-
PDF
- Split View
-
Views
-
Cite
Cite
Carrie A Smith, Charles P Woloshuk, Dominique Robertson, Gary A Payne, Silencing of the Aflatoxin Gene Cluster in a Diploid Strain of Aspergillus flavus Is Suppressed by Ectopic aflR Expression, Genetics, Volume 176, Issue 4, 1 August 2007, Pages 2077–2086, https://doi.org/10.1534/genetics.107.073460
Close - Share Icon Share
Abstract
Aflatoxins are toxic secondary metabolites produced by a 70-kb cluster of genes in Aspergillus flavus. The cluster genes are coordinately regulated and reside as a single copy within the genome. Diploids between a wild-type strain and a mutant (649) lacking the aflatoxin gene cluster fail to produce aflatoxin or transcripts of the aflatoxin pathway genes. This dominant phenotype is rescued in diploids between a wild-type strain and a transformant of the mutant containing an ectopic copy of aflR, the transcriptional regulator of the aflatoxin biosynthetic gene cluster. Further characterization of the mutant showed that it is missing 317 kb of chromosome III, including the known genes for aflatoxin biosynthesis. In addition, 939 kb of chromosome II is present as a duplication on chromosome III in the region previously containing the aflatoxin gene cluster. The lack of aflatoxin production in the diploid was not due to a unique or a mis-expressed repressor of aflR. Instead a form of reversible silencing based on the position of aflR is likely preventing the aflatoxin genes from being expressed in 649 × wild-type diploids. Gene expression analysis revealed the silencing effect is specific to the aflatoxin gene cluster.
ASPERGILLUS flavus is an asexual filamentous fungus with one of the best-characterized pathways of secondary metabolism in fungi. This pathway, resulting in the production of the carcinogenic polyketide aflatoxin, has been studied extensively through the use of genetic analysis and chemistry (Bennett and Klich 2003; Yu et al. 2004, 2005; Keller et al. 2005). Genetic analysis of aflatoxin biosynthesis was pioneered by K. E. Papa, who generated a large collection of mutants by UV and chemical methods using a single isolate from pecan (PC-7) (Leaich and Papa 1974; Papa 1979, 1984). Papa described the parasexual cycle in A. flavus in 1973, a genetic event originally discovered by Pontecorvo and Roper (1952) in A. nidulans. Parasexual crosses involve the pairing of two haploid strains to form a synthetic or somatic diploid. Through this cycle, mutations can be assessed for dominance and loci can be mapped as linkage groups. Papa performed parasexual crosses between wild-type PC-7 and mutant strains derived from PC-7, and was able to assign 36 loci to eight linkage groups (Papa 1973, 1979, 1980, 1982, 1986). The loci included genes for spore color, nutrient utilization, and aflatoxin biosynthesis.
Most of the mutations for aflatoxin biosynthesis mapped to genes in linkage group VII, which we now know represents chromosome III (http://www.aspergillusflavus.org). The functions of most of the genes required for aflatoxin production have been identified by genetic complementation (Chang et al. 1992, 1995, 2000; Skory et al. 1992; Payne et al. 1993; Yu et al. 1993, 1997, 1998, 2000, 2003; Mahanti et al. 1996; Mcguire et al. 1996; Silva et al. 1996; Kelkar et al. 1997; Silva and Townsend 1997; Meyers et al. 1998; Motomura et al. 1999). One mutation, however, has remained uncharacterized. This mutation, identified in strain 649 as afl-1 by Papa, has continued to intrigue aflatoxin researchers because it remains the only dominant mutation known for aflatoxin biosynthesis. When strain 649 was paired with several wild-type strains, aflatoxin levels were reduced by an average of 87% in the diploids (Papa 1980). More recently, a molecular characterization of strain 649 revealed a deletion of the entire aflatoxin biosynthetic gene cluster (Woloshuk et al. 1995; Prieto et al. 1996). CHEF gel analysis also indicated that strain 649 contains additional DNA as a result from a possible rearrangement during the original mutagenesis of PC-7 (Woloshuk et al. 1995). Furthermore, expression of genes that are involved in aflatoxin biosynthesis are repressed in diploids derived from 649 (Woloshuk et al. 1995).
