-
PDF
- Split View
-
Views
-
Cite
Cite
Todd Nystul, Allan Spradling, Regulation of Epithelial Stem Cell Replacement and Follicle Formation in the Drosophila Ovary, Genetics, Volume 184, Issue 2, 1 February 2010, Pages 503–515, https://doi.org/10.1534/genetics.109.109538
Close - Share Icon Share
Abstract
Though much has been learned about the process of ovarian follicle maturation through studies of oogenesis in both vertebrate and invertebrate systems, less is known about how follicles form initially. In Drosophila, two somatic follicle stem cells (FSCs) in each ovariole give rise to all polar cells, stalk cells, and main body cells needed to form each follicle. We show that one daughter from each FSC founds most follicles but that cell type specification is independent of cell lineage, in contrast to previous claims of an early polar/stalk lineage restriction. Instead, key intercellular signals begin early and guide cell behavior. An initial Notch signal from germ cells is required for FSC daughters to migrate across the ovariole and on occasion to replace the opposite stem cell. Both anterior and posterior polar cells arise in region 2b at a time when ∼16 cells surround the cyst. Later, during budding, stalk cells and additional polar cells are specified in a process that frequently transfers posterior follicle cells onto the anterior surface of the next older follicle. These studies provide new insight into the mechanisms that underlie stem cell replacement and follicle formation during Drosophila oogenesis.
THE Drosophila ovary is a highly favorable system for studying epithelial cell differentiation downstream from a stem cell (reviewed in Blanpain et al. 2007; Kirilly and Xie 2007). New follicles consisting of 16 interconnected germ cells surrounded by an epithelial (follicle cell) monolayer are continuously produced during adult life and develop sequentially within ovarioles (reviewed in Spradling 1993). Follicle formation begins in the germarium (Figure 1A), a structure at the tip of each ovariole that houses 2–3 germline stem cells (GSCs) and 2 follicle stem cells (FSCs) within stable niches (reviewed in Morrison and Spradling 2008). Successive GSC daughters known as cystoblasts are enclosed by a thin covering of squamous escort cells and divide asymmetrically four times in sucession to produce 16-cell germline cysts, comprising 15 presumptive nurse cells and a presumptive oocyte (reviewed in de Cuevas et al. 1997). At the junction between region 2a and region 2b, cysts are forced into single file as they encounter the FSCs, lose their escort cell covering, and begin to acquire a follicular layer. Follicle cells derived from both FSCs soon mold them into a “lens shape” characteristic of region 2b. Under the influence of continued somatic cell growth, cysts and their surrounding cells round up, enter region 3 (also known as stage 1), and bud from the germarium as new follicles that remain connected to their neighbors by short cellular stalks (Figure 1B).
A complex sequence of signaling and adhesive interactions between follicular and germline cells is required for follicle budding, oocyte development, and patterning (reviewed in Huynh and St. Johnston 2004). However, the mechanisms orchestrating the initial association between follicle cells and cysts within the germarium are less well understood. While lineage analysis indicates the presence of two FSCs (Margolis and Spradling 1995; Nystul and Spradling 2007), low fasciclin III (FasIII) expression has been claimed to specifically mark FSCs, leading to the conclusion that more FSCs are present under some conditions (Zhang and Kalderon 2001; Vied and Kalderon 2009).
The differentiation of polar cells at both their anterior and posterior ends is required for normal follicle production (Ruohola et al. 1991; Larkin et al. 1996; Grammont and Irvine 2001), and depends on Notch signals received from the germline (Lopez-Schier and St. Johnston 2001). Subsequently, anterior polar cells send JAK-STAT and Notch signals that specify stalk cells (McGregor et al. 2002; Torres et al. 2003; Assa-Kunik et al. 2007). While the source of these signals and their effects are clear, the timing of polar cell specification and its dependence on cell lineage are not. Some anterior and posterior polar cells (but not stalk cells) were inferred by lineage analysis to arise and cease division within region 2b (Margolis and Spradling 1995). In contrast, on the basis of marker gene expression it was concluded that anterior polar cells are specified later, in stage 1, and posterior polar cells in stage 2 (Torres et al. 2003). Up to four polar cells may eventually form, but apoptosis reduces their number to a single pair at each end by stage 5 (Besse and Pret 2003). Moreover, polar and stalk are believed to arise exclusively from “polar/stalk” precursors that separate from the rest of the FSC lineage (Larkin et al. 1996; Tworoger et al. 1999; Besse and Pret 2003) and these cells were proposed to invade between the last region 2b cyst to affect follicle budding (Torres et al. 2003; Assa-Kunik et al. 2007).
Here we have analyzed the detailed behavior of FSCs and their daughters in the germarium. No evidence of polar/stalk precursors was observed, and we show that the first anterior and posterior polar cells are specified in region 2b, prior to the previously accepted time of follicle cell specialization. Additional polar cells are also formed later during stages 1 and 2. Follicle cell differentiation appears to be independent of cell lineage, but is orchestrated by sequential cell interactions, and in particular by Notch signaling. Our results reveal the sophisticated, self-correcting behavior of an epithelial stem cell lineage at close to single-cell resolution.
METHODS
Drosophila stocks:
Drosophila stocks were maintained at 20–25°. The following stocks were used to generate mitotic clones: β-gal+ positive clones, yw, P[(hsFlp)12, ry+]; X.15.29 and yw; X.15.33 (Harrison and Perrimon 1993); MARCM, P[neoFRT]19A, P[tubP-GAL80]LL1, P[(hsFLP)1, w*]; Pin(Yt)/CyO (Bloomington Drosophila Stock Center), w; UAS-N(act), w; UAS-N(Dom. Neg.) (Fortini et al., 1993) (stocks obtained from Ilaria Rebay), and P[neoFRT]19A/FM7; ; P[Tub-GAL4], P[UAS-CD8∷GFP]/TM3, Ser. GFP− negative clones for the Notch experiments were as follows: y1, w[*] P[FRT, w+]101 (Bloomington Drosophila Stock Center), w, N(55e11), FRT 101/FM7/Y, N+ (stock obtained from Ken Irvine), and w, Ubi-GFP, FRT 101/FM7; ; MKRS, hsFlp/TM3, sb. GFP− clones for the Delta experiments were as follows: P[neoFRT]82B ry[506], FRT 82b, Dl(revF10)/TM3, Sb, and P[(hsFlp)12, ry+]; ; P[neoFRT]82B/TM3, Sb. To visualize Notch activation, we used Gbe+Su(H)-lacZ/FM7; TM2/TM6b (Furriols and Bray 2001) (stock obtained from Sarah Bray). Imp-GFP is line CB02137. Further information on symbols and genes can be found in FlyBase (http://flybase.org) and the Bloomington Drosophila Stock Center (http://fly.bio.indiana.edu/).
Identification of the insertion site in PZ02954 at coordinate 18298271 (R3) was determined by sequencing of an inverse PCR product generated using primers specific to the insertion as previously described (Bellen et al. 2004).
Clonal analysis:
Dividing cells were clonally labeled using one of three different methods to generate mitotic clones: β-gal+ positive clones, MARCM, or GFP− negative clones (see above for the pairs of stocks used for each type). Standard crosses were set up between paired stocks and F1 flies were maintained at 25° in bottles with fresh, wet yeast (∼2 g dry yeast/3 ml water), changed daily for at least 2 days prior to heat shock. Mitotic clones were generated by heat-shocking the flies in a 37° water bath to induce FLP expression and allowing the flies to recover at 25°. Flies were given fresh wet yeast every day and tossed to new bottles every other day. Depending on the aim of the experiment, we varied both the severity of the heat-shock treatment and the length of the recovery. For analysis of the wild-type FSC lineage using the β-gal+ clonal system we used a mild heat-shock treatment of 30 min and allowed the flies to recover at 25° for 3, 4, 5, 8, or 10 days post-heat shock (dphs). For Notch mutant analysis, we used a moderate heat-shock treatment of 1 hr and allowed the flies to recover at 25° for 5, 9, or 14 dphs. For Delta mutant analysis, we used a 1-hr heat-shock treatment and allowed the flies to recover for 4 dphs.
