-
PDF
- Split View
-
Views
-
Cite
Cite
Radostina Stamenova, Patrick H Maxwell, Alison E Kenny, M Joan Curcio, Rrm3 Protects the Saccharomyces cerevisiae Genome From Instability at Nascent Sites of Retrotransposition, Genetics, Volume 182, Issue 3, 1 July 2009, Pages 711–723, https://doi.org/10.1534/genetics.109.104208
Close - Share Icon Share
Abstract
The DNA helicase Rrm3 promotes replication fork progression through >1000 discrete genomic regions and represses the cDNA-mediated mobility of the Ty1 retrotransposon. We explored the connection between DNA replication and Ty1 retromobility by investigating the basis of increased retromobility in an rrm3 mutant. Even though Ty1 cDNA levels are increased in the absence of RRM3, neither the level nor target-site specificity of cDNA integration was altered. Instead, cDNA was incorporated into the genome by a Rad52-dependent mechanism that did not involve gene conversion of genomic Ty1 sequences. In rrm3 isolates, incorporated cDNA was often present in tandem arrays. Multimeric cDNA arrays probably arise during chromosomal break repair, since their appearance was strongly correlated with the formation of gross chromosomal rearrangements. Moreover, Ty1 multimers were invariantly located on rearranged chromosomes, when present. Overexpression of a cellular RNase H, which degrades RNA in an RNA:DNA hybrid, completely suppressed the increase in Ty1 multimer formation in an rrm3 mutant. We propose that RNA:DNA hybrid regions within nascent retrotransposition events block replication in an rrm3 mutant, leading to chromosome breaks within Ty1 sequences. Multiple extragenomic Ty1 cDNA molecules are then used as donors in recombinational repair of the break before it is healed.
ALL of the mobile genetic elements in the nuclear genome of the yeast, Saccharomyces cerevisiae, are long terminal repeat (LTR) retrotransposons known as Ty elements. In their structure and replication cycle, Ty elements resemble infectious retroviruses, except that there is no extracellular phase in replication. The Ty RNA is encapsulated into cytoplasmic virus-like particles (VLPs), which consist of structural subunits encoded by TYA and three enzymatic proteins encoded by TYB: protease, integrase, and reverse transcriptase/RNase H. Inside the VLP, Ty RNA is reverse transcribed into a linear, double-stranded cDNA. The cDNA, in association with integrase, is transported back to the nucleus, where it is integrated into chromosomal DNA. While integration is the primary mode of Ty retromobility, Ty cDNA can also be incorporated into the genome by homologous recombination with genomic Ty elements (Melamed et al. 1992; Ke et al. 1999).
Ty1, present in ∼30 copies per haploid genome, is the most abundant and most active of the retrotransposon families in budding yeast. The retromobility of Ty1 is repressed by a variety of proteins involved in genome stability. Ty1 repressors block cDNA synthesis, promote degradation of cDNA, or suppress cDNA recombination (Maxwell and Curcio 2007a). Examples of the latter class are Sgs1, a DNA helicase, and Rad27 (Fen1p), a FLAP endonuclease (Bryk et al. 2001; Sundararajan et al. 2003). In sgs1Δ and rad27Δ mutants, a large fraction of retromobility events are tandem arrays of Ty1 elements with a single LTR at the junction between elements. Multimeric Ty1 transposition events also occur at lower but easily detectable frequencies in wild-type strains. They are thought to form by recombination between Ty1 cDNAs prior to, during, or immediately after integration (Weinstock et al. 1990; Bryk et al. 2001). Multimeric Ty1 cDNA arrays can also be incorporated into the genome by homologous recombination with preexisting Ty1 sequences (Sharon et al. 1994; Maxwell and Curcio 2007b). Strikingly, Ty1 multimers that are inserted by homologous recombination are observed at chromosomal breakpoint junctions, raising the possibility that Ty1 cDNA arrays form in the process of chromosome break repair (Umezu et al. 2002; Maxwell and Curcio 2007b).
The Saccharomyces cerevisiae DNA helicases, Sgs1, Srs2, and Rrm3, are thought to have partially overlapping functions in maintaining genome stability during replication (Gangloff et al. 2000; Klein 2001; Fabre et al. 2002; Ooi et al. 2003; Schmidt and Kolodner 2004, 2006; Torres et al. 2004). These helicases are proposed to suppress inappropriate recombination intermediates that arise when replication forks stall or break. Sgs1 is a 3′- to 5′-DNA helicase in the RecQ family with a recently described role in resection of DNA ends at double-strand breaks (Gravel et al. 2008; Mimitou and Symington 2008; Zhu et al. 2008). The 3′- to 5′-DNA helicase Srs2 is an ortholog of UvrD that displaces the strand exchange protein Rad51 from single-stranded DNA (Krejci et al. 2003; Veaute et al. 2003). Rrm3 is a 5′–3′ helicase in the Pif1 family, which is conserved from yeast to humans. Rrm3 interacts with Orc5, PCNA, and Pol2 (epsilon), travels with the replication fork, and is involved in chromosomal DNA replication through nonnucleosomal protein:DNA complexes (Schmidt et al. 2002; Ivessa et al. 2003; Azvolinsky et al. 2006; Matsuda et al. 2007). In the absence of Rrm3, replication fork pausing and chromosomal breaks occur in the rDNA, telomeric and subtelomeric DNA, transcriptionally active tRNA genes, centromeres, inactive replication origins, the silent mating-type loci, and finally, in RNA polymerase II transcribed genes when the direction of transcription is opposed to the direction of replication (Ivessa et al. 2000, 2002, 2003; Prado and Aguilera 2005). Paradoxically, there is no increase in chromosome loss or gross chromosomal rearrangements in rrm3 mutants (Ivessa et al. 2003; Schmidt and Kolodner 2006).
Rrm3 was identified as a repressor of the mobility of endogenous Ty1 elements in a genetic screen and was subsequently shown to repress Ty2 and Ty3 retromobility as well (Scholes et al. 2001; Irwin et al. 2005; Curcio et al. 2007). A marked increase in the level of unintegrated Ty1 cDNA accompanies the increased Ty1 retromobility in an rrm3Δ mutant, but surprisingly, integration of Ty1 into known hotspots is not elevated (Scholes et al. 2001; Curcio et al. 2007). In this work, we ask how this large pool of unintegrated Ty1 cDNA is incorporated into the genome in the rrm3Δ mutant. We show that deletion of RRM3 increases the formation of tandem Ty1 cDNA arrays but does not increase integration or gene conversion of endogenous Ty1 elements. A high proportion of multimeric Ty1 arrays are present on rearranged chromosomes, supporting the idea that Ty1 multimers form during chromosome break repair. Given the ability of a related helicase, Pif1, to unwind RNA:DNA hybrids (Boule and Zakian 2007), we hypothesized that RNA:DNA hybrid regions in nascent Ty1 retrotransposition events block replication forks in the absence of Rrm3. Chromosome breaks ensue, and multimeric Ty1 cDNA arrays are incorporated into the genome during break repair. In support of this hypothesis, we show that RNA:DNA hybrids are involved in Ty1 multimer formation in rrm3 mutants. Together, our findings suggest that Ty1 retrotransposition events containing RNA:DNA hybrid regions can function as replication fork blocks in rrm3 mutants.
MATERIALS AND METHODS
Yeast strains:
The genotypes of S. cerevisiae strains used in this study are listed in Table 1. All strains are derivatives of congenic strains BY4741 and BY4742 (Brachmann et al. 1998). Strain JC3212 contains the chromosomal Ty1his3AI[Δ1]-3114 element, which was introduced into strain BY4741 by galactose induction of the pGTy1his3AI[Δ1] element, as described previously (Curcio and Garfinkel 1991). Strains JC3900, JC3946, and JC3956 are spores derived from a cross between strain JC3212 and the rtt101Δ∷kanMX derivative, the rad51Δ∷kanMX derivative, or the rad52Δ∷kanMX derivative of strain BY4742, respectively.
