitored using two procedures: calculation of β-galactosidase activities using an sdhB∷lac translational fusion and comparison of sdhA mRNA levels by quantitative RT–PCR. Data shown in Figure 7 can be summarized as follows: (i) In the wild type, the sdh operon was expressed at a lower level under microaerobiosis than under aerobiosis, as previously described in E. coli (Parket al. 1995), and (ii) lack of ArcA increased both the activity of the sdhB∷lac fusion and the level of sdhA mRNA under microaerobiosis (Figure 7). The conclusion from these experiments was that ArcA is a repressor of sdh expression in S. enterica, especially in the absence of oxygen. This conclusion is in agreement with E. coli studies (Parket al. 1995). Repression of sdh by ArcAB also explains why microaerobic expression of traJ decreases in an ArcA− background (Figure 3): In the absence of ArcAB, SdhABCD represses microaerobic traJ expression down to levels similar to those found in aerobiosis. The evidence that ArcA activates traJ transcription indirectly (by inhibiting SdhABCD synthesis) is supported by epistasis analysis: A traJ∷lac transcriptional fusion is expressed at similar levels in ArcA+ SdhA− and ArcA− SdhA− hosts (Figure 7C). ArcA thus plays a dual role in microaerobic activation of pSLT transfer, as a direct activator of the tra operon and as an indirect activator of traJ (by repressing sdhCDAB).
(A) Activity of an sdhB∷lac translational fusion in the wild type (SV5867) and in an ArcA− derivative (SV5868), grown under aerobiosis (open histograms) and under microaerobiosis (solid histograms). β-Galactosidase activities are averages from five independent experiments. (B) Relative amounts of sdhA mRNA, normalized to ompA mRNA, in the wild type (LT2) and in an isogenic ArcA− mutant (SV5067) grown under microaerobiosis. Data are averages and standard deviations from three independent experiments. (C) Activity of a traJ∷lac transcriptional fusion in FinO−, FinO− SdhA−, and FinO− SdhA− ArcA− backgrounds (SZ114, SV4914, and SZ116, respectively). β-Galactosidase activities are averages from five independent experiments. In A and C, histograms are as follows: open, aerobiosis; solid, microaerobiosis.
DISCUSSION
Expression of mating functions in the S. enterica virulence plasmid (pSLT) is tightly repressed by a functional FinOP system of fertility inhibition (Smithet al. 1973; Camacho and Casadesus 2002). However, mating conditions have a strong influence on the frequency of pSLT transfer in the laboratory, suggesting the existence of controls that regulate conjugation in response to environmental cues. In LB and other rich media, pSLT transconjugants appear at a frequency ∼10−8 per donor bacterium, a frequency so low that can be easily overlooked (Ahmeret al. 1999). Higher frequencies of mating are obtained in minimal medium (Ahmeret al. 1999; Camacho and Casadesus 2002). Incubation of the mating mixture in LB medium under microaerobiosis also yields relatively high mating frequencies, ∼10−5 transconjugants per donor (Garcia-Quintanillaet al. 2008). Slightly alkaline pH and high osmolarity also increase pSLT transfer, albeit mildly if compared with the effect of microaerobiosis (Garcia-Quintanillaet al. 2008). In analogy with studies of Salmonella pathogenesis that employ high osmolarity and microaerobiosis to mimic the environment of the animal intestine (Ohl and Miller 2001), high rates of pSLT transfer under such conditions may reflect the high frequency of matings that occur in the gut of infected mice (Garcia-Quintanillaet al. 2008). In murine ileal loops, frequencies can be as high as 10−3 transconjugants per donor (Garcia-Quintanillaet al. 2008).
Conjugal transfer of pSLT in microaerobiosis is under the control of ArcAB, a signal transduction system responsive to the oxygen level (Iuchi and Lin 1991). ArcA binds the upstream activating sequence of the pSLT main tra promoter (Figure 4), as previously described in other F-like plasmids (Silvermanet al. 1991; Strohmaieret al. 1998). ArcA is a typical response regulator, whose activity is modulated by phosphorylation by the cognate microaerobic sensor histidine kinase, ArcB (Cecchiniet al. 2002). Hence, the need of both ArcA and ArcB to activate the pSLT ptraY promoter under microaerobiosis (Figure 1) makes sense from a physiological point of view. ArcA also activates aerobic expression of the pSLT tra operon, albeit at a lower level than in microaerobiosis (Figure 1). ArcA-mediated aerobic activation of tra is ArcB independent, as previously described in the F sex factor (Buxton and Drury 1984; Silvermanet al. 1991). Like pSLT, F is transferred in the absence of oxygen (Stallions and Curtiss 1972). Hence, signal transduction by the ArcAB system might control microaerobic transfer of F as it does in pSLT. To our knowledge, this possibility has not been examined.