For lineage labeling we exposed flies to a mild heat-shock protocol of 30 min at 37°. This resulted in an initial rate of 0.2 clones/follicle in stage 3 to stage 10 follicles at 3dphs and subsequent rates of 0.19 clones/follicle and 0.09 clones/follicle at 5 and 8 dphs, respectively. At this low rate of clone induction, clones would be expected to occur together in the same follicle at most only 4% of the time (0.22). Furthermore, even when two clones do occur together in the same follicle, they are frequently in different parts of the follicle and thus do not touch each other. Therefore, under these experimental conditions, the chance that two independently induced clones will appear as a single clone derived from a common progenitor is very low.
Immunostaining and fluorescence microcopy:
Ovaries were dissected and stained as described previously (Nystul and Spradling 2007). Briefly, ovaries were dissected in 1× Grace's medium (Cambrex Bio Sciences), fixed in 4% paraformaldehyde fixation solution for 10 min, and blocked with 1× PBST + 0.5% BSA. Primary and secondary antibodies were diluted in 1× PBST + 0.5% BSA and samples were washed after each step in 1× PBST. The following primary antibodies were used: rabbit polyclonal anti-GFP, 1:5000 (Torrey Pines, no. TP-401); rabbit polyclonal anti-β-gal, preabsorbed against paraformaldehyde fixed yw ovaries, 1:2000 (Cappel, no. 55976); mouse monoclonal anti-Fasciclin III, 1:50 (Developmental Studies Hybridoma Bank, antibody 7G10); mouse monoclonal anti-slit (Developmental Studies Hybridoma Bank, antibody C555.6D); rabbit anti-vasa, 1:1000 (generated by Dianne Williams); and guinea pig anti-traffic jam, 1:1000 (generated by Lucy Morris). The following secondary antibodies were used: goat anti-mouse and goat anti-rabbit IgG conjugated to either Alexa 488 or Alexa 568, 1:1000, and goat anti-guinea pig IgG conjugated to Alexa 568 (Invitrogen, Carlsbad, CA). All images were taken by a Zeiss Axioimager Z1 equipped with an Apotome system. Serial reconstructions were performed using Zeiss Axiovision software. Intensity adjustments, rotations, and cropping of images were performed using Adobe Photoshop 7.0.
RESULTS
Cells derived from FSCs associate with cysts in a regular manner:
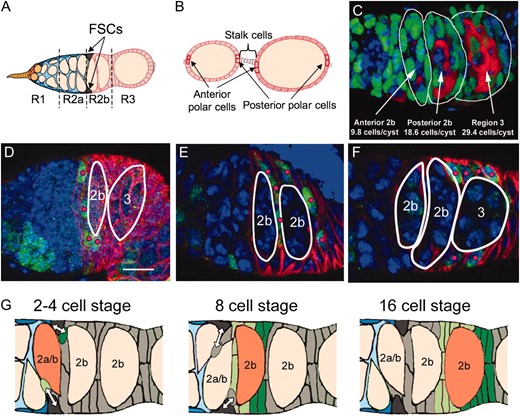
An imprecise understanding of how follicle cells associate with cysts in the germarium has limited the study of ovarian follicular cells. We quantified the average number of follicle cells surrounding the two region 2b cysts, and a single region 3 cyst characteristic of the wild-type germaria was analyzed. By staining with markers specific for the somatic cells and germ cells (Figure 1C), we found that in 21 such germaria examined, an average of 9.8 (± 2.2) follicle cells cover anterior region 2b cysts, 18.6 (± 5.2) follicle cells cover posterior 2b cysts, which have begun to round and acquire more surface area, and 29.4 (± 4.5) cells cover region 3 cysts, which are fully rounded.
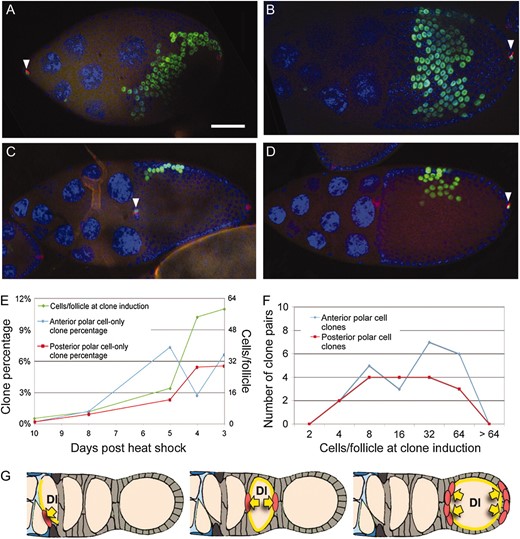
Prefollicle cells associate with cysts in an ordered fashion downstream from the FSCs. (A) A diagram of the Drosophila germarium showing the four subregions: 1, 2a, 2b, and 3. Two GSCs (orange) reside in region 1 and produce cysts (yellow ovals). Two FSCs reside at the border of regions 2a and 2b and produce follicle cells that encapsulate region 2b and region 3 cysts. (B) A diagram of two follicles that have budded from the germarium showing their pairs of anterior and posterior polar cells as well as the interconnecting 4–6 stalk cells. (C) Germaria stained with anti-traffic jam (green) to mark somatic cells, anti-vasa (red) to mark germ cells, and DAPI (blue). The numbers of somatic cells associated with each cyst (indicated) were reconstructed from three-dimensional image stacks. (D–F) Small transient clones stained with anti-LacZ (green, the clonal marker), anti-FasIII (red), and DAPI (blue). Regions 2b and 3 cysts are outlined in white. Pink dots indicate labeled FSC daughters; however, not all labeled cells are marked because some are not visible in the presented plane of focus. (D) A 4-cell clone associated with the first cyst in region 2b. (E) An 8-cell clone associated with the second region 2b cyst. (F) A 15-cell clone associated with the region 3 cyst. (G) Model of follicle layer acquisition. One FSC daughter, the cmc (light green, left) contacts the anterior face of the incoming cyst (2a/b, orange) and founds mostly anterior follicle cells (light green). Another FSC daughter, the pmc (dark green, left) contacts the posterior cyst face and founds mostly posterior follicle cells (dark green). Bar, 10 μm; anterior is to the left.
Next, we analyzed how marked clones associate with cysts. We generated clones (Harrison and Perrimon 1993) at a low frequency, so that each follicle would usually contain only 0 or 1 clone. This allows transient (i.e., nonstem cell) clones to be distinguished easily from stem cell clones and removes the ambiguity that two or more clones might contact each other and appear as a single clone. Prior to follicle formation, the cysts are so close together that marked follicle cells contact more than one cyst. Nonetheless, we observed that transient clones with ∼4 cells usually all contact the anterior region 2b cyst (Figure 1D), transient clones with ∼8 cells usually all adjoin the posterior region 2b cyst (Figure 1E), and transient clones with ∼16 cells usually all associate with the region 3 cyst (Figure 1F). The fact that a cyst's follicle cells can be half-labeled by an individual transient clone suggests that as a whole they derive from just two cellular precursors. To verify that the cells of these early clones maintain contact with a single cyst during subsequent development, we also examined older budded follicles.