Strains used in this study
Strain . | Genotype . | Source . |
|---|---|---|
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems |
| JC3884 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rrm3Δ∷kanMX | Open Biosystems |
| JC3212 | MATa ura3Δ0 met15Δ0 leu2Δ0 his3Δ1 Ty1his3AI-3114 | Mou et al. (2006) |
| JC3900 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rtt101Δ∷kanMX Ty1his3AI-3114 | This study |
| JC3917 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMX Ty1his3AI-3114 | Curcio et al. (2007) |
| JC3946 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rad51Δ∷kanMX Ty1his3AI-3114 | This study |
| JC3956 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rad52Δ∷kanMX Ty1his3AI-3114 | This study |
| JC4415 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMX rad52Δ∷hisG-URA3-hisG Ty1his3AI-3114 | This study |
| JC4522 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 URA3-tG(GCC)B | This study |
| JC4526 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rrm3Δ∷kanMX URA3-tG(GCC)B | This study |
| JC4733 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ste12Δ∷kanMX URA3-tG(GCC)B | This study |
| JC4915 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 sgs1Δ∷kanMX Ty1his3AI-3114 | This study |
| JC4916 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 srs2Δ∷kanMX Ty1his3AI-3114 | This study |
| JC5266 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMXΔNsi1 rad51Δ:KanMX Ty1his3AI-3114 | This study |
Strain . | Genotype . | Source . |
|---|---|---|
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems |
| JC3884 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rrm3Δ∷kanMX | Open Biosystems |
| JC3212 | MATa ura3Δ0 met15Δ0 leu2Δ0 his3Δ1 Ty1his3AI-3114 | Mou et al. (2006) |
| JC3900 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rtt101Δ∷kanMX Ty1his3AI-3114 | This study |
| JC3917 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMX Ty1his3AI-3114 | Curcio et al. (2007) |
| JC3946 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rad51Δ∷kanMX Ty1his3AI-3114 | This study |
| JC3956 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rad52Δ∷kanMX Ty1his3AI-3114 | This study |
| JC4415 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMX rad52Δ∷hisG-URA3-hisG Ty1his3AI-3114 | This study |
| JC4522 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 URA3-tG(GCC)B | This study |
| JC4526 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rrm3Δ∷kanMX URA3-tG(GCC)B | This study |
| JC4733 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ste12Δ∷kanMX URA3-tG(GCC)B | This study |
| JC4915 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 sgs1Δ∷kanMX Ty1his3AI-3114 | This study |
| JC4916 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 srs2Δ∷kanMX Ty1his3AI-3114 | This study |
| JC5266 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMXΔNsi1 rad51Δ:KanMX Ty1his3AI-3114 | This study |
Strains used in this study
Strain . | Genotype . | Source . |
|---|---|---|
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems |
| JC3884 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rrm3Δ∷kanMX | Open Biosystems |
| JC3212 | MATa ura3Δ0 met15Δ0 leu2Δ0 his3Δ1 Ty1his3AI-3114 | Mou et al. (2006) |
| JC3900 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rtt101Δ∷kanMX Ty1his3AI-3114 | This study |
| JC3917 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMX Ty1his3AI-3114 | Curcio et al. (2007) |
| JC3946 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rad51Δ∷kanMX Ty1his3AI-3114 | This study |
| JC3956 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rad52Δ∷kanMX Ty1his3AI-3114 | This study |
| JC4415 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMX rad52Δ∷hisG-URA3-hisG Ty1his3AI-3114 | This study |
| JC4522 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 URA3-tG(GCC)B | This study |
| JC4526 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rrm3Δ∷kanMX URA3-tG(GCC)B | This study |
| JC4733 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ste12Δ∷kanMX URA3-tG(GCC)B | This study |
| JC4915 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 sgs1Δ∷kanMX Ty1his3AI-3114 | This study |
| JC4916 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 srs2Δ∷kanMX Ty1his3AI-3114 | This study |
| JC5266 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMXΔNsi1 rad51Δ:KanMX Ty1his3AI-3114 | This study |
Strain . | Genotype . | Source . |
|---|---|---|
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems |
| JC3884 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rrm3Δ∷kanMX | Open Biosystems |
| JC3212 | MATa ura3Δ0 met15Δ0 leu2Δ0 his3Δ1 Ty1his3AI-3114 | Mou et al. (2006) |
| JC3900 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rtt101Δ∷kanMX Ty1his3AI-3114 | This study |
| JC3917 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMX Ty1his3AI-3114 | Curcio et al. (2007) |
| JC3946 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rad51Δ∷kanMX Ty1his3AI-3114 | This study |
| JC3956 | MATa ura3Δ0 lys2Δ0 leu2Δ0 his3Δ1 rad52Δ∷kanMX Ty1his3AI-3114 | This study |
| JC4415 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMX rad52Δ∷hisG-URA3-hisG Ty1his3AI-3114 | This study |
| JC4522 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 URA3-tG(GCC)B | This study |
| JC4526 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rrm3Δ∷kanMX URA3-tG(GCC)B | This study |
| JC4733 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ste12Δ∷kanMX URA3-tG(GCC)B | This study |
| JC4915 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 sgs1Δ∷kanMX Ty1his3AI-3114 | This study |
| JC4916 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 srs2Δ∷kanMX Ty1his3AI-3114 | This study |
| JC5266 | MATa ura3Δ0 lys2Δ0 leu2Δ0 met15Δ0 his3Δ1 rrm3Δ∷kanMXΔNsi1 rad51Δ:KanMX Ty1his3AI-3114 | This study |
The sgs1Δ∷kanMX allele in strain JC4915 and the srs2Δ∷kanMX allele in strain JC4916 were each introduced by PCR-mediated gene disruption of strain JC3212 (Table 2). Gene replacements were confirmed by two independent PCR analyses. Oligomer sequences used to construct all deletion alleles and confirm gene replacements are available upon request. Strain JC4415 was constructed by introduction of the rad52Δ:hisG-URA3-hisG allele (Curcio and Garfinkel 1994) into strain JC3917. Strain JC5266 was constructed by two-step gene disruption of the kanMX gene that replaces the RRM3 ORF in JC3917, followed by PCR-mediated introduction of the rad51∷kanMX allele. Two-step gene disruption of kanMX was performed using plasmid pBJC934, which contains a XhoI–XbaI fragment of kanMX bearing an internal NsiI deletion cloned into the URA3-based integrating vector, pRS406.
Oligonucleotide primers used in this study
Primer . | Sequence . |
|---|---|
| AD1 | 5′-NTCGASTWTSGWGTT-3′ |
| AD2 | 5′-NGTCGASWGANAWGAA-3′ |
| H3HOPA2 | 5′-TCTCCTACTTTCTCCCTTTGCAAACC-3′ |
| HISOUT3 | 5′-CTTCGTTTATCTTGCCTGCTC-3′ |
| PJ4 | 5′-ACCACTTGCGCTTATTTCTTGGAAGTGTTGTATCTCAAAATGAGACTGTGCGGTATTTCACACCG-3′ |
| PJ5 | 5′-ATGTTATTATAAACTACTTACCAAAAAAAGTGTTGTATTACGGGCAGATTGTACTGAGAGTGCAC-3′ |
| PJ37 | 5′-GACTGGGCCCGAATTCGACAGGTTATCAGCAAC-3′ |
| PJ38 | 5′-TAGAAGTTCTCCTCGAGGAATAAG-3′ |
| PJ111 | 5′-GTTTTTCTTCTTCAAGCACGTTAACCAG-3′ |
| PJ143 | 5′-GATCTACCACCGCTCTGGAAAGTG-3′ |
| TRPHOP-SE | 5′-AGATTGTACTGAGAGTGCACC-3′ |
| Ty1LTRF | 5′-TCTTTGCCTTCGTTTATCTTGCCTG-3′ |
| TyA1OUT | 5′-CCTTAGAAGTAACCGAAGCACAGGCG-3′ |
| TyAOUT2 | 5′-TCTCTGGAACAGCTGATGAAG-3′ |
| TyBOUT2 | 5′-GTGATGACAAAACCTCTTCCG-3′ |
| U3SEQ | 5′-GGCTATAATATTAGGTATACAGAATATACTAGAAGTTCTCCTC-3′ |
Primer . | Sequence . |
|---|---|
| AD1 | 5′-NTCGASTWTSGWGTT-3′ |
| AD2 | 5′-NGTCGASWGANAWGAA-3′ |
| H3HOPA2 | 5′-TCTCCTACTTTCTCCCTTTGCAAACC-3′ |
| HISOUT3 | 5′-CTTCGTTTATCTTGCCTGCTC-3′ |
| PJ4 | 5′-ACCACTTGCGCTTATTTCTTGGAAGTGTTGTATCTCAAAATGAGACTGTGCGGTATTTCACACCG-3′ |
| PJ5 | 5′-ATGTTATTATAAACTACTTACCAAAAAAAGTGTTGTATTACGGGCAGATTGTACTGAGAGTGCAC-3′ |
| PJ37 | 5′-GACTGGGCCCGAATTCGACAGGTTATCAGCAAC-3′ |
| PJ38 | 5′-TAGAAGTTCTCCTCGAGGAATAAG-3′ |
| PJ111 | 5′-GTTTTTCTTCTTCAAGCACGTTAACCAG-3′ |
| PJ143 | 5′-GATCTACCACCGCTCTGGAAAGTG-3′ |
| TRPHOP-SE | 5′-AGATTGTACTGAGAGTGCACC-3′ |
| Ty1LTRF | 5′-TCTTTGCCTTCGTTTATCTTGCCTG-3′ |
| TyA1OUT | 5′-CCTTAGAAGTAACCGAAGCACAGGCG-3′ |
| TyAOUT2 | 5′-TCTCTGGAACAGCTGATGAAG-3′ |
| TyBOUT2 | 5′-GTGATGACAAAACCTCTTCCG-3′ |
| U3SEQ | 5′-GGCTATAATATTAGGTATACAGAATATACTAGAAGTTCTCCTC-3′ |
Oligonucleotide primers used in this study
Primer . | Sequence . |
|---|---|
| AD1 | 5′-NTCGASTWTSGWGTT-3′ |
| AD2 | 5′-NGTCGASWGANAWGAA-3′ |
| H3HOPA2 | 5′-TCTCCTACTTTCTCCCTTTGCAAACC-3′ |
| HISOUT3 | 5′-CTTCGTTTATCTTGCCTGCTC-3′ |
| PJ4 | 5′-ACCACTTGCGCTTATTTCTTGGAAGTGTTGTATCTCAAAATGAGACTGTGCGGTATTTCACACCG-3′ |
| PJ5 | 5′-ATGTTATTATAAACTACTTACCAAAAAAAGTGTTGTATTACGGGCAGATTGTACTGAGAGTGCAC-3′ |
| PJ37 | 5′-GACTGGGCCCGAATTCGACAGGTTATCAGCAAC-3′ |
| PJ38 | 5′-TAGAAGTTCTCCTCGAGGAATAAG-3′ |
| PJ111 | 5′-GTTTTTCTTCTTCAAGCACGTTAACCAG-3′ |
| PJ143 | 5′-GATCTACCACCGCTCTGGAAAGTG-3′ |
| TRPHOP-SE | 5′-AGATTGTACTGAGAGTGCACC-3′ |
| Ty1LTRF | 5′-TCTTTGCCTTCGTTTATCTTGCCTG-3′ |
| TyA1OUT | 5′-CCTTAGAAGTAACCGAAGCACAGGCG-3′ |
| TyAOUT2 | 5′-TCTCTGGAACAGCTGATGAAG-3′ |
| TyBOUT2 | 5′-GTGATGACAAAACCTCTTCCG-3′ |
| U3SEQ | 5′-GGCTATAATATTAGGTATACAGAATATACTAGAAGTTCTCCTC-3′ |
Primer . | Sequence . |
|---|---|
| AD1 | 5′-NTCGASTWTSGWGTT-3′ |
| AD2 | 5′-NGTCGASWGANAWGAA-3′ |
| H3HOPA2 | 5′-TCTCCTACTTTCTCCCTTTGCAAACC-3′ |
| HISOUT3 | 5′-CTTCGTTTATCTTGCCTGCTC-3′ |
| PJ4 | 5′-ACCACTTGCGCTTATTTCTTGGAAGTGTTGTATCTCAAAATGAGACTGTGCGGTATTTCACACCG-3′ |
| PJ5 | 5′-ATGTTATTATAAACTACTTACCAAAAAAAGTGTTGTATTACGGGCAGATTGTACTGAGAGTGCAC-3′ |
| PJ37 | 5′-GACTGGGCCCGAATTCGACAGGTTATCAGCAAC-3′ |
| PJ38 | 5′-TAGAAGTTCTCCTCGAGGAATAAG-3′ |
| PJ111 | 5′-GTTTTTCTTCTTCAAGCACGTTAACCAG-3′ |
| PJ143 | 5′-GATCTACCACCGCTCTGGAAAGTG-3′ |
| TRPHOP-SE | 5′-AGATTGTACTGAGAGTGCACC-3′ |
| Ty1LTRF | 5′-TCTTTGCCTTCGTTTATCTTGCCTG-3′ |
| TyA1OUT | 5′-CCTTAGAAGTAACCGAAGCACAGGCG-3′ |
| TyAOUT2 | 5′-TCTCTGGAACAGCTGATGAAG-3′ |
| TyBOUT2 | 5′-GTGATGACAAAACCTCTTCCG-3′ |
| U3SEQ | 5′-GGCTATAATATTAGGTATACAGAATATACTAGAAGTTCTCCTC-3′ |
The URA3-tG(GCC)B locus in strains JC4522, JC4526, and JC4733 was constructed by insertion of the URA3 gene 34 bp upstream of tG(GCC)B in strain BY4741 and the rrm3ΔkanMX and ste12ΔkanMX derivatives. The URA3 gene was amplified from plasmid pRS406 DNA using oligomers PJ4 and PJ5.