While these studies provided evidence as to why strain 649 does not make aflatoxin, they did not explain why diploids made between 649 and 86 (wild type for aflatoxin production) did not produce significant amounts of aflatoxin. Papa entertained the idea that a mutation in 649 enabled the strain to degrade aflatoxin, but this was disproved (Papa 1980). Two other hypotheses were suggested by Woloshuk et al. (1995). One hypothesis states that a repressor of aflatoxin gene expression is produced by strain 649. The repressor would likely affect aflR, which is the transcriptional regulator of the aflatoxin biosynthetic genes. Diploids derived from a wild-type strain and transformants of 649 that contain multiple-copy insertions of aflR produce wild-type levels of aflatoxin, suggesting that more copies of aflR can suppress the effects of this hypothetical repressor. The second hypothesis of Woloshuk et al. (1995) suggests that a trans-sensing mechanism may be responsible for the lack of aflatoxin production in 649-derived diploids. Trans-sensing, which was identified in Drosophila melanogaster and referred to as transvection, results in changes in gene expression when alleles are unpaired as a result of genomic translocations (Tartof and Henikoff 1991). This process has also been shown to be responsible for an ascus-dominant mutation in Neurospora crassa (Aramayo and Metzenberg 1996).
Without the complete characterization of the mutation in 649, it has been difficult to explain the dominant effect of afl-1. New approaches for addressing this question are now possible as the whole genome sequence of A. flavus is available (http://www.aspergillusflavus.org). The high degree of correspondence between A. flavus and A. oryzae, which has chromosome structure based on an optical map, also provides a physical map of A. flavus (Payne et al. 2006). These resources allowed us to determine the size of the deletion in 649 and to determine if other rearrangements occurred in the genome. We were able to analyze transcription of genes adjacent to the aflatoxin gene cluster that were also single copy in the 649 × 86 diploid. Here we also show evidence that the ectopic insertion of a single copy of aflR into the genome of strain 649 activates transcription of the aflatoxin pathway genes and alleviates the repression of aflatoxin biosynthesis in diploids. Our results show that silencing was restricted to the aflatoxin gene cluster in 649 × wild type diploids. The results of this study provide a better understanding of the molecular genetics surrounding the novel phenotype of a secondary metabolite pathway in an asexual fungus.
MATERIALS AND METHODS
Fungal strains:
A. flavus strains 649 (tan leu-7 afl-1) and 86 (whi arg-7) were obtained from USDA National Center for Agricultural Utilization Research, Peoria, Illinois. Strain 649 WAF2 (tan leu-7 afl-1 pyr), referred to hereafter as 649 pyr, was backcrossed twice to strain 86 (Prieto et al. 1996). Strain 29-10D (whi arg-7 pyr), referred to hereafter as 86 pyr, is a UV-mutagenized strain of 86 (Meyers et al. 1998). The wild-type strain NRRL 3357 was obtained from the National Center for Agricultural Utilization Research. Diploid 86 × 649 was previously described (Woloshuk et al. 1995). Strain 3357 is the sequenced strain of A. flavus and it is not derived from PC-7. Cultures were maintained on potato dextrose agar and when needed 10 mm uracil was added to the medium. Cultures were incubated at 37°.
Fungal growth conditions:
Mother cultures (400 ml) containing A&M medium and 0.4% agar were inoculated with 1 × 106 conidia/ml of 649 × 86 and 86. After 16 hr of growth at 28°, 20 ml of the mother cultures were used to seed 200-ml daughter cultures. The daughter cultures were grown at 28° for 24 hr. The tissue was harvested and lyophilized.