Besides ptraY activation, the S. enterica ArcAB system plays a second role in the regulation of pSLT transfer: repression of the sdhCDAB operon, which encodes succinate dehydrogenase, an enzyme identified in this study as a repressor of pSLT transfer in aerobiosis. Preliminary evidence suggests that Sdh may be repressor of traJ transcription (Figure 5). Because succinate dehydrogenase is a membrane-bound protein complex (Cecchiniet al. 2002), it seems a priori unlikely that the SdhABCD complex may repress traJ transcription directly (e.g., binding to the traJ promoter region). An indirect effect may thus be postulated, via a hitherto unknown transcriptional regulator responsive to signals produced by central metabolism. ArcA-mediated repression of sdh is mainly observed in microaerobiosis (Figure 7), as previously described in E. coli (Parket al. 1995).
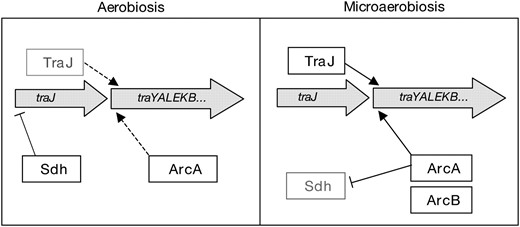
The model outlined in Figure 8 summarizes the mechanisms of conjugation control discussed in this study. In aerobiosis, ArcA-mediated activation of tra has low efficiency, and TraJ is scarce because traJ expression is directly or indirectly repressed by SdhABCD. In microaerobiosis, the ArcB oxygen sensor triggers activation of tra operon transcription mediated by ArcA. In addition, sdhCDAB expression is repressed by ArcAB, thus relieving traJ repression. Maximal activation of ptraY in other F-like plasmids requires both ArcA and TraJ (Strohmaieret al. 1998). Repression of sdhCDAB may therefore be crucial to increase the TraJ level and to boost tra expression under microaerobiosis. The level of ArcA transcription factor is less likely to be limiting, because ArcA is abundant in the cell (Salmonet al. 2005).
Model for the regulation of pSLT mating functions in response to oxygen availability. Under aerobiosis, low TraJ level may be a limiting factor for tra operon expression, even if ArcA (SfrA) is abundant. A factor that contributes to traJ repression is SdhABCD. Under microaerobiosis, however, ArcAB-mediated repression of the sdhCDAB operon may indirectly increase TraJ synthesis. As a consequence, TraJ and ArcA may efficiently activate transcription from the ptraY promoter.
The regulators of pSLT conjugal transfer included in Figure 8 are part of a wider regulatory network whose dimensions we may know only partially (Torreblancaet al. 1999; Camacho and Casadesus 2002; Camachoet al. 2005b). Host-encoded regulators may adjust conjugal transfer to favorable circumstances, optimizing the balance between its cost and its benefits (Bingle and Thomas 2001). In the case of the ArcAB signal transduction system, control of tra transcription under microaerobiosis may be viewed as an adaptation to the animal gut, an environment where the density of potential pSLT recipients is high (Garcia-Quintanillaet al. 2008).
Footnotes
Present address: Instituto de Parasitologia y Biomedicina López-Neyra, Avda. Conocimiento s/n, Parque Tecnológico Ciencias de la Salud, Armilla 18100, Spain.
Present address: Departamento de Ciencias Ambientales, Universidad Pablo de Olavide, Carretera de Utrera km 1, Seville 41013, Spain.
Footnotes
Communicating editor: S. Gottesman
Acknowledgements
We are grateful to Silvia Marqués and Joaquín Torreblanca for early experiments on traY regulation, to Francisco Ramos-Morales and Joaquín Bernal-Bayard for help in GST-ArcA construction and purification, and to Roberto Balbontín for the construction of strain SV5867. Manuel Espinosa, Meritxell García-Quintanilla, and Javier López-Garrido participated in insightful discussions and suggested crucial experiments. Clara García-Calderón and Javier López-Garrido helped also in the preparation of the manuscript. Finally, we thank Modesto Carballo for assistance in experiments performed at the Centro de Investigación, Tecnología e Innovación de la Universidad de Sevilla. This study was supported by grants BIO2007-67457-CO2-02 and CSD2008-00013 from the Spanish Ministry of Science and Innovation and the European Regional Fund.