Plasmids:
Plasmid pGTy1his3AI[Δ1] was described previously (Scholes et al. 2001). The plasmid pOY1, a URA3-CEN vector carrying a Ty1his3AI element, was described in Lee et al. (1998). Plasmid pJC838 is a LEU2-CEN vector carrying GAL1p:Ty1his3AI[Δ1]. To construct pJC838, the GAL1 promoter in plasmid pGTy1his3AI[Δ1] was amplified by PCR with oligomers PJ37 and PJ38. The PCR product was digested with ApaI and XhoI and cloned into pRS415 digested with ApaI and XhoI. The resulting plasmid, pBJC837, was digested with XhoI and EagI, and the 7513-bp XhoI–EagI fragment of pGTy1his3AI[Δ1] was inserted. The pGAL1:RNH1 plasmid was a gift of Robert Crouch (National Institutes of Health). Plasmids pBDG542 (Curcio and Garfinkel 1994) and pJC573 (Bryk et al. 2001) were described previously.
Frequency of chromosomal Ty1his3AI element mobility:
Cells from a single colony of each strain (JC3212, JC3917, JC3946, JC3956, JC4415, JC4915, JC4916, and JC5266) were grown to saturation in YPD broth at 30°, diluted 1:1000 into 8-ml YPD broth, divided into four cultures, and grown to saturation at 20°. Aliquots of each culture were plated on YPD agar and SC −His agar and the plates were incubated for 3 days at 30°, at which time colonies were counted.
PCR-based detection of multimeric cDNA arrays:
Independent His+ colonies that sustained an insertion of Ty1HIS3 cDNA were obtained by spreading strains JC3212, JC3900, JC3917, JC4415, JC4915, and JC4916 on YPD agar. Following growth for 1 day at 30°, cells were replicated to YPD agar, grown at 20° for 3 days, and then replicated to SC −His agar and grown at 30°. His+ papillae were single-colony purified. To test the effect of inducing the pGAL1:RNH1 plasmid, strains JC3212, JC3917, and JC4915 transformed with plasmid pRS416 or pGAL1:RNH1 were spread on SC −Ura agar and grown for 1 day at 30°. The lawns were replicated to YP agar containing 2% galactose and grown at 20° for 3 days. Subsequently, the lawns were replicated to SC −Ura −His agar to select His+ isolates that had maintained the pGAL1:RNH1 plasmid throughout the induction. His+ papillae were single-colony purified.
Independent His+ colonies were suspended in Lyse-N-Go PCR reagent (Pierce Chemical), according to the manufacturer's specifications. Cell suspensions were used as templates in PCR reactions with HISOUT3 and TyAOUT2 as primers. Isolates that yielded a band were retested by PCR using primers H3HOPA2 and TyA1OUT. Isolates that yielded a PCR product in both tests are included in Table 4 or Table 7. Several strains yielding products larger than predicted by the presence of two LTRs were subjected to DNA sequencing using primer HISOUT3. Differences between strains in the fraction of His+ isolates harboring Ty1HIS3:Ty1 multimers were analyzed using Fisher's exact test (www.langsrud.com/fisher.htm). The two-tailed P-value is reported.
Rate of Ty1 integration at URA3-tG(GCC)B locus:
Strains JC4522, JC4526, and JC4733 grown on SC −Ura agar were transferred to YPD broth and grown overnight at 30°. Each culture was diluted 1:1000 into YPD broth, and aliquoted into 1-ml cultures, which were grown to saturation at 20°. A 1-μl aliquot was removed from each of four cultures per strain and plated on YPD. Subsequently, each culture was plated on 5-FOA agar. The rate of FOAr was determined from the median number of FOAr colonies in 11 cultures divided by the average number of colonies in 4 cultures (Foster 2006). One independent FOAr colony from each of 17 to 50 plates was single-colony purified. Following lysis of FOAr colonies in Pierce Lyse-N-Go reagent, PCR reactions were performed with primers PJ111 and TRPHOP-SE, which generate a 1.4-kb band including the URA3 ORF and promoter. In isolates lacking the 1.4-kb band, two subsequent PCR reactions using primer PJ111 and either TYA1OUT or TYBOUT2 were performed to confirm the presence of a Ty1 element in either orientation in URA3. In isolates in which the 1.4-kb band was shifted to 1.7 kb, two subsequent PCR reactions with primer U3SEQ and either PJ111 or TRPHOP-SE were performed to confirm the presence of a solo LTR within URA3.
Frequency of gene conversion of a Ty1his3AI element by Ty1HIS3 cDNA:
Plasmid pJC573, a URA3 integrating vector that harbors Ty1his3AI[Δ1] upstream of the BIK1-HIS4 intergenic region, was integrated into strain BY4741. The rrm3Δ∷kanMX, rad52Δ∷kanMX, and rad27Δ∷kanMX alleles were subsequently introduced into a pJC573 integrant by PCR-mediated gene disruption. Following growth of each strain on YPD agar at 20°, independent His+ Ura+ isolates were obtained. The fraction of His+ Ura+ derivatives of each strain in which Ty1HIS3 cDNA had replaced the Ty1his3AI[Δ1] allele was determined as described previously (Bryk et al. 2001).
TAIL PCR to identify the 3′ junction of Ty1HIS3 cDNA with genomic DNA:
Plasmid pOY1 was introduced into BY4741 and the rrm3Δ∷kanMX derivative of BY4741. Large lawns of independent transformants were grown on SC −Ura agar overnight at 30°. The lawns were replicated to two YPD plates and each lawn was grown at 20° or 30° for 3 days. Lawns on YPD were replicated to SC −His agar and grown for 3 days at 30°. No His+ colonies arose following growth on YPD agar at 30°, indicating that the His+ colonies that arose following growth on YPD agar at 20° were independent. His+ colonies were single-colony purified and replicated to FOA −His agar to select segregants lacking the pOY1 plasmid. Genomic DNA was isolated from His+ Ura− isolates, and the 3′ junction between Ty1HIS3 and genomic DNA was amplified by thermal asymmetric interlaced (TAIL) PCR (Liu and Whittier 1995). TAIL-PCR utilized each of three nested primers, H3HOPA2, PJ143, and HISOUT3 in successive reactions with an arbitrary degenerate primer (AD1 or AD2). Primary TAIL-PCR reaction mixtures contained 50 ng genomic DNA, primer H3HOPA2, and primer AD1 or AD2 in a 25-μl reaction. The product of the first reaction was diluted 1:20, and a 1-μl aliquot was used in a 25-μl PCR reaction containing primer PJ143 and primer AD1 or AD2. The secondary PCR reaction product was diluted 1:10, and 1 μl was used in a 25-μl PCR reaction containing primer HISOUT3 and AD1 or AD2. Thermal cycling conditions were those described previously (Liu and Whittier 1995) except that the annealing temperature varied between 62° and 67°, depending on the specific primer used. PCR products were visualized on a 1% agarose gel. If secondary and tertiary reactions yielded one major band, the tertiary reaction product was purified using the QIAquick PCR purification kit. If both the series of reactions with primer AD1 and the series of reactions with primer AD2 yielded a product, the larger of the two products was purified. Purified PCR products were cloned into the pCR2.1-TOPO vector using the InVitrogen TOPO TA cloning kit and sequenced. Sequences were compared to the Saccharomyces Genome Database (www.yeastgenome.org) to identify the Ty1HIS3 insertion site.
CHEF gel analysis:
Strains JC3212 was inoculated into 1 ml of YPD broth, while His+ derivatives of strains JC3212, JC3917, and JC4915 were inoculated into 8 ml of SC −His broth, and the strains were grown overnight at 30°. Agarose-embedded chromosomal DNA was prepared from each culture (Gerring et al. 1991). Using a Biorad CHEF Mapper apparatus, samples were subject to electrophoresis on a 1% agarose gel in 0.5% TBE buffer at 6 V/cm for 24 hr. Pulse times of 45–120 sec were used to analyze native yeast chromosomes. The gels were stained with ethidium bromide and photographed. DNA from CHEF gels was transferred onto a Hybond-N+ membrane (Amersham), which was hybridized in NorthernMax buffer (Ambion) at 50° overnight with 32P-labeled HIS3 riboprobe synthesized using plasmid pGEM-HIS3 (Curcio et al. 1990).
RESULTS
Retromobility and formation of tandem Ty1 cDNA arrays are increased in rrm3Δ mutants:
Considering that Sgs1, Rrm3, and Srs2 have partially overlapping functions in genome maintenance, and that the increased formation of cDNA multimers is correlated with increased retromobility in sgs1Δ mutants (Bryk et al. 2001), we compared the levels of Ty1 retromobility and multimer formation in rrm3Δ and srs2Δ mutants to that in sgs1Δ and wild-type strains. We used a chromosomal Ty1his3AI element to measure Ty1 retromobility (Curcio and Garfinkel 1991). The his3AI retrotranscript indicator gene consists of the HIS3 gene interrupted by an artificial intron (AI) in antisense orientation. The his3AI gene is inserted in the 3′-UTR of a genomic Ty1 element in the opposing transcriptional orientation; consequently, splicing of AI from the Ty1his3AI RNA followed by reverse transcription of the spliced transcript generates Ty1 cDNA carrying a functional HIS3 gene. When Ty1HIS3 cDNA is incorporated into the genome by integration or by recombination with genomic Ty1 sequences, the cell becomes a His+ prototroph. Therefore, the frequency of His+ prototroph formation is a measure of the retromobility of the Ty1his3AI element. The frequency of Ty1his3AI retromobility was increased 42-fold in the rrm3Δ mutant (Table 3). This effect was substantially larger than that caused by sgs1Δ, which increased retromobility 12-fold. On the other hand, the srs2Δ mutation caused only a 3-fold increase in retromobility.