Most cysts are encapsulated by the progeny of exactly two FSC daughters:
Examining large clones on fully formed follicles confirmed that the early association of follicle cells with individual cysts was quite stable. Stem cell clones labeled 49% ± 11% of each follicle's cells on average (Table 1). The largest transient clones also frequently included ∼50% of a follicle's cells, as predicted if follicles are founded by a single daughter of two different FSCs. Seventy-seven percent of these putative FSC daughter clones (i.e., clones with about half as many cells as comparably aged follicles) were located on a single follicle. In an additional 18% of clones, >90% of the cells were on one follicle, but a few of the marked cells extended onto the anterior region of the next oldest follicle. The remaining 5% of clones were spread more equally between the posterior of one follicle and the anterior of the next (older) follicle. These results show that successive FSC daughter cells usually associate singly with successive follicles and generate half of their follicle cell layer with little spillover to the adjacent follicle. However, the atypical origin of the follicular layer on occasional follicles indicated that equal contributions from two FSC daughter progenitors are not necessary to construct a morphologically normal follicle.
Position on the follicle(s) and polar cell content of large follicle cell clones
. | . | . | Anterior follicle . | . | Posterior follicle . | |||
|---|---|---|---|---|---|---|---|---|
. | n . | % . | PCa (SD) . | % FC (% SD) . | PCp (SD) . | SC (SD) . | PCa (SD) . | % FC (% SD) . |
| Daughter, single follicle | ||||||||
| Anterior bias | 16 | 42 | 1.13 (0.7) | 50 (8) | 0.5 (0.7) | 1.2 (2.0) | 0 (0) | 0 (0) |
| Posterior bias | 13 | 34 | 0.23 (0.4) | 49 (9) | 1.31 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Posterior overlap | 7 | 18 | 0 (0) | 46 (5) | 0.86 (0.7) | 2.8 (1.5) | 1.29 (0.5) | 5 (7) |
| Daughter, multifollicle | 2 | 5 | 0 (0) | 25 (0) | 1.5 (0.7) | 3.8 (1.8) | 1 (1.4) | 25 (0) |
| FSC clone | 34 | 1.2 (0.9) | 49 (11) | 1.3 (0.8) | 1.2 (0.4) | |||
| Daughter, single follicle (overall average) | 36 | 0.83 (0.7) | 49 (8) | 0.86 (0.8) | 1.1 (1.7) | |||
| Daughter, multifollicle (overall average) | 2 | 1 (1.4) | 50 (0) | 1.5 (0.7) | 3.8 (1.8) | |||
. | . | . | Anterior follicle . | . | Posterior follicle . | |||
|---|---|---|---|---|---|---|---|---|
. | n . | % . | PCa (SD) . | % FC (% SD) . | PCp (SD) . | SC (SD) . | PCa (SD) . | % FC (% SD) . |
| Daughter, single follicle | ||||||||
| Anterior bias | 16 | 42 | 1.13 (0.7) | 50 (8) | 0.5 (0.7) | 1.2 (2.0) | 0 (0) | 0 (0) |
| Posterior bias | 13 | 34 | 0.23 (0.4) | 49 (9) | 1.31 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Posterior overlap | 7 | 18 | 0 (0) | 46 (5) | 0.86 (0.7) | 2.8 (1.5) | 1.29 (0.5) | 5 (7) |
| Daughter, multifollicle | 2 | 5 | 0 (0) | 25 (0) | 1.5 (0.7) | 3.8 (1.8) | 1 (1.4) | 25 (0) |
| FSC clone | 34 | 1.2 (0.9) | 49 (11) | 1.3 (0.8) | 1.2 (0.4) | |||
| Daughter, single follicle (overall average) | 36 | 0.83 (0.7) | 49 (8) | 0.86 (0.8) | 1.1 (1.7) | |||
| Daughter, multifollicle (overall average) | 2 | 1 (1.4) | 50 (0) | 1.5 (0.7) | 3.8 (1.8) | |||
Clones derived from FSC daughters are typically confined to a single follicle, though some extend onto the next older (more posterior) follicle. The first category (“Daughter, single follicle”) shows the distribution of labeled cells in clones that are primarily located on a single follicle, by subcategory. The second category (“Daughter, multifollicle”) shows the distribution of labeled cells in clones that are approximately equally distributed across two follicles. For comparison, the overall fractions of anterior polar cells, follicle cells, posterior polar cells, and stalk cells labeled in FSC clones and FSC daughter clones are listed. PCa, number of anterior polar cells labeled; PCp, number of posterior polar cells labeled; SC, number of stalk cells labeled; FC, percentage of follicle cells in a single follicle labeled.
Position on the follicle(s) and polar cell content of large follicle cell clones
. | . | . | Anterior follicle . | . | Posterior follicle . | |||
|---|---|---|---|---|---|---|---|---|
. | n . | % . | PCa (SD) . | % FC (% SD) . | PCp (SD) . | SC (SD) . | PCa (SD) . | % FC (% SD) . |
| Daughter, single follicle | ||||||||
| Anterior bias | 16 | 42 | 1.13 (0.7) | 50 (8) | 0.5 (0.7) | 1.2 (2.0) | 0 (0) | 0 (0) |
| Posterior bias | 13 | 34 | 0.23 (0.4) | 49 (9) | 1.31 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Posterior overlap | 7 | 18 | 0 (0) | 46 (5) | 0.86 (0.7) | 2.8 (1.5) | 1.29 (0.5) | 5 (7) |
| Daughter, multifollicle | 2 | 5 | 0 (0) | 25 (0) | 1.5 (0.7) | 3.8 (1.8) | 1 (1.4) | 25 (0) |
| FSC clone | 34 | 1.2 (0.9) | 49 (11) | 1.3 (0.8) | 1.2 (0.4) | |||
| Daughter, single follicle (overall average) | 36 | 0.83 (0.7) | 49 (8) | 0.86 (0.8) | 1.1 (1.7) | |||
| Daughter, multifollicle (overall average) | 2 | 1 (1.4) | 50 (0) | 1.5 (0.7) | 3.8 (1.8) | |||
. | . | . | Anterior follicle . | . | Posterior follicle . | |||
|---|---|---|---|---|---|---|---|---|
. | n . | % . | PCa (SD) . | % FC (% SD) . | PCp (SD) . | SC (SD) . | PCa (SD) . | % FC (% SD) . |
| Daughter, single follicle | ||||||||
| Anterior bias | 16 | 42 | 1.13 (0.7) | 50 (8) | 0.5 (0.7) | 1.2 (2.0) | 0 (0) | 0 (0) |
| Posterior bias | 13 | 34 | 0.23 (0.4) | 49 (9) | 1.31 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Posterior overlap | 7 | 18 | 0 (0) | 46 (5) | 0.86 (0.7) | 2.8 (1.5) | 1.29 (0.5) | 5 (7) |
| Daughter, multifollicle | 2 | 5 | 0 (0) | 25 (0) | 1.5 (0.7) | 3.8 (1.8) | 1 (1.4) | 25 (0) |
| FSC clone | 34 | 1.2 (0.9) | 49 (11) | 1.3 (0.8) | 1.2 (0.4) | |||
| Daughter, single follicle (overall average) | 36 | 0.83 (0.7) | 49 (8) | 0.86 (0.8) | 1.1 (1.7) | |||
| Daughter, multifollicle (overall average) | 2 | 1 (1.4) | 50 (0) | 1.5 (0.7) | 3.8 (1.8) | |||
Clones derived from FSC daughters are typically confined to a single follicle, though some extend onto the next older (more posterior) follicle. The first category (“Daughter, single follicle”) shows the distribution of labeled cells in clones that are primarily located on a single follicle, by subcategory. The second category (“Daughter, multifollicle”) shows the distribution of labeled cells in clones that are approximately equally distributed across two follicles. For comparison, the overall fractions of anterior polar cells, follicle cells, posterior polar cells, and stalk cells labeled in FSC clones and FSC daughter clones are listed. PCa, number of anterior polar cells labeled; PCp, number of posterior polar cells labeled; SC, number of stalk cells labeled; FC, percentage of follicle cells in a single follicle labeled.