RNA isolation and cDNA synthesis:
RNA was extracted using the RNeasy plant mini kit (QIAGEN). The samples were DNase treated using the RNase-Free DNase set (QIAGEN). RNA (1 μg) was used for reverse transcriptase reactions using Stratascript reverse transcriptase (Stratagene).
DNA isolation and analysis:
Genomic DNA was isolated from fungal cultures grown as previously described (Flaherty et al. 1995). Southern analysis was performed as previously described (Woloshuk et al. 1989). DNA probes were 32P-labeled with Prime-It II random primer labeling kit (Stratagene). The Universal Genome Walker kit (Clontech) was used to identify the break point and the addition in strain 649. PCR products were visualized on 1% agarose gels stained with ethidium bromide. Secondary Genome Walker PCR products were gel extracted using the QIAquick gel extraction kit (QIAGEN). Gel-purified products were cloned into a plasmid using the TOPO TA cloning kit (Invitrogen). Plasmid and cosmid DNA from Escherichia coli were isolated following the protocol described by Sambrook and Russell (2001). Plasmid DNA from E. coli was isolated using the Wizard Midi prep kit (Promega). Sequencing was performed on both DNA strands at the DNA Sequencing Facilities at Purdue University, Ohio State University, and Iowa State University. DNA sequences were analyzed with the MacDNAsis program (Hitachi Software Engineering, San Bruno, CA) and Vector NTI software (Invitrogen). The blastn program from the A. flavus BLAST server (http://www.aspergillusflavus.org) was used to identify the genomic location of DNA sequences. Correspondence between the A. flavus and A. oryzae chromosomes was determined by blastn processing of the two genomes as performed by the HPC GridRdbBlast.pl program with the complexity filtering turned off (D. E. Brown, unpublished data). The resulting relational database was processed with the GenomicDNAmappingV2.pl program (D. E. Brown, unpublished data). Genes were predicted using the TIGR gene models associated with the genome browser http://www.aspergillusflavus.org.
PCR reactions:
Primers were designed using Primer3 from the Whitehead Institute for Biomedical Research (Rozen and Skaletsky 2000) and the genomic sequence of A. flavus strain NRRL 3357 (http://www.aspergillusflavus.org). The Advantage genomic polymerase mix (Clontech) was used in Genome Walker PCR reactions. The sequences of all primers used in this study are listed in Table 1 and supplemental Table S1 at http://www.genetics.org/supplemental/.
Primer sequences used in this study
Primer name . | Primer sequence . |
|---|---|
| GSP1-649 | GTACATGTAATAGGTGCGTTTCCTAGA |
| GSP2-649 | CCATTAGTCCCCAGTATAGCTTTTATC |