Frequency of Ty1his3AI retromobility in DNA helicase mutants
Genotype . | Frequency of Ty1his3AI retromobilitya ± SE × 10−7 . | Fold increase in the absence of RRM3, SGS1, or SRS2 . |
|---|---|---|
| WT | 4.8 ± 0.3 | 1 |
| rrm3Δ | 204 ± 3 | 42 |
| sgs1Δ | 60.0 ± 2.9 | 12 |
| srs2Δ | 15.0 ± 0.8 | 3 |
| rad52Δ | 35.4 ± 0.5 | 1 |
| rad52Δ rrm3Δ | 66.6 ± 5.0 | 2 |
| rad51Δ | 18.0 ± 2.3 | 1 |
| rad51Δ rrm3Δ | 152 ± 20 | 8 |
Genotype . | Frequency of Ty1his3AI retromobilitya ± SE × 10−7 . | Fold increase in the absence of RRM3, SGS1, or SRS2 . |
|---|---|---|
| WT | 4.8 ± 0.3 | 1 |
| rrm3Δ | 204 ± 3 | 42 |
| sgs1Δ | 60.0 ± 2.9 | 12 |
| srs2Δ | 15.0 ± 0.8 | 3 |
| rad52Δ | 35.4 ± 0.5 | 1 |
| rad52Δ rrm3Δ | 66.6 ± 5.0 | 2 |
| rad51Δ | 18.0 ± 2.3 | 1 |
| rad51Δ rrm3Δ | 152 ± 20 | 8 |
Ty1his3AI retromobility occurs when the Ty1his3AI RNA is spliced and reverse transcribed, and the resulting cDNA is incorporated into the genome by integration or recombination. The frequency of Ty1his3AI retromobility is the average of the number of His+ prototrophs divided by the total number of cells plated for each of four cultures.
Frequency of Ty1his3AI retromobility in DNA helicase mutants
Genotype . | Frequency of Ty1his3AI retromobilitya ± SE × 10−7 . | Fold increase in the absence of RRM3, SGS1, or SRS2 . |
|---|---|---|
| WT | 4.8 ± 0.3 | 1 |
| rrm3Δ | 204 ± 3 | 42 |
| sgs1Δ | 60.0 ± 2.9 | 12 |
| srs2Δ | 15.0 ± 0.8 | 3 |
| rad52Δ | 35.4 ± 0.5 | 1 |
| rad52Δ rrm3Δ | 66.6 ± 5.0 | 2 |
| rad51Δ | 18.0 ± 2.3 | 1 |
| rad51Δ rrm3Δ | 152 ± 20 | 8 |
Genotype . | Frequency of Ty1his3AI retromobilitya ± SE × 10−7 . | Fold increase in the absence of RRM3, SGS1, or SRS2 . |
|---|---|---|
| WT | 4.8 ± 0.3 | 1 |
| rrm3Δ | 204 ± 3 | 42 |
| sgs1Δ | 60.0 ± 2.9 | 12 |
| srs2Δ | 15.0 ± 0.8 | 3 |
| rad52Δ | 35.4 ± 0.5 | 1 |
| rad52Δ rrm3Δ | 66.6 ± 5.0 | 2 |
| rad51Δ | 18.0 ± 2.3 | 1 |
| rad51Δ rrm3Δ | 152 ± 20 | 8 |
Ty1his3AI retromobility occurs when the Ty1his3AI RNA is spliced and reverse transcribed, and the resulting cDNA is incorporated into the genome by integration or recombination. The frequency of Ty1his3AI retromobility is the average of the number of His+ prototrophs divided by the total number of cells plated for each of four cultures.
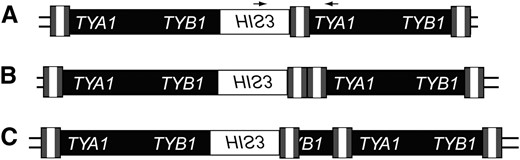
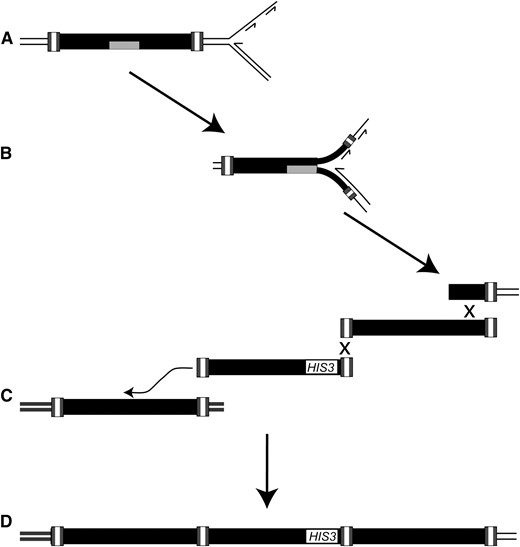
To measure the formation of Ty1 cDNA multimers in these strains, we isolated independent His+ derivatives of strains containing a chromosomal Ty1his3AI element and used a PCR assay to detect Ty1HIS3 cDNA that was a component of a multimeric Ty1 array (Figure 1). Using one primer that anneals to the splice junction in HIS3 and another that anneals in TYA1, we detected junctions between the newly transposed Ty1HIS3 element and a downstream Ty1 element containing a single LTR or two adjoining LTRs (Figure 1, A and B). In addition, irregular junctions with rearranged Ty1 sequences between adjoining elements were detected (Figure 1C). Each multimeric cDNA array that was detected contained at least two, but possibly more Ty1 cDNAs. In agreement with previous results in a different strain background (Bryk et al. 2001), 13% of independent His+ derivatives of the wild-type strain were Ty1HIS3:Ty1 multimers (Table 4). Junctions containing one LTR were the most common, but 2-LTR junctions and irregular junctions were also observed. Deletion of RRM3 or SGS1 caused a significant increase in the fraction of Ty1HIS3:Ty1 multimers to 30% (P < 0.001) or 31% (P < 0.01) of total Ty1HIS3 retromobility events, respectively. In contrast, deletion of SRS2 caused only a minor increase in Ty1HIS3:Ty1 multimers. As in the wild-type strain, the majority of Ty1HIS3:Ty1 multimers in the rrm3Δ, sgs1Δ, and srs2Δ strains had 1-LTR junctions.
Schematic of Ty1HIS3:Ty1 multimers. Simple Ty1 elements consist of LTRs (tripartite shaded and open rectangle) flanking a central coding region (solid rectangle) containing two open reading frames, TYA1 and TYB1. The HIS3 gene, which is in the opposite orientation relative to Ty1 ORFs, is represented by an open rectangle. (A) 1-LTR Ty1HIS3:Ty1 multimer. Primers used to detect all classes of Ty1HIS3:Ty1 multimers are represented by small arrows. (B) 2-LTR Ty1HIS3:Ty1 multimer. (C) A single representative of the diverse class of Ty1HIS3:Ty1 multimers with irregular junctions (Weinstock et al. 1990).
Ty1HIS3:Ty1 multimer formation in His+ isolates
Genotype . | 1-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | 2-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Irregular Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Total Ty1HIS3:Ty1 multimers/His+ isolate (%) . |
|---|---|---|---|---|
| WT | 13/188 (7) | 7/188 (4) | 4/188 (2) | 24/188 (13) |
| rrm3Δ | 46/213 (22) | 5/213 (2) | 13/213 (6) | 64/213 (30) |
| sgs1Δ | 27/102 (26) | 3/102 (3) | 2/102 (2) | 32/102 (31) |
| srs2Δ | 21/143 (15) | 4/143 (3) | 1/143 (2) | 26/143 (18) |
| rtt101Δ | 6/64 (9) | 3/64 (5) | 0/64 (0) | 9/64 (14) |
| rrm3Δ rad52Δ | 1/32 (3) | 0/32 (0) | 0/32 (0) | 1/32 (3) |
Genotype . | 1-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | 2-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Irregular Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Total Ty1HIS3:Ty1 multimers/His+ isolate (%) . |
|---|---|---|---|---|
| WT | 13/188 (7) | 7/188 (4) | 4/188 (2) | 24/188 (13) |
| rrm3Δ | 46/213 (22) | 5/213 (2) | 13/213 (6) | 64/213 (30) |
| sgs1Δ | 27/102 (26) | 3/102 (3) | 2/102 (2) | 32/102 (31) |
| srs2Δ | 21/143 (15) | 4/143 (3) | 1/143 (2) | 26/143 (18) |
| rtt101Δ | 6/64 (9) | 3/64 (5) | 0/64 (0) | 9/64 (14) |
| rrm3Δ rad52Δ | 1/32 (3) | 0/32 (0) | 0/32 (0) | 1/32 (3) |
Ty1HIS3:Ty1 multimer formation in His+ isolates
Genotype . | 1-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | 2-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Irregular Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Total Ty1HIS3:Ty1 multimers/His+ isolate (%) . |
|---|---|---|---|---|
| WT | 13/188 (7) | 7/188 (4) | 4/188 (2) | 24/188 (13) |
| rrm3Δ | 46/213 (22) | 5/213 (2) | 13/213 (6) | 64/213 (30) |
| sgs1Δ | 27/102 (26) | 3/102 (3) | 2/102 (2) | 32/102 (31) |
| srs2Δ | 21/143 (15) | 4/143 (3) | 1/143 (2) | 26/143 (18) |
| rtt101Δ | 6/64 (9) | 3/64 (5) | 0/64 (0) | 9/64 (14) |
| rrm3Δ rad52Δ | 1/32 (3) | 0/32 (0) | 0/32 (0) | 1/32 (3) |
Genotype . | 1-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | 2-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Irregular Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Total Ty1HIS3:Ty1 multimers/His+ isolate (%) . |
|---|---|---|---|---|
| WT | 13/188 (7) | 7/188 (4) | 4/188 (2) | 24/188 (13) |
| rrm3Δ | 46/213 (22) | 5/213 (2) | 13/213 (6) | 64/213 (30) |
| sgs1Δ | 27/102 (26) | 3/102 (3) | 2/102 (2) | 32/102 (31) |
| srs2Δ | 21/143 (15) | 4/143 (3) | 1/143 (2) | 26/143 (18) |
| rtt101Δ | 6/64 (9) | 3/64 (5) | 0/64 (0) | 9/64 (14) |
| rrm3Δ rad52Δ | 1/32 (3) | 0/32 (0) | 0/32 (0) | 1/32 (3) |
The homologous recombination protein, Rad52, is required for elevated retromobility and Ty1 multimer formation in sgs1Δ mutants (Bryk et al. 2001); therefore, we asked whether Rad52 is required in the rrm3Δ mutant. Ty1his3AI mobility was only twofold higher in the rrm3Δ rad52Δ mutant than in the rad52Δ mutant (Table 3). Thus, the rrm3Δ mutation causes increased Ty1 retromobility primarily by a Rad52-dependent mechanism. Furthermore, the fraction of Ty1HIS3:Ty1 multimers in the rrm3Δ rad52Δ mutant was reduced to 3%, which was significantly lower than that in the rrm3Δ mutant (P < 0.01; Table 4) and comparable to the fraction previously reported for a rad52Δ mutant (Bryk et al. 2001). Therefore, both increased retromobility and the formation of Ty1 cDNA arrays require Rad52 in the rrm3Δ mutant. We also examined the role of Rad51, which is required for gene conversion but not strand annealing, in retromobility. Retromobility was increased eightfold by deletion of RRM3 in a rad51Δ mutant. Therefore, Rad51 is not critical for increasing retromobility of an rrm3Δ mutant.