FSCs preferentially contribute to the anterior or posterior of successive cysts:
FSC daughters alternate between the production of a cross-migrating cell (cmc), which migrates across the anterior face of the first region 2b cyst, and a posterior-migrating cell (pmc), whose progeny migrate across the posterior face of the same cyst (Nystul and Spradling 2007). If one FSC produces a cmc and one FSC produces a pmc as each cyst moves into region 2b, then follicles would be founded by one FSC daughter of each type. An initial anterior or posterior bias in the migratory path of early prefollicle cells might result in a bias of the position of their daughters toward the anterior or posterior half of the follicle. Consistent with this model, we found that putative FSC daughter clones do indeed exhibit an anterior or a posterior positional bias on the follicle and that the two classes of clones occur at approximately equal frequencies (Figure 2, A and B; Table 1).
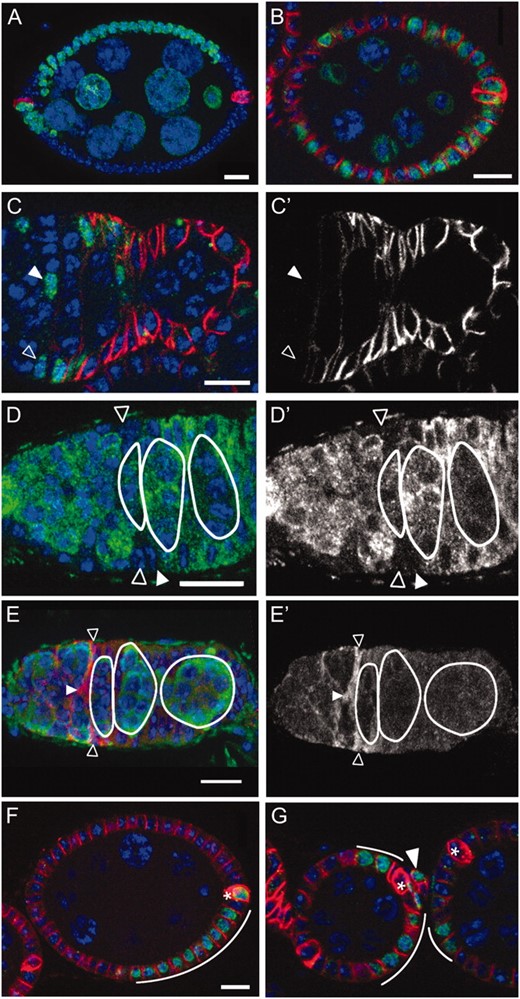
A/P spatial organization and cell type differentiation downstream from the FSCs. (A) An “anterior-biased” clone (green, LacZ), which encompasses ∼50% of total follicle cells (red, FasIII). (B) A “posterior-biased” clone, which encompasses ∼50% of the follicle cells. (C) An FSC clone 8 dphs (green, LacZ) stained with FasIII (red). Neither the FSC (open triangle) nor the daughter cmc (solid triangle) is labeled above background with FasIII. C′ shows the red (anti-FasIII) channel only. (D) An ovariole expressing Imp-GFP stained with anti-GFP (green) and DAPI (blue). Imp-GFP expression is absent in the FSCs (open triangles) and immediate daughters (solid triangle). D′ shows the anti-GFP (green) channel only. (E) A wild-type germarium stained with anti-slit (red) and anti-vasa (green). The FSCs (open triangles) and cmc (solid triangle) have high levels of slit. E′ shows the red (anti-slit) channel only. (F) A clone (green, lacZ) 3 dphs that includes both a polar cell (asterisk) and several follicle cells (curved line) but not stalk cells. (G) A clone (green, lacZ) 3 dphs that contains both stalk cells (solid triangle) and follicle cells (curved white lines) but not polar cells (asterisks). The follicle cells in this clone extend from the posterior of one follicle to the anterior of the adjacent older follicle. Bars, 10 μm; anterior is to the left, and DAPI is pseudocolored in blue.
Every FSC clone examined included both the anterior-biased and the posterior-biased patterns, indicating that single FSCs produce both types of progenitors. Moreover, anterior and posterior biases tended to alternate. For example, 28 of the 36 follicles examined with posterior-biased cells were immediately followed in the ovariole by a follicle with anterior-biased cells. Conversely, 22 of the 37 follicles examined with anterior-biased cells were followed by follicles with posterior-biased cells (χ2 = 21.7, P < 0.001). Likewise, the labeling status of the anterior polar cells in one follicle frequently mirrored the labeling status of the posterior polar cells in the adjacent younger follicle (not shown). Taken together, these observations suggest a simple picture of how the two FSCs and their progeny associate with cysts in the germarium (Figure 1G model).
Interestingly, we also found that in ovarioles that had recently undergone an FSC loss event (as evidenced by a long chain of labeled follicles connected to a germarium with two unlabeled FSCs), the youngest labeled follicle cells were posterior biased 71% (P < 0.05, N = 24) of the time (supporting information, Figure S1). Since the labeled cells in the most anterior follicle were probably derived from the former FSC itself following its ejection from the niche, this suggests that ejected FSCs typically do not cross-migrate, but move toward the posterior.
Gene expression changes slowly in the early FSC lineage:
Gene expression changes rapidly downstream from several characterized Drosophila stem cells, due either to factors that segregate asymmetrically during mitosis or to the location of daughters outside the niche (Kirilly and Xie 2007; Morrison and Spradling 2008). Consequently, we looked for genes whose expression differed in FSCs and their daughters. Low expression of FasIII has been claimed to distinguish FSCs from the daughters and other early follicle cells (Zhang and Kalderon 2001; Vied and Kalderon 2009). To test this, we examined FasIII expression in clonally marked FSCs and their progeny. We found that not only the FSCs, but also cmcs and a variable number of additional early cells near the region 2a/2b border exhibit low levels of FasIII expression (Figure 2, C and C′, and Figure S2). This also confirms a previous report for enhancer trap PZ02954 (Margolis and Spradling 1995), which is inserted in FasIII. Similar patterns were observed with other genes that are expressed strongly in later follicle cells. For example, GFP expressed from a protein trap insertion within IMP (Buszczak et al. 2007) was absent from FSCs and one to four immediate daughters, but was expressed subsequently in follicle cells surrounding the first region 2b cyst (Figure 2, D and D′).
We also found several examples where only the FSCs and their early daughters expressed a particular gene. Slit was expressed in posterior escort cells, FSCs, and immediate daughter cells, but not in older germarial follicle cells (Figure 2, E and E′). A similar tendency of escort cell genes to be expressed in the FSCs and first few downstream cells was also noted in the case of ptc-lacZ and PZ01444 (Forbes et al. 1996; Decotto and Spradling 2005; T. Nystul, unpublished data). Thus, the onset of follicle cell gene expression takes place gradually over the course of several divisions, suggesting that the first few daughters briefly remain less differentiated and closer to the FSC state, particularly if they remain near the 2a/2b junction. A corollary of these observations is that neither FasIII nor any other currently known gene can be used as an FSC marker.
Downstream follicle cell fates are not specified by lineage:
To look for lineage restrictions in the production of polar and stalk cells we induced clones at a low frequency and analyzed the cell types they contained in stage 9–10 follicles, which is a well-defined point in oogenesis after the cessation of follicle division at stage 6 (Table 2). Because new follicles are continuously formed in the germarium and move through the ovariole in an ordered fashion, the first clones to reach stages 9 and 10 will be those that formed at the last follicle division (stage 6). These will also be the smallest clones because there were the fewest divisions between the point of clone induction and the final division in the lineage. Subsequent clones to arrive will be larger and correspond to earlier points in the lineage, and the latest to arrive will be the largest and correspond to the earliest points in the lineage.