| 5′add649 | TAAGCAGGACGCAGTGTGAC |
| 3′nat&add | GTTGGAGGCTGTTTGTTGGT |
| 5′nat649 | GGAGCTGGTTCAGAGACTGG |
| 5′gpdA | TCTGTTGTCGACCTCACCTG |
| 3′gpdA | GTCAATTTCAAGGGGTGGTG |
| 5′aflD | GCACTAACCGTCGCTGAAG |
| 3′aflD | CGTGAGCCATTTGTTCTCAA |
| 5′27TV | TTGGGGATTGTCCTGAATGT |
| 3′27TV | GCCCCGAGTAAATCCTCCTA |
| 5′nest27TV | CCTTCAACAGTCTGGTCTCC |
| 3′nest27TV | AAACGAGCGAGAAGACATTC |
| 5′16TV | CCCTCTGATTTGGCATCGTA |
| 3′16TV | GTGGCCATCACGACACATAG |
| 5′nest16TV | CGAGCCACAGATCTATTTCC |
| 3′nest16TV | GAAAGCTTCGAAGGTATGGA |
| 5′laeA | ATGGGGTGTGGAAGTGTGAT |
| 3′laeA | GCAACCTTTCTTTCGTGCTC |
Primer name . | Primer sequence . |
|---|---|
| GSP1-649 | GTACATGTAATAGGTGCGTTTCCTAGA |
| GSP2-649 | CCATTAGTCCCCAGTATAGCTTTTATC |
| 5′add649 | TAAGCAGGACGCAGTGTGAC |
| 3′nat&add | GTTGGAGGCTGTTTGTTGGT |
| 5′nat649 | GGAGCTGGTTCAGAGACTGG |
| 5′gpdA | TCTGTTGTCGACCTCACCTG |
| 3′gpdA | GTCAATTTCAAGGGGTGGTG |
| 5′aflD | GCACTAACCGTCGCTGAAG |
| 3′aflD | CGTGAGCCATTTGTTCTCAA |
| 5′27TV | TTGGGGATTGTCCTGAATGT |
| 3′27TV | GCCCCGAGTAAATCCTCCTA |
| 5′nest27TV | CCTTCAACAGTCTGGTCTCC |
| 3′nest27TV | AAACGAGCGAGAAGACATTC |
| 5′16TV | CCCTCTGATTTGGCATCGTA |
| 3′16TV | GTGGCCATCACGACACATAG |
| 5′nest16TV | CGAGCCACAGATCTATTTCC |
| 3′nest16TV | GAAAGCTTCGAAGGTATGGA |
| 5′laeA | ATGGGGTGTGGAAGTGTGAT |
| 3′laeA | GCAACCTTTCTTTCGTGCTC |
Primer sequences used in this study
Primer name . | Primer sequence . |
|---|---|
| GSP1-649 | GTACATGTAATAGGTGCGTTTCCTAGA |
| GSP2-649 | CCATTAGTCCCCAGTATAGCTTTTATC |
| 5′add649 | TAAGCAGGACGCAGTGTGAC |
| 3′nat&add | GTTGGAGGCTGTTTGTTGGT |
| 5′nat649 | GGAGCTGGTTCAGAGACTGG |
| 5′gpdA | TCTGTTGTCGACCTCACCTG |
| 3′gpdA | GTCAATTTCAAGGGGTGGTG |
| 5′aflD | GCACTAACCGTCGCTGAAG |
| 3′aflD | CGTGAGCCATTTGTTCTCAA |
| 5′27TV | TTGGGGATTGTCCTGAATGT |
| 3′27TV | GCCCCGAGTAAATCCTCCTA |
| 5′nest27TV | CCTTCAACAGTCTGGTCTCC |
| 3′nest27TV | AAACGAGCGAGAAGACATTC |
| 5′16TV | CCCTCTGATTTGGCATCGTA |
| 3′16TV | GTGGCCATCACGACACATAG |
| 5′nest16TV | CGAGCCACAGATCTATTTCC |
| 3′nest16TV | GAAAGCTTCGAAGGTATGGA |
| 5′laeA | ATGGGGTGTGGAAGTGTGAT |
| 3′laeA | GCAACCTTTCTTTCGTGCTC |
Primer name . | Primer sequence . |
|---|---|
| GSP1-649 | GTACATGTAATAGGTGCGTTTCCTAGA |
| GSP2-649 | CCATTAGTCCCCAGTATAGCTTTTATC |
| 5′add649 | TAAGCAGGACGCAGTGTGAC |
| 3′nat&add | GTTGGAGGCTGTTTGTTGGT |
| 5′nat649 | GGAGCTGGTTCAGAGACTGG |
| 5′gpdA | TCTGTTGTCGACCTCACCTG |
| 3′gpdA | GTCAATTTCAAGGGGTGGTG |
| 5′aflD | GCACTAACCGTCGCTGAAG |
| 3′aflD | CGTGAGCCATTTGTTCTCAA |
| 5′27TV | TTGGGGATTGTCCTGAATGT |
| 3′27TV | GCCCCGAGTAAATCCTCCTA |
| 5′nest27TV | CCTTCAACAGTCTGGTCTCC |
| 3′nest27TV | AAACGAGCGAGAAGACATTC |
| 5′16TV | CCCTCTGATTTGGCATCGTA |