To determine whether elevated Ty1 multimer formation is a general feature of mutants that have defects in replication fork progression, we also measured the fraction of Ty1HIS3:Ty1 multimers in an rtt101Δ mutant (Table 4). Like the rrm3Δ mutant, the rtt101Δ mutant has an elevated level of Ty1 cDNA and increased replication fork pausing at tRNA genes and other sites (Luke et al. 2006; Curcio et al. 2007). However, the fraction of Ty1HIS3:Ty1 multimers in an rtt101Δ mutant was equivalent to that in the wild-type strain. Therefore, Ty1 multimer formation is not elevated in all mutants that have increased replication fork pausing. Together, these findings implicate Ty1 multimer formation in the increased retromobility of rrm3Δ mutants as well as sgs1Δ mutants.
Neither integration nor recombination of Ty1 cDNA is substantially elevated in rrm3Δ mutants:
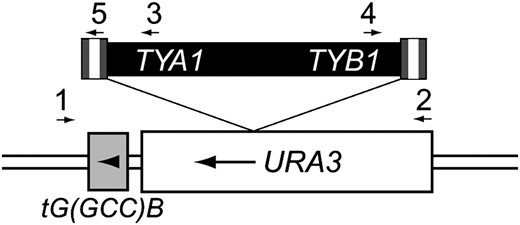
To ascertain whether the formation of Ty1 cDNA multimers is a major cause of elevated retromobility in rrm3Δ mutants, we asked whether either cDNA integration or recombination of cDNA with genomic Ty1 elements was also elevated. First, we developed a quantitative assay for Ty1 integration upstream of a known hotspot, namely, the tG(GCC)B gene. This tRNA gene is the most strongly preferred target site for Ty1 among 16 different tRNAgly genes in the genome. Furthermore, insertion of a URA3 gene either 15 or 60 bases upstream of tG(GCC)B does not significantly alter the frequency of Ty1 integration (Bachman et al. 2004). Therefore, we introduced the URA3 gene 34 bp upstream of tG(GCC)B, in the same transcriptional orientation, and measured the rate of Ty1 insertion into URA3 (Figure 2). The rate of Ty1 insertion upstream of tG(GCC)B is the product of the rate of FOAr and the fraction of independent FOAr colonies in which Ty1 had integrated into URA3. Integration of Ty1 in either orientation was detected by PCR (Figure 2). Retrotransposition into URA3-tG(GCC)B occurred at a rate of 1.9 × 10−7 per generation in the wild-type strain (Table 5). This rate was reduced at least 200-fold by deletion of the gene encoding the Ty1 transcription factor, Ste12, confirming that Ty1 integration is a major cause of URA3 inactivation. While the rate of FOAr increased modestly in the rrm3Δ mutant, the fraction of Ty1 insertions into URA3 decreased, resulting in nearly equivalent rates of Ty1 integration into URA3-tG(GCC)B in the wild-type and rrm3Δ strains (Table 5). This result is consistent with our previous finding in a study that employed a semi-quantitative PCR assay to detect Ty1 integration upstream of all 16 tRNAgly genes (Scholes et al. 2001). Therefore, the rrm3Δ mutation does not increase integration into preferred target sites.
Integration of a Ty1 element into the URA3-tG(GCC)B locus. The URA3 gene was inserted 34 bp upstream of the tG(GCC)B gene. FOAr colonies were screened by PCR using primers that flank the locus (denoted by arrows 1 and 2) to detect the presence of an insertion within the URA3 gene. Subsequent PCR reactions using primers 1 and 3 or 1 and 4 were performed to detect the specific presence of a Ty1 element. In the case of a 0.3-kb insertion into URA3, PCR reactions using primers 1 and 5 and 2 and 5 were performed to detect solo LTR insertions.
Ty1 retrotransposition into the URA3-tG(GCC)B locus
Genotype . | Rate of FOAr ± SE (× 10−7) . | Ty1 or solo LTR integrants in URA3a/no. of FOAr colonies . | Rate of Ty1 integration upstream of tG(GCC)B (× 10−7) . |
|---|---|---|---|
| WT | 2.5 ± 0.8 | 37/50 | 1.9 |
| rrm3Δ | 10.2 ± 2.9 | 8/27 | 3.0 |
| ste12Δ | <0.04 | 4/17 | <0.009 |
Genotype . | Rate of FOAr ± SE (× 10−7) . | Ty1 or solo LTR integrants in URA3a/no. of FOAr colonies . | Rate of Ty1 integration upstream of tG(GCC)B (× 10−7) . |
|---|---|---|---|
| WT | 2.5 ± 0.8 | 37/50 | 1.9 |
| rrm3Δ | 10.2 ± 2.9 | 8/27 | 3.0 |
| ste12Δ | <0.04 | 4/17 | <0.009 |
As determined by the PCR assays described in Figure 2. Two isolates with a solo LTR in URA3, presumably the product of Ty1 integration followed by recombination between the LTRs, were identified in the wild-type strain background.
Ty1 retrotransposition into the URA3-tG(GCC)B locus
Genotype . | Rate of FOAr ± SE (× 10−7) . | Ty1 or solo LTR integrants in URA3a/no. of FOAr colonies . | Rate of Ty1 integration upstream of tG(GCC)B (× 10−7) . |
|---|---|---|---|
| WT | 2.5 ± 0.8 | 37/50 | 1.9 |
| rrm3Δ | 10.2 ± 2.9 | 8/27 | 3.0 |
| ste12Δ | <0.04 | 4/17 | <0.009 |
Genotype . | Rate of FOAr ± SE (× 10−7) . | Ty1 or solo LTR integrants in URA3a/no. of FOAr colonies . | Rate of Ty1 integration upstream of tG(GCC)B (× 10−7) . |
|---|---|---|---|
| WT | 2.5 ± 0.8 | 37/50 | 1.9 |
| rrm3Δ | 10.2 ± 2.9 | 8/27 | 3.0 |
| ste12Δ | <0.04 | 4/17 | <0.009 |
As determined by the PCR assays described in Figure 2. Two isolates with a solo LTR in URA3, presumably the product of Ty1 integration followed by recombination between the LTRs, were identified in the wild-type strain background.
Alternatively, Ty1 cDNA may be incorporated into the genome of rrm3Δ mutants by recombination with genomic Ty1 elements. To test this idea, we measured the frequency of gene conversion of a chromosomal Ty1his3AI element by Ty1HIS3 cDNA using a previously described genetic assay (Bryk et al. 2001). RRM3, RAD52, and RAD27 were deleted from a strain containing 1.2-kb direct repeats of the BIK1-HIS4 intergenic region flanking a Ty1his3AI element and the URA3 gene. Independent His+ Ura+ derivatives of each strain were selected. His+ Ura+ isolates that became His− as a result of selection for the loss of URA3 were scored as recombinants formed through gene conversion of Ty1his3AI by Ty1HIS3 cDNA. The frequency of cDNA recombination with Ty1his3AI in the wild-type strain was 1.3% (Table 6). The frequency was threefold lower in the recombination-defective rad52Δ mutant and twofold higher in the rad27Δ mutant, which has previously been reported to have an elevated level of Ty1 cDNA recombination (Sundararajan et al. 2003). However, deletion of RRM3 had no effect on the frequency of cDNA recombination in our assay.
Recombination between Ty1HIS3 cDNA and a genomic Ty1his3AI element
Genotype . | No. of His− Ura− recombinants/no. of His+ Ura+ isolates (%) . |
|---|---|
| WT | 10/758 (1.3) |
| rad52Δ | 3/672 (0.4) |
| rad27Δ | 25/902 (2.8) |
| rrm3Δ | 9/799 (1.1) |
Genotype . | No. of His− Ura− recombinants/no. of His+ Ura+ isolates (%) . |
|---|---|
| WT | 10/758 (1.3) |
| rad52Δ | 3/672 (0.4) |
| rad27Δ | 25/902 (2.8) |
| rrm3Δ | 9/799 (1.1) |
Recombination between Ty1HIS3 cDNA and a genomic Ty1his3AI element
Genotype . | No. of His− Ura− recombinants/no. of His+ Ura+ isolates (%) . |
|---|---|
| WT | 10/758 (1.3) |
| rad52Δ | 3/672 (0.4) |
| rad27Δ | 25/902 (2.8) |
| rrm3Δ | 9/799 (1.1) |
Genotype . | No. of His− Ura− recombinants/no. of His+ Ura+ isolates (%) . |
|---|---|
| WT | 10/758 (1.3) |
| rad52Δ | 3/672 (0.4) |
| rad27Δ | 25/902 (2.8) |
| rrm3Δ | 9/799 (1.1) |
An independent method was used to explore the possibility that Ty1 cDNA integration at novel target sites, or cDNA recombination with other genomic Ty1 sequences such as solo LTRs, contributes to elevated retromobility in the absence of Rrm3. Sites of Ty1HIS3 insertion into the genome were identified by TAIL-PCR followed by sequencing of the PCR products. To perform this analysis, a Ty1his3AI element on a CEN-based vector was introduced into strain BY4741 and the isogenic rrm3Δ strain. Fifteen independent His+ derivatives of strain BY4741 and 41 His+ derivatives of the rrm3Δ mutant were isolated. After segregation of the Ty1his3AI donor plasmid, genomic DNA was isolated and used as a template in TAIL-PCR to amplify genomic sequences adjacent to the 3′-LTR of the Ty1HIS3 element. In the rrm3Δ mutant, 10 of the 41 TAIL-PCR products (24%) contained TYA1 sequences immediately downstream of the Ty1HIS3 LTR, consistent with the presence of Ty1HIS3 cDNA in a dimeric array with a 1-LTR junction (see supporting information, Table S1 A). Three other TAIL-PCR products contained the junction between the 3′ end of Ty1HIS3 and sequences within a Ty1, Ty2, or Ty4 LTR at a genomic location that could not be determined due to the short length of the TAIL-PCR products (Table S1 B). Of the remaining 28 events, none of the Ty1HIS3 LTR:genomic DNA junctions was at a preexisting LTR:genomic DNA junction. Therefore, gene conversion of genomic Ty1 sequences by Ty1HIS3 cDNA accounted for <4% of Ty1HIS3 mobility events in the rrm3Δ mutant. Furthermore, the specificity of Ty1 integration in the rrm3Δ mutant was similar to that in a wild-type strain. In 24 of 28 rrm3Δ isolates (86%), Ty1HIS3 integrated within an 825-bp window upstream of a gene transcribed by RNA polymerase III. This window is a well-characterized hotspot for Ty1 integration (Devine and Boeke 1996; Bachman et al. 2004). In 1 of 28, Ty1HIS3 integrated downstream of a tRNA gene, while in 3 others, Ty1HIS3 integrated between two divergently transcribed open reading frames. Bidirectional promoter regions have not previously been described as preferred sites of Ty1 integration, raising the possibility that they become stronger targets in the absence of Rrm3.