Cell type composition of transient clones, 3–8 dphs
. | 3 dphs . | 5 dphs . | 8 dphs . | |||
|---|---|---|---|---|---|---|
. | n . | % . | n . | % . | n . | % . |
| FC only | 151 | 57.2 | 90 | 45.9 | 37 | 24.3 |
| PC only | 26 | 9.8 | 12 | 6.1 | 4 | 2.6 |
| SC only | 35 | 13.3 | 21 | 10.7 | 2 | 1.3 |
| FC + PC only | 13 | 4.9 | 27 | 13.8 | 67 | 44.1 |
| FC + SC only | 18 | 6.8 | 15 | 7.7 | 5 | 3.3 |
| PC + SC only | 8 | 3.0 | 5 | 2.6 | 2 | 1.3 |
| FC + PC + SC | 13 | 4.9 | 26 | 13.3 | 35 | 23.0 |
| Total | 264 | 196 | 152 | |||
| Clone frequency | 0.20 clones/follicle | 0.19 clones/follicle | 0.09 clones/follicle | |||
. | 3 dphs . | 5 dphs . | 8 dphs . | |||
|---|---|---|---|---|---|---|
. | n . | % . | n . | % . | n . | % . |
| FC only | 151 | 57.2 | 90 | 45.9 | 37 | 24.3 |
| PC only | 26 | 9.8 | 12 | 6.1 | 4 | 2.6 |
| SC only | 35 | 13.3 | 21 | 10.7 | 2 | 1.3 |
| FC + PC only | 13 | 4.9 | 27 | 13.8 | 67 | 44.1 |
| FC + SC only | 18 | 6.8 | 15 | 7.7 | 5 | 3.3 |
| PC + SC only | 8 | 3.0 | 5 | 2.6 | 2 | 1.3 |
| FC + PC + SC | 13 | 4.9 | 26 | 13.3 | 35 | 23.0 |
| Total | 264 | 196 | 152 | |||
| Clone frequency | 0.20 clones/follicle | 0.19 clones/follicle | 0.09 clones/follicle | |||
FC, follicle cell; PC, polar cell; SC, stalk cell.
Cell type composition of transient clones, 3–8 dphs
. | 3 dphs . | 5 dphs . | 8 dphs . | |||
|---|---|---|---|---|---|---|
. | n . | % . | n . | % . | n . | % . |
| FC only | 151 | 57.2 | 90 | 45.9 | 37 | 24.3 |
| PC only | 26 | 9.8 | 12 | 6.1 | 4 | 2.6 |
| SC only | 35 | 13.3 | 21 | 10.7 | 2 | 1.3 |
| FC + PC only | 13 | 4.9 | 27 | 13.8 | 67 | 44.1 |
| FC + SC only | 18 | 6.8 | 15 | 7.7 | 5 | 3.3 |
| PC + SC only | 8 | 3.0 | 5 | 2.6 | 2 | 1.3 |
| FC + PC + SC | 13 | 4.9 | 26 | 13.3 | 35 | 23.0 |
| Total | 264 | 196 | 152 | |||
| Clone frequency | 0.20 clones/follicle | 0.19 clones/follicle | 0.09 clones/follicle | |||
. | 3 dphs . | 5 dphs . | 8 dphs . | |||
|---|---|---|---|---|---|---|
. | n . | % . | n . | % . | n . | % . |
| FC only | 151 | 57.2 | 90 | 45.9 | 37 | 24.3 |
| PC only | 26 | 9.8 | 12 | 6.1 | 4 | 2.6 |
| SC only | 35 | 13.3 | 21 | 10.7 | 2 | 1.3 |
| FC + PC only | 13 | 4.9 | 27 | 13.8 | 67 | 44.1 |
| FC + SC only | 18 | 6.8 | 15 | 7.7 | 5 | 3.3 |
| PC + SC only | 8 | 3.0 | 5 | 2.6 | 2 | 1.3 |
| FC + PC + SC | 13 | 4.9 | 26 | 13.3 | 35 | 23.0 |
| Total | 264 | 196 | 152 | |||
| Clone frequency | 0.20 clones/follicle | 0.19 clones/follicle | 0.09 clones/follicle | |||
FC, follicle cell; PC, polar cell; SC, stalk cell.
Thus, if at some point in the FSC lineage, a lineage restricted polar/stalk precursor formed, we would expect that shortly after clone induction, when stage 9–10 clones derive from late in the follicle cell lineage, polar and stalk cells would be frequently found together in the same clone and these polar/stalk clones would rarely or never contain main-body follicle cells. At late time points, when clones derived from points in the lineage prior to the polar/stalk lineage restriction have arrived at stage 9–10, all three cell types may be together in the same clone, but individual clones should rarely contain polar cells without stalk cells or vice versa.
The results of this experiment were unambiguous (Table 2). At the latest time point, 8 dphs, we observed clones containing all combinations of cell types and saw no significant association between polar and stalk cells. Nearly half of the clones we scored (48.7%) had polar cells or stalk cells, but not both, while only 24.3% contained both polar and stalk cells. The clone size at 8 dphs averaged 102.7 ± 75.0 cells, which is about one-eighth of the follicle, indicating that these clones must have formed two to three divisions downstream from the FSC when eight cells are present on the first region 2b cyst. This strongly argues against the formation of a lineage-restricted polar/stalk precursor in the first two to three divisions downstream of the FSC.
Examining younger clones also failed to reveal evidence of a polar/stalk precursor. At 5 dphs, when clones averaged 35.2 ± 32.5 cells or 1/24th of the follicle, corresponding to budding stage 1 follicles, polar or stalk cells were still found alongside main body cells far more frequently than with each other (Table 2; Figure 2, F and G). Even at 3 dphs, when clones contained 10.9 ± 8.6 cells indicating that they formed on follicles that had about the 64 cells (which corresponds to stage 2, a time when some polar and stalk cells have clearly been specified), clones containing follicle cells along with polar or stalk cells were more common than polar/stalk clones (Table 2). Thus, few if any polar or stalk cells derive from a lineage-restricted polar/stalk precursor. Instead, our data indicate that these cells arise from lineage unrestricted progenitors, most likely in response to localized signals.
Notch signaling is required for lateral migration of FSC daughters:
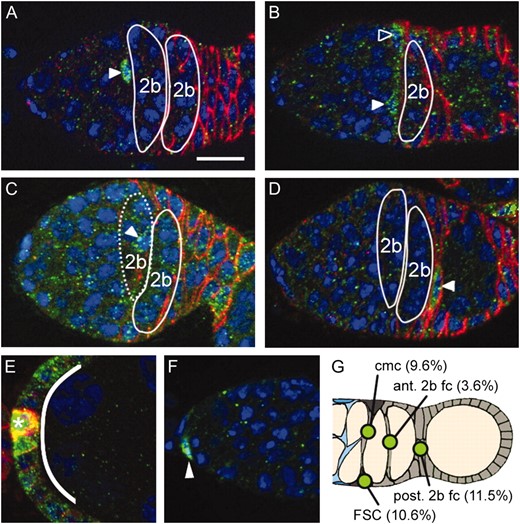
To investigate signals that might influence the cell fate decisions and differentiation of FSC daughters, we examined expression of a sensitive Notch reception reporter (Furriols and Bray 2001) in somatic cells within the undifferentiated region. We found that in 9.6% of Gbe Su(H)-lacZ germaria, a single cmc was lacZ positive (Figure 3A) and in 10.6%, a single FSC was LacZ positive. In 2.4%, both an FSC and a cmc were LacZ positive (Figure 3B). Interestingly, we also observed evidence of Notch signal reception by prefollicle cells downstream from the FSC daughters at times earlier than those predicted for polar cell induction (Torres et al. 2003). These included individual cells near the anterior (Figure 3C) and posterior of the last region 2b cyst (Figure 3D) and at other sites. Reporter labeling at these sites probably represents true Notch signals because strong expression was also observed at known sites of Notch signaling including stage 6 follicle cells (Figure 3E) (Lopez-Schier and St. Johnston 2001), cap cells (Figure 3F) (Ward et al. 2006; Song et al. 2007), and stage 2 and 3 polar cells (data not shown).