| 3′16TV | GTGGCCATCACGACACATAG |
| 5′nest16TV | CGAGCCACAGATCTATTTCC |
| 3′nest16TV | GAAAGCTTCGAAGGTATGGA |
| 5′laeA | ATGGGGTGTGGAAGTGTGAT |
| 3′laeA | GCAACCTTTCTTTCGTGCTC |
Transformation of A. flavus:
A reporter cassette in pGAP13 (Flaherty et al. 1995) containing the promoter of aflM (ver-1) fused to the beta-glucuronidase (GUS) gene (uidA) from E. coli (Jefferson et al. 1987) was inserted into the SspI site in pGAP3BB-pAF (Payne et al. 1993; Woloshuk et al. 1994). This plasmid also contains aflR from A. flavus and pyr-4 from N. crassa, each driven by its native promoter. The resulting vector, WE64 (Figure 3A), was linearized by ScaI prior to transformation into strain 649 pyr by the method of Woloshuk et al. (1989). Genomic DNA (5 μg) was screened by dot-blot analysis to identify transformants containing low copy numbers of WE64 (Sambrook and Russell 2001). Dot blots were probed with radiolabeled aflR and amy1, the single copy α-amylase gene (Fakhoury and Woloshuk 1999). Copy numbers of aflR were determined by Southern analysis with PstI-digested genomic DNA and an aflR probe (Sambrook and Russell 2001).
Aflatoxin analysis:
Aflatoxin production on coconut agar medium was detectable as blue fluorescence around the fungal colony when observed under UV irradiation (Davis et al. 1987). Aflatoxin was extracted from the culture medium by soaking 10 mycelial plugs (0.6 mm diameter) overnight in 5 ml of chloroform. Extracts were spotted onto thin-layer chromatography (TLC) plates (K5 silica gel; Whatman, Clifton, NJ), and the plates were developed in ether/methanol/water (96:3:1). Aflatoxin B1 was visualized under UV and compared to an aflatoxin standard provided by Sigma.
Histochemical analysis:
Histochemical staining for GUS activity was performed as described by Jefferson et al. (1987). Fungal tissue was immersed into a histochemical substrate solution containing 1 mm 5-bromo-4-chloro-3-indoly β–d-glucuronic acid, 50 mm phosphate, pH 7.0, 10 mm EDTA, 0.1% Triton X-100, 0.1% sodium lauryl sarcosine, and 10 mm β–mercaptoethanol and incubated at 37°. GUS expression in fungal tissue resulted in the production of a blue product. Tissue from a non-transformed strain of A. flavus was used as a control.
Parasexual mating:
Diploids were formed between transformant T25, containing a single ectopic copy of aflR, and strain 86 by the method described previously (Papa 1973; Woloshuk et al. 1989). Stable diploids were prototrophs with green conidial heads. Haploidization of diploids was induced by the addition of 1 μg/ml of benomyl to the growth medium (Woloshuk et al. 1989). Both parental phenotypes were recovered from the resulting haploid sectors.