In comparison to 10 of 41 Ty1HIS3 insertions in the rrm3Δ mutant, none of 15 Ty1HIS3 insertions was present as a Ty1HIS3:Ty1 multimer in the wild-type strain (Table S1 C). In 3 of 15, Ty1HIS3 integrated into a Ty1 or Ty4 LTR at an undetermined genomic location. In the remaining 12, the upstream regions of genes transcribed by RNA polymerase III were targeted 11 times (92%), while one Ty1HIS3 element integrated into the CHK1 open reading frame, which is not in the vicinity of an RNA polymerase III transcription unit. We conclude that neither gene conversion of Ty1 elements by Ty1 cDNA nor a greatly expanded integration target range is a major factor in the elevated Ty1 cDNA mobility in rrm3 mutants. Since the rate of Ty1 integration into a preferred target is also not increased (Table 5), it is likely that most of the Ty1 cDNA in rrm3 mutants is inserted into the genome in multimeric arrays.
We analyzed the 28 rrm3 His+ isolates with characterized Ty1HIS3:genomic DNA junctions (Table S1 B) by Southern analysis and by PCR to determine whether the integrated Ty1HIS3 element was part of a Ty1 multimer. None of these 28 integration events were multimeric (data not shown). This finding suggests that when Ty1HIS3 cDNA is incorporated into a multimer, it is rarely if ever present at the 3′ junction with genomic DNA. Therefore, we cannot use genomic insertion site data to determine whether Ty1 multimers are present at sites of integration into the genome or recombination with the genome.
Ty1 cDNA multimers are associated with chromosomal rearrangements:
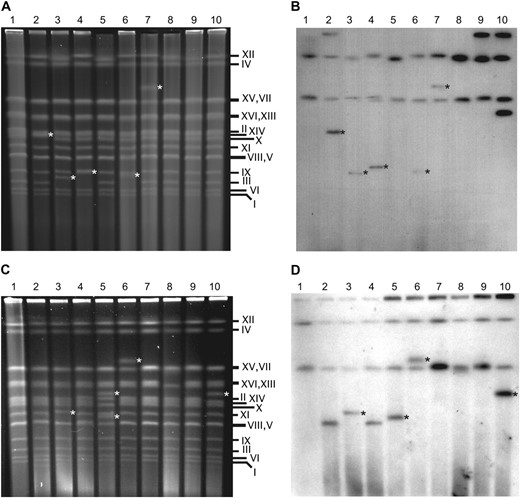
Tandem arrays of Ty1 elements have been found at chromosomal breakpoint junctions, suggesting that cDNA multimers can form during chromosomal break repair (Umezu et al. 2002; Maxwell and Curcio 2007b). To determine whether there is a connection between Ty1 multimer formation and chromosomal break repair in rrm3Δ mutants, we asked whether isolates containing Ty1HIS3:Ty1 multimers have an increased incidence of chromosomal rearrangements. The chromosomal banding pattern of His+ isolates with or without a Ty1HIS3:Ty1 multimer (Table 4) was analyzed using clamped homogeneous electric field (CHEF) gels. Chromosome bands with a novel migration position in His+ isolates relative to the bands in a wild-type His− strain (prior to Ty1his3AI transposition) were scored as chromosomal rearrangements (Figure 3). In the wild-type strain, 4 of 18 His+ isolates with a Ty1HIS3:Ty1 multimer (22%) harbored one chromosomal rearrangement (Table 7). In contrast, no chromosomal rearrangements were observed in 30 His+ isolates that had a nonmultimeric Ty1HIS3 insertion (0%), which is a significantly lower percentage (P < 0.05). In the rrm3Δ mutant, 38% of isolates with a Ty1HIS3:Ty1 multimer had a chromosomal rearrangement compared to 3% of isolates with a monomeric Ty1HIS3 insertion (P < 0.01). The percentage of rrm3Δ isolates with chromosomal rearrangements was even higher (47%) when only those harboring 1-LTR Ty1HIS3:Ty1 multimers were considered. In the sgs1Δ strain, His+ isolates with multimeric Ty1HIS3:Ty1 insertions also had markedly higher levels of chromosomal rearrangements compared to isolates with monomeric Ty1HIS3 insertions (30 and 6%, respectively). Together, the data in Table 7 suggest that a substantial fraction of isolates with Ty1 cDNA multimers, particularly 1-LTR multimers, are associated with chromosomal rearrangements in a wild-type strain, and this fraction is increased when either SGS1 or RRM3 is deleted.
Ty1HIS3:Ty1 multimers are located on chromosomes that have undergone rearrangement. (A) Intact chromosomes separated by CHEF gel electrophoresis. Lane 1, the His− wild-type strain, JC3212; lanes 2–10, His+ derivatives of the isogenic rrm3Δ strain, JC3917, harboring a 1-LTR Ty1HIS3:Ty1 multimer. (B) Southern blot of the gel in A probed with HIS3. (C) CHEF analysis of intact chromosomes. Lane 1, the His− wild-type strain JC3212; lanes 2–10, His+ derivatives of the isogenic sgs1Δ strain, JC4915, harboring a 1-LTR Ty1HIS3:Ty1 multimer. The His+ isolate in lane 5 is the only one in which two rearranged chromosomes were detected. (D) Southern blot of the gel in C probed with HIS3. White asterisks in A and C indicate new chromosome bands. In B and D, bands that correspond to novel chromosome bands in A and C are marked with a black asterisk. The his3Δ1 allele present in every strain is located on chromosome XV. The Ty1his3AI-3114 element present in every strain is located on chromosome XII.
Chromosomal rearrangements in His+ isolates
. | Fraction of His+ isolates with a chromosomal rearrangementa . | ||||
|---|---|---|---|---|---|
| Genotype . | Nonmultimeric Ty1HIS3 (%) . | 1-LTR Ty1HIS3:Ty1 multimer (%) . | 2-LTR Ty1HIS3:Ty1 multimer (%) . | Irregular Ty1HIS3:Ty1 multimer (%) . | Total Ty1HIS3:Ty1 multimers (%) . |
| WT | 0/30 (0) | 3/10 (30) | 0/5 (0) | 1/3 (33) | 4/18 (22) |
| rrm3Δ | 1/34 (3) | 16/34 (47) | 0/3 (0) | 1/8 (13) | 17/45 (38) |
| sgs1Δ | 1/18 (6) | 8/23 (35) | 0/3 (0) | 0/1 (0) | 8/27 (30) |
. | Fraction of His+ isolates with a chromosomal rearrangementa . | ||||
|---|---|---|---|---|---|
| Genotype . | Nonmultimeric Ty1HIS3 (%) . | 1-LTR Ty1HIS3:Ty1 multimer (%) . | 2-LTR Ty1HIS3:Ty1 multimer (%) . | Irregular Ty1HIS3:Ty1 multimer (%) . | Total Ty1HIS3:Ty1 multimers (%) . |
| WT | 0/30 (0) | 3/10 (30) | 0/5 (0) | 1/3 (33) | 4/18 (22) |
| rrm3Δ | 1/34 (3) | 16/34 (47) | 0/3 (0) | 1/8 (13) | 17/45 (38) |
| sgs1Δ | 1/18 (6) | 8/23 (35) | 0/3 (0) | 0/1 (0) | 8/27 (30) |
Chromosome bands of a novel size relative to the wild-type strain prior to selection for Ty1HIS3 retromobility.
Chromosomal rearrangements in His+ isolates
. | Fraction of His+ isolates with a chromosomal rearrangementa . | ||||
|---|---|---|---|---|---|
| Genotype . | Nonmultimeric Ty1HIS3 (%) . | 1-LTR Ty1HIS3:Ty1 multimer (%) . | 2-LTR Ty1HIS3:Ty1 multimer (%) . | Irregular Ty1HIS3:Ty1 multimer (%) . | Total Ty1HIS3:Ty1 multimers (%) . |
| WT | 0/30 (0) | 3/10 (30) | 0/5 (0) | 1/3 (33) | 4/18 (22) |
| rrm3Δ | 1/34 (3) | 16/34 (47) | 0/3 (0) | 1/8 (13) | 17/45 (38) |
| sgs1Δ | 1/18 (6) | 8/23 (35) | 0/3 (0) | 0/1 (0) | 8/27 (30) |
. | Fraction of His+ isolates with a chromosomal rearrangementa . | ||||
|---|---|---|---|---|---|
| Genotype . | Nonmultimeric Ty1HIS3 (%) . | 1-LTR Ty1HIS3:Ty1 multimer (%) . | 2-LTR Ty1HIS3:Ty1 multimer (%) . | Irregular Ty1HIS3:Ty1 multimer (%) . | Total Ty1HIS3:Ty1 multimers (%) . |
| WT | 0/30 (0) | 3/10 (30) | 0/5 (0) | 1/3 (33) | 4/18 (22) |
| rrm3Δ | 1/34 (3) | 16/34 (47) | 0/3 (0) | 1/8 (13) | 17/45 (38) |
| sgs1Δ | 1/18 (6) | 8/23 (35) | 0/3 (0) | 0/1 (0) | 8/27 (30) |
Chromosome bands of a novel size relative to the wild-type strain prior to selection for Ty1HIS3 retromobility.
If Ty1HIS3:Ty1 multimers form at chromosomal breakpoints, we would expect to find the Ty1HIS3 cDNA on the rearranged chromosome. We performed Southern blot analysis of chromosomal DNA from 3 wild-type, 14 rrm3Δ, and 7 sgs1Δ isolates that harbored a 1-LTR Ty1HIS3:Ty1 multimer as well as a chromosomal band of a novel size. HIS3 was located on a novel chromosome band in all 24 isolates (Figure 3 and data not shown). Together, these findings provide evidence that 1-LTR Ty1 multimers form at chromosomal break sites in all three of the strains, whereas monomeric Ty1 integration events are significantly less likely to occur at breakpoints.