A Notch signaling reporter is activated at multiple steps in the FSC lineage. Gbe-Su(H) LacZ (green) is expressed in (A) a cross-migrating FSC daughter, (B) an FSC (open triangle) and cross-migrating daughter (solid triangle), (C) a cell between the two region 2b cysts (the anterior 2b cyst, indicated by dotted line, is in a different plane of focus), (D) a cell at the posterior of the second region 2b cyst, (E) multiple stage 6 follicle cells (curved line) and polar cells (asterisk), and (F) cap cells (solid triangle). (G) A diagram summarizing sites in regions 2b and 3 where activity was observed. The types of cells and frequency with which they are labeled are indicated (n = 139). FSC, follicle stem cell; cmc, cross-migrating cell; fc, follicle cell. Ovarioles were stained with anti-FasIII (red), anti-β-galactosidase (green), and DAPI (blue). Bar, 10 μm; anterior is to the left.
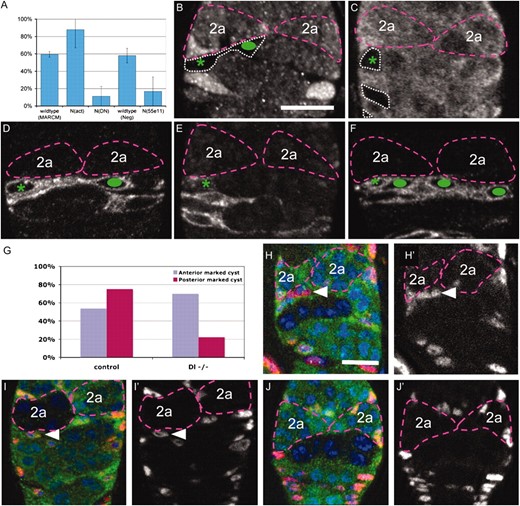
To investigate whether Notch signaling was important for the behavior of the cmc, we analyzed negatively marked and MARCM (Lee and Luo 2001) clones in which the level of Notch signaling was genetically perturbed (Figure 4A). Control negatively marked FSC clones were indistinguishable from wild type and contained a single cmc in the undifferentiated region ∼60% of the time (Figure 4B). The remaining 40% of clones contained no cross-migrating cells and instead the youngest FSC daughter was migrating toward the posterior. In contrast, negatively marked clones of N(55e11) rarely contained a cmc (Figure 4C). Similar reductions in the frequency of cmcs were observed when comparing wild-type (Figure 4D) to Notch dominant negative (Figure 4E) MARCM clones. In contrast, 90% of MARCM clones expressing activated Notch contained cmcs. Furthermore, these clones contained three to six cmcs in the undifferentiated region (Figure 4F), rather than one, as we typically saw in wild type.
A Notch signal from anterior region 2b germ cells is required for cmc migration. (A) Graph summarizing the percentage of FSC clones of the indicated genotypes that contain a cross-migrating FSC daughter cell. (B–F) Germaria with wild-type or Notch mutant FSC clones, 5 dphs stained with anti-GFP (white, clonal marker). Region 2a cysts (purple dashed lines), negatively marked FSC clones (white dotted lines), FSCs (green asterisks), and cross-migrating FSC daughters (green ovals) are indicated. (B) Wild-type negatively marked clone with a cross-migrating FSC daughter. (C) N(55e11) negatively marked clone with no cross-migrating FSC daughter. (D) wild-type MARCM clones with a single cross-migrating daughter. (E) NDN MARCM clones with no cross-migrating FSC daughter. (F) Nact MARCM clone with several cross-migrating daughters. (G) Graph summarizing the frequency of FSC daughter cell cross-migration in wild-type and Delta germline mutant clones 7–9 dphs. (H–J) Germaria with wild-type or Delta mutant germline clones 7–9 dphs stained with anti-GFP (green, clonal marker), traffic jam (red, somatic cell marker) and DAPI (blue). Cross-migrating FSC daughters (solid triangles) and the region 2a cysts (purple dashed lines) are indicated. Cross-migrating FSC daughters are present when a negatively marked wild-type cyst is positioned posterior to the migration path (H) or a negatively marked Delta−/− cyst is positioned anterior to the migration path (I), but not when a negatively marked Delta−/− cyst is positioned posterior to the migration path (J). H′, I′, and J′ show the red (somatic cell marker) channel only. Bars, 10 μm; anterior is at the top.
Delta is expressed in the germline and activates Notch in the soma at several stages of oogenesis, including at early stages of follicle cell development (Lopez-Schier and St. Johnston 2001). To investigate whether germline Delta is also the ligand for the Notch signal received by cross-migrating FSC daughters, we examined the effect of removing Delta from cysts at various positions in the germarium (Figure 4G). The frequency of FSC daughter cell cross-migration was not affected when germ cells contained control clones (Figure 4, H and H′) or lacked Delta in the last cysts in region 2a, which lie just anterior to the cmc (Figure 4, I and I′). However, when the cyst immediately posterior to the migration path was mutant for Delta, the frequency of cross-migration was greatly reduced (Figure 4, J and J′). These results indicate that Delta within the first region 2b cyst is needed to induce and/or maintain cross-migrating FSC daughters on its anterior surface.
Notch signaling is also required for FSC replacement:
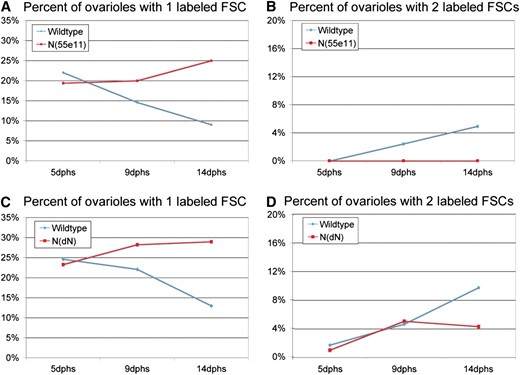
Previous studies indicated that the cmc produces a daughter cell that engages the opposite FSC and its niche and is capable of replacing it to become a functional FSC (Nystul and Spradling 2007). We reasoned that since cross-migration behavior requires Notch signaling, interfering with that signal early in the FSC lineage would reduce FSC replacement. To test this possibility, we generated wild-type and N(55e11) mutant clones using a moderate heat shock, sufficient to mark a single FSC in 22% of wild-type ovarioles at 5 dphs (Figure 5A). Normal stem cell replacement in these wild-type ovarioles then caused the number of ovarioles with two labeled FSCs to increase, reaching 2.4% by 9 dphs and 5% by 14 dphs (Figure 5B). When the marked clones were mutant for N(55e11), a very different result was observed. Although a similar number of ovarioles (19%) contained a single labeled FSC at 5 dphs, no ovarioles underwent replacement and became double labeled at either 9 or 14 dphs (Figure 5, A and B).
Notch signaling is required for FSC replacement. The percentage of ovarioles with one labeled FSC (A and C) or two labeled FSCs (B and D) is shown at 5, 9, and 14 days after clone induction by heat shock. (A and B) Negatively marked wild-type or N(55e11) clones. (C and D) MARCM wild type and Notch dominant negative (NdN). Notch mutant FSCs are not replaced by wild-type cells.