References
Ahmer, B. M. M., M. Tran and F. Heffron,
Aliabadi, Z., F. Warren, S. Mya and J. W. Foster,
Beutin, L., and M. Achtman,
Beuzon, C. R., S. Marques and J. Casadesus,
Bingle, L. E., and C. M. Thomas,
Buxton, R. S., and L. S. Drury,
Camacho, E. M., and J. Casadesus,
Camacho, E. M., and J. Casadesus,
Camacho, E. M., A. Serna and J. Casadesus,
Camacho, E. M., A. Serna, C. Madrid, S. Marques, R. Fernandez et al.,
Cecchini, G., I. Schroder, R. P. Gunsalus and E. Maklashina,
Chan, R. K., D. Botstein, T. Watanabe and Y. Ogata,
Datsenko, K. A., and B. L. Wanner,
Firth, N., K. Ippen-Ihler and R. A. Skurray,
Frost, L. S., and J. Manchak,
Frost, L. S., K. Ippen-Ihler and R. A. Skurray,
Garcia-Quintanilla, M., F. Ramos-Morales and J. Casadesus,
Garzon, A., D. A. Cano and J. Casadesus,
Garzón, A., C. R. Beuzón, M. J. Mahan and J. Casadesús,
Gubbins, M. J., I. Lau, W. R. Will, J. M. Manchak, T. L. Raivio et al.,
Iuchi, S., and E. C. Lin,
Kleckner, N., D. A. Steele, K. Reichardt and D. Botstein,
Kleckner, N., J. Bender and S. Gottesman,
Koraimann, G., K. Teferle, G. Markolin, W. Woger and G. Hogenauer,
Lau-Wong, I. C., T. Locke, M. J. Ellison, T. L. Raivio and L. S. Frost,
Lawley, T. D., W. A. Klimke, M. J. Gubbins and L. S. Frost,
Lum, P. L., M. E. Rodgers and J. F. Schildbach,
Lynch, A. S., and E. C. Lin,
McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille et al.,
Meynell, E., and N. Datta,
Miller, J. H.,
Mouslim, C., D. A. Cano, A. Flores and J. Casadesus,
Nakaya, R., A. Nakamura and Y. Murata,
Ohl, M. E., and S. I. Miller,
Park, S. J., C. P. Tseng and R. P. Gunsalus,
Penfold, S. S., J. Simon and L. S. Frost,
Polzleitner, E., E. L. Zechner, W. Renner, R. Fratte, B. Jauk et al.,
Rotger, R., and J. Casadesus,
Salmon, K. A., S. P. Hung, N. R. Steffen, R. Krupp, P. Baldi et al.,
Schmieger, H.,
Silverman, P. M., and A. Sholl,
Silverman, P., K. Nat, J. McEwen and R. Birchman,
Silverman, P. M., E. Wickersham and R. Harris,
Smith, D. B., and K. S. Johnson,
Smith, H. R., G. O. Humphreys, N. D. Grindley, J. N. Grindley and E. S. Anderson,
Spratt, B. G., and R. J. Rowbury,
Stallions, D. R., and R. Curtiss, 3rd,
Starcic, M., D. Zgur-Bertok, B. J. Jordi, M. M. Wosten, W. Gaastra et al.,
Starcic-Erjavec, M., J. P. van Putten, W. Gaastra, B. J. Jordi, M. Grabnar et al.,
Stockwell, D., V. Lelianova, T. Thompson and W. B. Dempsey,
Strohmaier, H., R. Noiges, S. Kotschan, G. Sawers, G. Hogenauer et al.,
Taki, K., T. Abo and E. Ohtsubo,
Torreblanca, J., and J. Casadesus,
Torreblanca, J., S. Marques and J. Casadesus,
Vogel, H., and D. Bonner,
Way, J. C., and N. Kleckner,
Wei, Y., and C. G. Miller,
Will, W. R., and L. S. Frost,
Will, W. R., J. Lu and L. S. Frost,
Willetts, N., and R. Skurray,
Wu, T. T.,
Yoshioka, Y., H. Ohtsubo and E. Ohtsubo,
Zahrl, D., M. Wagner, K. Bischof and G. Koraimann,
Zahrl, D., A. Wagner, M. Tscherner and G. Koraimann,