Expression of RNase H suppresses Ty1 multimer formation but not retromobility in an rrm3 mutant:
Insertion of multimeric Ty1 cDNA could occur during repair of chromosomal breaks at two different types of sites in the rrm3Δ mutant. The first type comprises sites occupied by nonnucleosomal protein:DNA complexes, which are common features of the sites where replication pausing and chromosomal breaks occur in the rrm3 mutant (Ivessa et al. 2000, 2002, 2003). The second type comprises RNA:DNA hybrid regions, which are proposed to be substrates of the Rrm3 helicase (Boule and Zakian 2006). Immediately following integration, Ty1 cDNA could contain RNA:DNA hybrid regions resulting from incomplete reverse transcription (Muller et al. 1991). If retrotransposition occurs prior to replication, Rrm3 could be required to traverse these regions. To test the idea that Ty1 cDNA multimers form at breaks initiated by RNA:DNA hybrids, we expressed a cellular RNase H, encoded by the RNH1 gene, from the inducible GAL1 promoter to degrade the RNA strand of RNA:DNA hybrids. The pGAL1-RNH1 plasmid was expressed in an rrm3Δ, sgs1Δ, or wild-type strain harboring Ty1his3AI, and the effects on Ty1 retromobility and Ty1 multimer formation were determined. Notably, expression of pGAL1:RNH1 had no effect on the level of Ty1 retromobility in a wild-type, rrm3Δ, or sgs1Δ strain (see Figure S1), suggesting that it did not interfere with the synthesis of Ty1 cDNA in cytoplasmic VLPs. However, expression of pGAL1:RNH1 completely suppressed Ty1HIS3:Ty1 multimer formation in the rrm3Δ mutant (P < 0.05), such that the level of Ty1HIS3:Ty1 multimers in the rrm3Δ mutant with RNase H expression was as low as the level of Ty1 multimers in the wild-type strain without pGAL1:RNH1 (Table 8). In contrast, expression of pGAL1:RNH1 did not alter the fraction of Ty1HIS3 mobility events that were multimeric in a wild-type strain or an sgs1Δ mutant. Therefore, RNA:DNA hybrid regions in the chromosome are likely to trigger the formation of Ty1 cDNA multimers specifically in the absence of Rrm3.
Effect of RNase H overexpression on Ty1HIS3:Ty1 multimer formation
Genotype . | Plasmid . | 1-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | 2-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Irregular Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Total Ty1HIS3:Ty1 multimers/His+ isolate (%) . |
|---|---|---|---|---|---|
| WT | Vector | 9/68 (13) | 2/68 (3) | 0/68 (0) | 11/68 (16) |
| WT | pGAL1:RNH1 | 6/58 (10) | 4/58 (7) | 1/58 (2) | 11/58 (19) |
| rrm3Δ | Vector | 14/56 (25) | 3/56 (5) | 2/56 (4) | 19/56 (34) |
| rrm3Δ | pGAL1:RNH1 | 4/56 (7) | 0/56 (0) | 1/56 (2) | 5/56 (9) |
| sgs1Δ | Vector | 12/66 (18) | 2/66 (3) | 3/66 (5) | 17/66 (26) |
| sgs1Δ | pGAL1:RNH1 | 16/66 (24) | 0/66 (0) | 2/66 (3) | 18/66 (27) |
Genotype . | Plasmid . | 1-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | 2-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Irregular Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Total Ty1HIS3:Ty1 multimers/His+ isolate (%) . |
|---|---|---|---|---|---|
| WT | Vector | 9/68 (13) | 2/68 (3) | 0/68 (0) | 11/68 (16) |
| WT | pGAL1:RNH1 | 6/58 (10) | 4/58 (7) | 1/58 (2) | 11/58 (19) |
| rrm3Δ | Vector | 14/56 (25) | 3/56 (5) | 2/56 (4) | 19/56 (34) |
| rrm3Δ | pGAL1:RNH1 | 4/56 (7) | 0/56 (0) | 1/56 (2) | 5/56 (9) |
| sgs1Δ | Vector | 12/66 (18) | 2/66 (3) | 3/66 (5) | 17/66 (26) |
| sgs1Δ | pGAL1:RNH1 | 16/66 (24) | 0/66 (0) | 2/66 (3) | 18/66 (27) |
Effect of RNase H overexpression on Ty1HIS3:Ty1 multimer formation
Genotype . | Plasmid . | 1-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | 2-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Irregular Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Total Ty1HIS3:Ty1 multimers/His+ isolate (%) . |
|---|---|---|---|---|---|
| WT | Vector | 9/68 (13) | 2/68 (3) | 0/68 (0) | 11/68 (16) |
| WT | pGAL1:RNH1 | 6/58 (10) | 4/58 (7) | 1/58 (2) | 11/58 (19) |
| rrm3Δ | Vector | 14/56 (25) | 3/56 (5) | 2/56 (4) | 19/56 (34) |
| rrm3Δ | pGAL1:RNH1 | 4/56 (7) | 0/56 (0) | 1/56 (2) | 5/56 (9) |
| sgs1Δ | Vector | 12/66 (18) | 2/66 (3) | 3/66 (5) | 17/66 (26) |
| sgs1Δ | pGAL1:RNH1 | 16/66 (24) | 0/66 (0) | 2/66 (3) | 18/66 (27) |
Genotype . | Plasmid . | 1-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | 2-LTR Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Irregular Ty1HIS3:Ty1 multimer/His+ isolate (%) . | Total Ty1HIS3:Ty1 multimers/His+ isolate (%) . |
|---|---|---|---|---|---|
| WT | Vector | 9/68 (13) | 2/68 (3) | 0/68 (0) | 11/68 (16) |
| WT | pGAL1:RNH1 | 6/58 (10) | 4/58 (7) | 1/58 (2) | 11/58 (19) |
| rrm3Δ | Vector | 14/56 (25) | 3/56 (5) | 2/56 (4) | 19/56 (34) |
| rrm3Δ | pGAL1:RNH1 | 4/56 (7) | 0/56 (0) | 1/56 (2) | 5/56 (9) |
| sgs1Δ | Vector | 12/66 (18) | 2/66 (3) | 3/66 (5) | 17/66 (26) |
| sgs1Δ | pGAL1:RNH1 | 16/66 (24) | 0/66 (0) | 2/66 (3) | 18/66 (27) |
DISCUSSION
DNA helicases act on a variety of substrates to carry out their diverse functions in the cell. Not only do they unwind double-stranded DNA, but they also unwind RNA:DNA hybrids (Shin and Kelman 2006; Boule and Zakian 2007) and remove protein from single-stranded DNA (Krejci et al. 2003; Veaute et al. 2003; Byrd and Raney 2004). Members of the Pif1 family of DNA helicases function in the maintenance of mitochondrial and nuclear genomes, but the precise nature of their substrates is an important and unresolved issue. S. cerevisiae has two members of this family, Pif1 and Rrm3, which have distinct functions in the cell (Ivessa et al. 2000). Both helicases have been implicated in the removal of protein complexes from DNA and in the unwinding of RNA:DNA hybrid regions (Ivessa et al. 2003; Boule et al. 2005; Boule and Zakian 2006, 2007). In this work, we explored the role of Rrm3 in repressing Ty1 retromobility. We found that formation of Ty1 cDNA multimers is a major pathway of retromobility in rrm3 mutants and that there is a significant correlation between cDNA multimers and chromosomal rearrangements. Moreover, Ty1 multimer formation in an rrm3 mutant is suppressed by the overexpression of RNase H. Together, these results suggest that RNA:DNA hybrid regions in nascent retrotransposition events are substrates of the Rrm3 helicase.
Ty1 cDNA levels are significantly elevated in the absence of Rrm3, in part because Ty1 cDNA synthesis is stimulated through the DNA damage checkpoint pathway (Scholes et al. 2001; Curcio et al. 2007). Despite the elevated levels of cDNA, deletion of RRM3 does not increase integration into a preferred target, namely the region upstream of tRNA genes (Table 5; see also Scholes et al. 2001). Furthermore, target-site specificity of Ty1 is similar in the presence and absence of Rrm3 (Table S1). Thus, increased recombination of Ty1 cDNA rather than elevated levels of integration may underlie the higher levels of retromobility in the absence of Rrm3. Consistent with this idea, we found only a twofold increase in retromobility when RRM3 was deleted in a rad52Δ strain (Table 3). However, three observations suggest that Ty1 cDNA is rarely a donor for gene conversion of genomic Ty1 sequences in the rrm3 mutant. First, Rad51, which is required for gene conversion, is not required for the increase in retromobility that results from deletion of RRM3. Second, gene conversion of a chromosomal Ty1his3AI element by its cDNA was not elevated in the absence of Rrm3 (Table 6). Third, gene conversion of 1 of the 32 Ty1 elements or 185 Ty1 LTRs in the S. cerevisiae genome (Kim et al. 1998) by Ty1HIS3 cDNA was not observed in the analysis of 41 Ty1HIS3 insertion sites in the rrm3Δ mutant, even though 12 of the 41 Ty1HIS3 insertions sites resulted from integration into Ty LTRs (Table S1). On the other hand, the formation of multimeric Ty1 cDNA arrays was significantly increased in the rrm3 mutant. Most of the multimers had one LTR at the junction between cDNAs, implicating the involvement of homologous recombination between LTRs of individual cDNAs in their formation. Together, our results point to Ty1 multimer formation as a major route of Ty1 retromobility in the rrm3Δ mutant. Multimer formation results in higher levels of retromobility by increasing the average number of cDNA molecules that are incorporated into the genome of each cell that sustains a retromobility event, rather than by increasing the proportion of cells in which cDNA is inserted. Interestingly, multimer formation is not a mandatory pathway for cDNA insertion in the rrm3 mutant, since cDNA can be incorporated via alternative pathways, such as integration, when multimer formation is blocked. For example, deletion of RAD52 in an rrm3 mutant resulted in only a small reduction in the level of retromobility. (Compare the retromobility frequency in the rrm3Δ mutant to the rrm3Δ rad52Δ mutant in Table 3.) Similarly, overexpression of RNase H suppressed the formation of Ty1 multimers in an rrm3 mutant without affecting the frequency of Ty1 retromobility.
The frequency of Ty1 multimer formation is similar between rrm3Δ and sgs1Δ mutants (Table 4), but the frequency of Ty1his3AI retromobility is substantially higher in an rrm3Δ mutant. We cannot yet explain this discrepancy, but it could be due to a higher average number of monomeric units within cDNA arrays in an rrm3Δ mutant relative to the sgs1Δ mutant. The fact that the rrm3Δ mutant has more Ty1 cDNA per cell than the sgs1Δ mutant supports this idea (Bryk et al. 2001; Scholes et al. 2001).
We have observed that the presence of chromosomal rearrangements, which could be large insertions or translocations, is correlated with the formation of multimeric Ty1 cDNA arrays in the rrm3Δ mutant as well as in the sgs1Δ mutant and the wild-type strain. This correlation is even stronger when only 1-LTR multimers are considered (Table 7). Moreover, in isolates that harbor both a 1-LTR multimer and a rearrangement, the HIS3-marked multimer is found on the rearranged chromosome (Figure 3 and data not shown). Together, these observations suggest that Ty1 multimers are frequently formed at chromosomal break sites. Notably, the fraction of multimer-containing isolates that harbor a chromosomal rearrangement is lowest in the wild-type strain, intermediate in the sgs1 strain, and highest in the rrm3 mutant (Table 7). Perhaps this observation reflects the fact that most rearrangements are large insertions of cDNA arrays, which would have to contain at least six or seven Ty1 cDNAs to cause a chromosomal band shift that is large enough to detect by CHEF gel analysis. Thus, the level of Ty1 cDNA, which is lowest in the wild-type strain and highest in the rrm3 mutant, may directly influence the fraction of Ty1 cDNA multimers that are large enough to be detected as rearrangements in each strain. His+ isolates of the rrm3Δ mutant can harbor Ty1 arrays consisting of as many as 25 cDNA monomers (data not shown), which are certainly long enough to result in a chromosomal band shift.