Studies of MARCM clones expressing a dominant-negative Notch transgene [N(DN)] further supported these conclusions. Wild-type FSCs turned over significantly between 5 and 14 dphs (Figure 5C). Reducing Notch activity by expressing N(DN) in clones halted the turnover of these FSCs by stem cell replacement. These changes were reflected in the generation of ovarioles with two labeled FSCs. The number of ovarioles containing two marked FSCs increased from 1.8 to 9.8% between 5 dphs and 14 dphs in the case of wild-type cells, but increased only from 1.1 to 4.3% when FSCs expressed N(DN) (Figure 5D). These results strongly suggest that regulated Notch signaling is required for efficient interniche FSC replacement. Furthermore, reducing the level of Notch signaling in an FSC does not destabilize it and may actually make it resistant to replacement by a wild-type FSC.
Polar cells are first specified early in follicle development:
Polar and stalk cells exit the mitotic cycle soon after specification and eventually express specific markers such as elevated FasIII and enhancer trap A101 (in neuralized). We used two methods to clarify when polar cells are specified and stop dividing. First, we measured the time interval between polar cell induction and stage 9-10 (where clone scoring is most favorable). Clones induced at the 8-cell stage (8 dphs) contained few polar cell-only clones. However, by the 24-cell stage (5 dphs) many clones were observed that labeled a single anterior or posterior polar cell. Additional clones of this type arose at later stages including the 64-cell stage (3 dphs), indicating that polar cells are specified over a considerable period of time, spanning late region 2b through stage 2 (Figure 6E).
Polar cells are specified at multiple points in the germarium. (A and B) Anterior (A) and posterior (B) polar cell-only clones coincident with large follicle cell-only clones that cover ∼12.5% (one-eighth) of the follicle. (C and D) Anterior (C) and posterior (D) polar cell-only clones coincident with small follicle cell-only clones that cover ∼3% (1/32nd) of the follicle cell population. Solid white triangles indicate polar cell-only clones. Ovarioles were stained with anti-FasIII (red), anti-β galactosidase (the clonal marker, green), and DAPI (blue). Bar, 50 μm; anterior is to the left. (E) Quantification of polar cell-only transient clones in stage 9–10 follicles at 3–10 dphs. Follicle cell-only clone size was used to estimate the number of cells per follicle at clone induction for each time point. (F) Number of anterior polar cell-only and posterior polar cell-only clones found coincident with follicle-cell only clones. The sizes of the follicle cell-only clones were used to estimate the number of cells per follicle at the time of clone induction. (G) Model showing the timing of Notch/Delta signaling in the germarium. A signal from cysts near the region 2a/2b border (left) induces FSC daughters to cross-migrate. A signal from the posterior region 2b cyst initiates the first round of polar cell specification (middle) and a later signal (right) maintains polar cell fate and initiates additional rounds of polar cell specification.
We next sought to refine this time interval, using an internal marker to correct for variations in cyst number and developmental rate within individual germaria. Rarely, postmitotic follicles (stages 6–14) contain both a polar cell-only and a noncontiguous follicle cell-only clone. Since the flies were heat-shocked only once, such clones must have arisen at approximately the same time. We observed many follicles containing both anterior and posterior polar cell clones whose partner clones covered one-eighth to one-sixteenth of the follicle (Figure 6, A and B). Clones this size would have been induced in region 2b when only 8 or 16 cells were associated with the cyst (Figure 1C). Other anterior and posterior polar cell clones were partnered with smaller clones that correspond to the 32- or 64-cell stages, indicating polar cell induction in region 3 or at stage 2 (Figure 6, C and D). Thus, polar cell specification begins when the cysts are still in region 2b and continues during and after budding (Figure 6F).
DISCUSSION
Regular origin of follicular precursors:
Our data provide a much clearer picture of the follicle stem cell lineage than was previously available (Figures 1F and 6G). They suggest that key aspects of FSC regulation depend on mechanisms that move cysts into a single file and program the loss of their escort cells precisely as they encounter FSCs and enter region 2b. Contact with incoming region 2a cysts likely induces FSC divisions, ensuring that cysts acquire a daughter cell from each stem cell as they stretch out to span the width of the germarium at the region 2a/2b junction. The asymmetry in cyst organization exposes FSC daughter cells to different cyst faces and, therefore, potentially to different signals. The FSC and daughter located on the same side as the entering cyst are exposed to the posterior face of the cyst while it is still in region 2a, covered by escort cells. In contrast, the opposite FSC and daughter contact the anterior face of the cyst as it migrates into 2b, at a time when the cyst is shedding its escort cell layer and exposing the Delta signal on the germ cell surface (Figures 1G and 6G). Since region 2a cysts tend to interdigitate in forming a single file, cyst entry will usually alternate sides as successive cysts pass, causing FSC daughters arising from the same side to alternate migration paths. An advantage to this system may be its flexibility, allowing follicles to form normally even if multiple cysts enter from the same side in succession.
Notch signaling in early FSC daughters promotes a “prefollicle” state by blocking follicle cell differentiation (Larkin et al. 1996; Tworoger et al. 1999). Consistent with this, we observed that FSCs and their early daughters have much lower levels of differentiation markers such as FasIII and IMP-GFP (Figure 2, C and D, and Figure S2). This developmental delay may prevent prefollicle cells from immediately incorporating into the differentiated follicular epithelium, allowing them to instead retain a more mesenchymal character conducive to cross-migration, and may also contribute to their ability to compete with the resident FSC for niche occupancy. Notch mutant daughters did not replace wild-type FSCs, most likely because they were unable to migrate into proximity. A role for Notch in suppressing differentiation downstream from the FSC might also explain why cells expressing activated Notch failed to migrate posteriorly.
Follicle cell fates are specified by intercellular signals rather than lineage:
The two FSC daughters and their descendants, with few exceptions, continue to associate with the cyst they first contact at the 2a/2b boundary throughout subsequent development. Their division rate increases briefly, because daughters divide four times in the time it takes to generate three new cysts. Despite their growing number, however, all the cells retain the ability to produce main body, stalk, and polar cells for at least the first two to three divisions (8- to 16-cell stage). In contrast to previous reports (Tworoger et al. 1999; Besse and Pret 2003), we found no evidence that polar and stalk cells derive from a lineage-restricted polar/stalk precursor population. Claims of a polar/stalk fate were based on experiments using higher rates of clone induction than in the experiments reported here. While many clones were also observed in these studies that contained both polar and follicle cells or both stalk and follicle cells, they were discounted as double clones.
By examining clones induced at low frequency (more than threefold lower than in previous studies) we were able to minimize the need for statistical correction for double clones. Furthermore, by studying clones induced at multiple times downstream from the FSC, we avoided overweighting small clones induced just as the polar and stalk cell fates are being determined by signaling within small cell groups, which has the effect of increasing the proportion of clones containing only one or two cell types even in the absence of any lineage restriction. At early, intermediate, and late times in somatic cell development in the germarium, we always observed clones that included all combinations of cell fates, indicating that follicle cells are multipotent prior to polar or stalk specification. This fits well with recent studies showing that many additional cells in the germarium can be induced to take on a polar cell fate by strong Notch signaling, while high levels of JAK-STAT signaling can induce more stalk cells (Assa-Kunik et al. 2007). In contrast, no mechanism, time, or location where putative polar/stalk precursor cells are specified has ever been documented. Previous models also did not explain how these cells would preferentially arrive in the zone of cells separating regions 2b and 3 (Torres et al. 2003) or what would become of the many extra cells that can sometimes be found in this region beyond the number needed for these fates.