RESULTS
Identifying the extent of the deletion in strain 649:
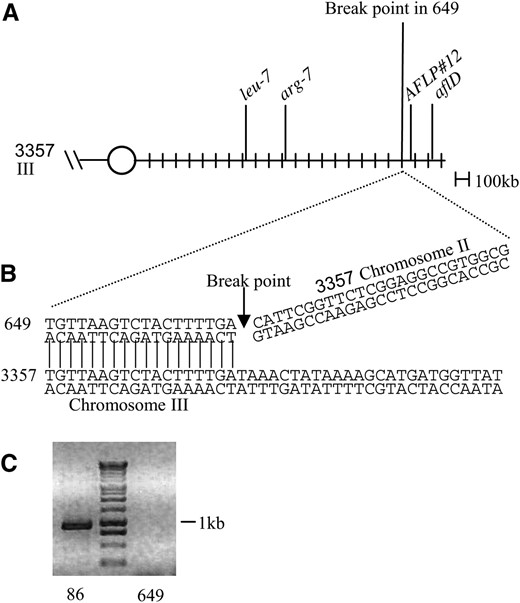
Utilizing the parasexual cycle in A. flavus, Papa mapped the relative position of several loci on linkage group VII, including the loci arg-7 and leu-7 as well as nor-1, afl-1, afl-15, and afl-17, which are defective in aflatoxin biosynthesis. We were able to locate three of the loci on linkage group VII to chromosome III using the newly available genome sequence of A. flavus (Figure 1A). The nor locus, which was identified because of a mutation in aflD (nor-1) within the aflatoxin gene cluster, is ∼101 kb away from the telomeric end of scaffold 1047283863273. This scaffold, together with scaffold 1047283863270, represents chromosome III. Cosmid 1911-A was shown to complement the arg-7 mutation in strain 796 (ATCC 60042) (Bennett and Papa 1988; Foutz et al. 1995). The genomic position of arg-7 was located on scaffold 1047283863273 ∼1 Mb away from the aflatoxin gene cluster. Cosmid 1812-B was found to complement the leu-7 mutation in strain 650 (ATCC 62633) (Papa 1979; Foutz et al. 1995) and was used as a probe on chromosome blots (Foutz et al. 1995; Woloshuk et al. 1995). The genomic position of leu-7 was located on scaffold 1047283863273 and is ∼1352 kb away from the aflatoxin gene cluster. The arg-7 and leu-7 gene order differs from Papa's findings (1984), but it is possible that rearrangements may have occurred in either NRRL 3357 (sequenced strain) or PC-7 (strain Papa worked with) to alter the gene order.
Identification and characterization of the deletion in strain 649. (A) Schematic of linkage group VII located on chromosome III in the wild-type strain NRRL 3357. (B) The exact break point is determined. A region of sequence obtained using Genome Walker is shown. Horizontal lines represent the region of 649 DNA that aligns with chromosome III in NRRL 3357. The arrow indicates where the 649 DNA no longer aligns with chromosome III and begins to align with chromosome II in NRRL 3357. (C) The region of DNA near the telomere of chromosome III has been deleted in strain 649. PCR primers AAU1F and AAU1R amplify a region that is ∼2 kb away from the end of the 1047283863273 scaffold. These primers amplify a 936-bp product from wild-type 86 genomic DNA. Ethidium bromide-stained agarose gel (1% w/v) is shown. The 1-kb ladder from Promega was used as a size standard.
Papa showed that the afl-1 locus in strain 649 was closely linked to the nor locus. Subsequent studies by Woloshuk et al. (1995) and Prieto et al. (1996) showed that strain 649 had a large deletion that included the entire aflatoxin biosynthetic gene cluster. Previously we attempted to locate the break in the genome of strain 649 by the amplified fragment length polymorphism technique, in which AFLP#12 was identified (5′ CAAATCGAATCAAATCCAACRGCCCTCAGAGTAATCGACGTAATCTGCAGCCMTCTCGAAAATCATTCGGATCGTGCCATATATTGCGCCAGTACTTTTC 3′) (M. Ramesh, G.-H. Huh and C. P. Woloshuk, unpublished data). This marker was located 83 kb upstream of the aflatoxin gene cluster and is also included in the deleted region of strain 649 (Figure 1A). Therefore the deletion break point must be between arg-7 and AFLP#12, which is a 917-kb region. To identify the break point, a series of PCR primers were designed using the available genomic sequence of strain 3357. Even though 649 is derived from PC-7 and not 3357, these primers were used to amplify segments within the 917-kb region between arg-7 and AFLP#12 from strain 649. The final round of PCR indicated that the break point was within a 3.3-kb region. To identify the exact break point, specific primers were designed within the 3.3-kb region to amplify a product from the Genome Walker libraries for both NRRL 3357 wild type and strain 649. Sequence analysis of the PCR product from the wild-type library indicated an alignment with scaffold 1047283863273; the point in which this alignment ends indicates the break point and the end of the deletion in strain 649 (Figure 1B). To determine if the deletion in strain 649 extended to the telomeric end of scaffold 1047283863273, PCR primers AAU1F and AAU1R were designed to amplify a region that is ∼2 kb away from the end of the scaffold. The primers amplified a product of predicted size from wild-type (NRRL 3357) genomic DNA (Figure 1C). No product was obtained with 649 genomic DNA, indicating that the deletion in strain 649 extends from the aflatoxin gene cluster to the telomere. On the basis of these analyses, the deletion in strain 649 appears to encompass a 317-kb region. Within this region there are a total of 114 predicted genes. A list of these deleted genes can be found in Table 2.