We took advantage of the fact that overexpression of a cellular RNase H does not inhibit the level of Ty1 retromobility in the wild-type, rrm3, or sgs1 strain to ask whether the presence of an RNA:DNA hybrid promotes the formation of Ty1 multimers. Despite the association of Ty1 multimers with chromosomal breaks in all three strains, overexpression of RNase H suppressed multimer formation only in the rrm3 mutant, and not in the wild-type or sgs1Δ strain (Table 8). This result strongly suggests that RNA:DNA hybrids promote multimer formation specifically in the rrm3 mutant. Other types of DNA lesions may be involved in initiating multimer formation in the wild-type strain and the sgs1 mutant.
How are multimeric arrays of Ty1 cDNAs incorporated into the genome in an rrm3Δ mutant? A model for these events must take into account the significant association between Ty1 multimers and genome rearrangements and the requirement for RNA:DNA hybrids in the increase in multimer formation in the rrm3Δ mutant. Our evidence favors a model in which Ty1 cDNA containing internal RNA:DNA hybrid regions is integrated into the genome prior to replication (Figure 4A). The presence of an RNA:DNA hybrid, which results from incomplete synthesis of the plus strand of cDNA, has been observed in the nucleic acids of Ty1 VLPs (Muller et al. 1991). If the RNA:DNA hybrid cannot be traversed by the replication fork in the absence of Rrm3 (Figure 4B), it could trigger the formation of a chromosome break within Ty1 sequences. The broken DNA end could then recombine with an extragenomic Ty1 cDNA molecule. Once one cDNA is incorporated at the break site, its free LTR end could recombine with the LTR of another extragenomic cDNA molecule, forming an array with 1-LTR at the junction. Subsequent recombination-mediated incorporation of cDNA at the break site would increase the size of the multimeric cDNA array. These recombination events could occur by strand annealing, consistent with the requirement for Rad52 but not for Rad51. Eventually, the chromosome end would be repaired by recombination with a genomic Ty1 element (Figure 4C), resulting in formation of a rearranged chromosome (Figure 4D). The genomic element with which the broken end recombines could be the newly retransposed element on the sister chromatid, or an ectopic Ty1 element. This model predicts that multimeric cDNA arrays and chromosomal rearrangements form at sites of retrotransposition, and thus, explains why a high level of chromosomal rearrangement is seen specifically when multimeric retromobility events are selected (Table 7). This model also provides an explanation for the low level of recombination between Ty1HIS3 cDNA and genomic Ty1 elements in the rrm3 mutant, since the breaks that are repaired by Ty1 cDNA occur at nascent sites of retrotransposition, rather than at Ty1 elements preexisting in the genome.
A model for the formation of Ty1 cDNA multimers in rrm3 mutants, and their association with chromosomal rearrangements. (A) Ty1 retrotransposition is proposed to occur directly before replication, resulting in incorporation of RNA (shaded rectangle):DNA hybrids into the genome. (B) The RNA:DNA hybrids function as replication fork blocks that give rise to a chromosome break in the absence of Rrm3. (C) The broken chromosomal fragment recombines with multiple copies of extrachromosomal Ty1 cDNA (illustrated by an “X”) prior to recombination with the Ty1 element on the sister chromatid or with an ectopic copy of Ty1 (illustrated by the curved arrow). (D) These events result in a multimeric Ty1 cDNA array at a chromosomal breakpoint junction.
It has previously been proposed that Rrm3 is required for replication through nonnucleosomal protein-DNA complexes rather than RNA:DNA hybrids (Ivessa et al. 2003). This putative role is not necessarily conflicting with our observation that RNA:DNA hybrids trigger Ty1 cDNA multimer formation in the rrm3Δ mutant. Perhaps it is not the RNA:DNA hybrid per se that stalls the replication fork in rrm3 mutants, but instead the presence of a protein that is specifically bound to this region. If so, one possible protein that could be bound to the RNA:DNA hybrid region within nascent retrotransposition events is the Ty1 reverse transcriptase. Reverse transcription of the double-strand Ty1 cDNA may be a very inefficient process (Muller et al. 1991); therefore, it is conceivable that reverse transcriptase remains bound to the RNA:DNA hybrid regions in nascent retrotransposition events.
Two alternative models for the formation of Ty1 multimers in rrm3 mutants cannot be eliminated by the data presented but are considered unlikely. The first is that Ty1 cDNA forms multimeric arrays by homologous recombination prior to its incorporation into the genome by integration. If Ty1 arrays in the genome are refractory to progression of the replication fork, they could give rise to chromosomal rearrangements. In this model, Rrm3 might repress Ty1 multimer formation by unwinding RNA:DNA heteroduplexes in Ty1 cDNA so that the cDNA can be repaired, thereby preventing its use as a substrate for multimer formation. It is important to note however, that this model invokes a role for Rrm3 outside of replication, which is inconsistent with studies showing its involvement in replication fork progression (Schmidt et al. 2002; Azvolinsky et al. 2006; Matsuda et al. 2007).
The second alternative is that following a chromosomal break at a stalled replication fork in the rrm3 mutant, the ends are resected until Ty1 sequences are reached. Subsequently, the chromosome end would recombine with multiple Ty1 cDNAs before recombining with the allelic Ty1 sequences on the sister chromatid or with an ectopic Ty1 element. The high levels of chromosomal breaks in rrm3 mutants render this an appealing model. However, the model does not provide an obvious rationale for the role of RNA:DNA hybrids in Ty1 multimer formation and is inconsistent with several observations. First, chromosomal rearrangements are rare in the absence of selection for retromobility events, despite the high level of chromosomal breaks in an rrm3 mutant (Ivessa et al. 2003; Schmidt and Kolodner 2006). Second, gene conversion of genomic Ty1 elements or LTRs by cDNA occurs rarely in rrm3 mutants (Table 6 and Table S1), whereas this model predicts that gene conversion events would be common. Third, no increase in Ty1 multimer formation was observed in an rtt101Δ mutant, even though this mutant also has an elevated level of replication fork stalling. In contrast, this model predicts that other mutants with increased replication fork pausing might also have higher levels of Ty1 multimers.
The results presented here raise the possibility that retrotransposition occurs shortly before replication, precluding repair of the RNA:DNA hybrid before the replication fork passes through the newly transposed element. This idea is supported by previous findings that retrotransposition is inhibited by arresting yeast cells in G1 and stimulated by prolonging S phase with hydroxyurea (Xu and Boeke 1991; Curcio et al. 2007). This mode of retrotransposition is optimal for the spread of the retrotransposon, since it results in the presence of the replicated retrotransposon in both the mother and daughter cells.
Footnotes
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.104208/DC1.
Footnotes
Communicating editor: D. Voytas
Acknowledgements
We are grateful to Suzanne Sandmeyer for comments on the manuscript and to the Wadsworth Applied Genomic Technologies Core for DNA sequencing. This work was supported by National Institutes of Health grant GM52072.
References
Azvolinsky, A., S. Dunaway, J. Z. Torres, J. B. Bessler and V. A. Zakian,
Bachman, N., Y. Eby and J. D. Boeke,
Boule, J. B., L. R. Vega and V. A. Zakian,
Boule, J. B., and V. A. Zakian,
Boule, J. B., and V. A. Zakian,
Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li et al.,
Bryk, M., M. Banerjee, D. Conte, Jr. and M. J. Curcio,
Byrd, A. K., and K. D. Raney,
Curcio, M. J., and D. J. Garfinkel,
Curcio, M. J., and D. J. Garfinkel,
Curcio, M. J., A. M. Hedge, J. D. Boeke and D. J. Garfinkel,
Curcio, M. J., A. E. Kenny, S. Moore, D. J. Garfinkel, M. Weintraub et al.,
Devine, S. E., and J. D. Boeke,
Fabre, F., A. Chan, W. D. Heyer and S. Gangloff,
Foster, P. L.,
Gangloff, S., C. Soustelle and F. Fabre,
Gerring, S. L., C. Connelly and P. Hieter,
Gravel, S., J. R. Chapman, C. Magill and S. P. Jackson,
Irwin, B., M. Aye, P. Baldi, N. Beliakova-Bethell, H. Cheng et al.,
Ivessa, A. S., B. A. Lenzmeier, J. B. Bessler, L. K. Goudsouzian, S. L. Schnakenberg et al.,
Ivessa, A. S., J. Q. Zhou, V. P. Schulz, E. K. Monson and V. A. Zakian,
Ivessa, A. S., J. Q. Zhou and V. A. Zakian,
Ke, N., X. Gao, J. B. Keeney, J. D. Boeke and D. F. Voytas,
Kim, J. M., S. Vanguri, J. D. Boeke, A. Gabriel and D. F. Voytas,
Klein, H. L.,
Krejci, L., S. Van Komen, Y. Li, J. Villemain, M. S. Reddy et al.,
Lee, B. S., C. P. Lichtenstein, B. Faiola, L. A. Rinckel, W. Wysock et al.,
Liu, Y.-G., and R. F. Whittier,
Luke, B., G. Versini, M. Jaquenoud, I. W. Zaidi, T. Kurz et al.,
Matsuda, K., M. Makise, Y. Sueyasu, M. Takehara, T. Asano et al.,
Maxwell, P. H., and M. J. Curcio,
Maxwell, P. H., and M. J. Curcio,
Melamed, C., Y. Nevo and M. Kupiec,
Mimitou, E. P., and L. S. Symington,
Mou, Z., A. E. Kenny and M. J. Curcio,
Muller, F., W. Laufer, U. Pott and M. Ciriacy,
Ooi, S. L., D. D. Shoemaker and J. D. Boeke,
Prado, F., and A. Aguilera,
Schmidt, K. H., K. L. Derry and R. D. Kolodner,
Schmidt, K. H., and R. D. Kolodner,
Schmidt, K. H., and R. D. Kolodner,
Scholes, D. T., M. Banerjee, B. Bowen and M. J. Curcio,
Sharon, G., T. J. Burkett and D. J. Garfinkel,
Shin, J. H., and Z. Kelman,
Sundararajan, A., B. S. Lee and D. J. Garfinkel,
Torres, J. Z., S. L. Schnakenberg and V. A. Zakian,
Umezu, K., M. Hiraoka, M. Mori and H. Maki,
Veaute, X., J. Jeusset, C. Soustelle, S. C. Kowalczykowski, E. Le Cam et al.,
Weinstock, K. G., M. F. Mastrangelo, T. J. Burkett, D. J. Garfinkel and J. N. Strathern,
Xu, H., and J. D. Boeke,
Zhu, Z., W. H. Chung, E. Y. Shim, S. E. Lee and G. Ira,