Follicle cell specialization begins earlier than previously supposed:
Our finding that polar cells are initially specified in region 2b suggests that more spatial information is available within region 2b follicles than has been detected in earlier experiments. We find that the first anterior and posterior polar cells are specified when cysts are associated with 8- to 16-cell follicle cells, in mid-to-late region 2b. This agrees closely with previous studies (Margolis and Spradling 1995; Besse and Pret 2003), which found that polar cells were first specified at the 14-cell stage. The early polar cells are detected by lineage because they cease dividing; however, no gene expression markers specific for these cells have been identified. Consequently, it remains uncertain where they are located at the time of induction or whether they function while remaining in region 2b. Since we observed evidence of Notch signal reception within individual follicle cells located at the anterior and posterior regions of stage 2b cysts, the simplest model is that these polar cells are induced by Delta signaling from the germline in a normal anterior/posterior (A/P) orientation. Although we did not detect Upd expression at this time, these cells may nonetheless signal to the surrounding somatic cells to establish the graded levels of cadherin that define the initial anterior/posterior axis of the cyst (Godt and Tepass 1998; Gonzalez-Reyes and St. Johnston 1998).
Where does the information come from that allows a small number of polar cells to be specified at this time? One possibility is a “signal relay” from more posterior follicles (Torres et al. 2003; Assa-Kunik et al. 2007). Highly accurate timing of polar cell formation relative to the signaling events during follicle budding might help to further test this model. However, our observation of localized Notch signal reception and polar cell specification in region 2b follicles suggests that the germline at this stage is already sufficiently polarized to signal in a limited manner along the future A/P axis. Some of this information may come from the inherent asymmetry within the germline cyst whose cells differ systematically in their fusome content, organelle content, and microtubule organization. The future oocyte and its sister four-ring canal cell are always located in the center of the region 2b cysts and hence might be one source for this inductive signal (Gonzalez-Reyes and St. Johnston 1998). Alternatively, there may be additional differences within this region of the germarium that have yet to be detected and that may contribute.
Additional polar and stalk cells are signaled during budding:
Our studies confirm previous conclusions that additional polar cells are formed during the process of budding (Besse and Pret 2003; Torres et al. 2003) and provide new insight into the budding process itself. Anterior-biased clones were almost always confined to a single follicle, but a significant fraction of posterior-biased clones (∼33%) extended onto the next older follicle where they encompassed both an anterior polar cell and 2–30 anterior follicle cells (Table 1, “posterior overlap”). This suggests that cells at the posterior of the nascent follicle outgrow their cyst as it rounds up and are forced into the space between the posterior 2b cyst and the budding cyst. The origin of these cells has long been a mystery. A fraction of the interstitial cells likely contact and move onto the anterior of the downstream cyst where those that happen to lie adjacent to the existing polar cells are induced as new polar cells and stalk cells, as previously described. Any remaining interstitial cells likely rejoin the main body of follicle cells as budding is completed or are eliminated by apoptosis as the stalk resolves to its final size (Assa-Kunik et al. 2007).
Homeostatic developmental strategy of an epithelial stem cell lineage:
Our study of early follicle cell development provides a rare opportunity to analyze how epithelial cells behave downstream from a stem cell. Most characterized Drosophila stem cell daughters receive information asymmetrically from their mother stem cell and differentiate rapidly. Germline stem cells and their niches ensure that cystoblasts receive an asymmetric fusome segment as well as differential environmental signals that program exactly four stereotyped divisions prior to entering meiosis (de Cuevas et al. 1997). Under nonstressed conditions, intestinal stem cells utilize Notch signals to specify their daughters as either enterocytes or enteroendocrine cells and to terminate subsequent division (Ohlstein and Spradling 2007). Neuroblasts program a stereotyped sequence of daughter cell fates by differential division and signaling (Doe 2008). In contrast, FSC daughters undergo eight to nine divisions and differentiate independently of lineage over the course of several divisions and are capable of producing normal follicles even when the usual pattern of cellular interactions is altered. The increased resolution of follicle cell behavior afforded by these studies provides a valuable opportunity to study how epithelial cells are able to robustly bring about defined outcomes in the absence of the precise early programming.
Several mechanisms are likely to contribute to successful follicle formation. First, we documented that genes characteristic of a polarized epithelium turn on slowly downstream of the FSC. The cmc and several other cells frequently lacked such gene expression, but instead expressed genes characteristic of escort cells, suggesting that follicles are able to maintain some germline–soma interactions while completely replacing their somatic coverings. The early differentiation of polar cells may help guide subsequent cell behavior. In conjunction with the intrinsic asymmetric structure of germline cysts, differential adhesive interactions between germ and somatic cells across the follicle, differential pressures resulting from cell growth, and the resistive forces of the ovariolar wall, signals from these cells may be sufficient to ensure that the oocyte moves to the posterior and that cysts begin to round.
These characteristics of the FSC lineage, although unique among well-studied stem cells in Drosophila, may be closer to those governing the epithelial lineages within many mammalian tissues (Haegebarth and Clevers 2009). Thus, the mechanisms that give FSCs and their daughters their developmental flexibility and robustness are likely to be both widespread and medically relevant.
Footnotes
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.109538/DC1.
Present address: Center for Reproductive Sciences, Departments of Anatomy and OB/GYN-RS, University of California, San Francisco, CA 94143-0452.
Footnotes
Communicating editor: T. C. Kaufman
Acknowledgements
We thank Roger Hoskins and the Drosophila Gene Disruption Project for sequencing the flanks of PZ02954. Don Fox, Becky Frederick, Lucy Morris, and Judy Yanowitz made useful comments on the manuscript. T.N. is a Howard Hughes Fellow of the Life Sciences Research Foundation.
The authors declare that they have no competing financial interests.
References
Assa-Kunik, E., I. L. Torres, E. D. Schejter, D. St. Johnston and B.-Z. Shilo,
Bastock, R., and D. St. Johnston,
Bellen, H. J., R. W. Levis, G. Liao, Y. He, J. W. Carlson et al.,
Besse, F., and A. M. Pret,
Blanpain, C., V. Horsley and E. Fuchs,
Buszczak, M., S. Paterno, D. Lighthouse, J. Bachman, J. Planck et al.,
Decotto, E., and A. C. Spradling,
de Cuevas, M., M. A. Lilly and A. C. Spradling,
Doe, C. Q.,
Forbes, A. J., A. C. Spradling, P. W. Ingham and H. Lin,
Fortini M. E., I. Rebay, L. A. Caron and S. Artavanis-Tsakonas,
Furriols, M., and S. Bray,
Godt, D., and U. Tepass,
Gonzalez-Reyes, A., and D. St. Johnston,
Grammont, M., and K. D. Irvine,
Haegebarth, A., and H. Clevers,
Harrison, D. A., and N. Perrimon,
Huynh, J.-R., and D. St. Johnston,
Keller Larkin, M., W. M. Deng, K. Holder, M. Tworoger, N. Clegg et al.,
Kirilly, D., and T. Xie,
Larkin, M. K., K. Holder, C. Yost, E. Giniger and H. Ruohola-Baker,
Lee, T., and L. Luo,
Lin, H., and A. C. Spradling,
Lopez-Schier, H., and D. St. Johnston,
Margolis, J., and A. Spradling,
McGregor, J. R., R. Xi and D. A. Harrison,
Morrison, S., and A. C. Spradling,
Nystul, T., and A. Spradling,
Ohlstein, B., and A. Spradling,
Ruohola, H., K. A. Bremer, D. Baker, J. R. Swedlow, L. Y. Jan et al.,
Song, X., G. B. Call, D. Kirilly and T. Xie,
Spradling, A. C.,
Torres, I. L., H. López-Schier and D. St. Johnston,
Tworoger, M., M. K. Larkin, Z. Bryant and H. Ruohola-Baker,
Vied, C., and D. Kalderon,
Ward, E. J., H. R. Shcherbata, S. H. Reynolds, K. A. Fischer, S. D. Hatfield et al.,
Zhang, Y., and D. Kalderon,