Deleted genes in strain 649
Protein ID . | TIGR gene prediction . |
|---|---|
| 2911.m00707 | Cytochrome P450 family protein |
| 2911.m00708 | Fungal-specific transcription factor domain containing protein |
| 2911.m00709 | glnA4-related |
| 2911.m00710 | Glutamine synthetase, catalytic domain containing protein |
| 2911.m00711 | Hypothetical protein |
| 2911.m00712 | Hypothetical protein |
| 2911.m00713 | Hypothetical protein |
| 2911.m00714 | Hypothetical protein |
| 2911.m00715 | Hypothetical protein |
| 2911.m00716 | Emopamil binding protein |
| 2911.m00717 | Alkaline serine protease AorO, putative |
| 2911.m00718 | G protein-coupled receptor α-related |
| 2911.m00719 | Cysteine-type peptidase, putative |
| 2911.m00720 | Enoyl-CoA hydratase/isomerase family protein |
| 2911.m00721 | Hypothetical protein |
| 2911.m00722 | Hypothetical protein |
| 2911.m00723 | Major facilitator superfamily protein |
| 2911.m00724 | Amino acid permease family protein |
| 2911.m00725 | Hypothetical protein |
| 2911.m00726 | Hypothetical protein |
| 2911.m00727 | MIP family channel proteins containing protein |
| 2911.m00728 | FAD-dependent oxidoreductase family protein |
| 2911.m00729 | Hypothetical protein |
| 2911.m00730 | Hypothetical protein |
| 2911.m00731 | Amino acid permease family protein |
| 2911.m00732 | AMP-binding enzyme family protein |
| 2911.m00733 | GMC oxidoreductase family protein |
| 2911.m00734 | Hydantoinase/oxoprolinase N-terminal region family protein |
| 2911.m00735 | Thioredoxin family protein |
| 2911.m00736 | Permease, cytosine/purines, uracil, thiamine, allantoin family protein |
| 2911.m00737 | C6 transcription factor, putative |
| 2911.m00738 | Sterigmatocystin biosynthesis peroxidase stcC precursor-related |
| 2911.m00739 | Hypothetical protein |
| 2911.m00740 | Hypothetical protein |
| 2911.m00741 | Hypothetical protein |
| 2911.m00742 | Hypothetical protein |
| 2911.m00743 | Hypothetical protein |
| 2911.m00744 | Sugar transporter, putative |
| 2911.m00745 | Glycosyl hydrolases family 16 protein |
| 2911.m00746 | Conserved hypothetical protein |
| 2911.m00747 | Protein kinase domain containing protein |
| 2911.m00748 | Hypothetical protein |
| 2911.m00749 | Major facilitator superfamily protein |
| 2911.m00750 | Metallo-beta-lactamase superfamily protein |
| 2911.m00751 | Flavin-binding monooxygenase-like family protein |
| 2911.m00752 | Fungal-specific transcription factor domain containing protein |
| 2911.m00753 | Flavin-binding monooxygenase-like family protein |
| 2911.m00754 | Oxidoreductase, zinc-binding dehydrogenase family protein |
| 2911.m00755 | Oxidoreductase, short chain dehydrogenase/reductase family protein |
| 2911.m00756 | Hypothetical protein |
| 2911.m00757 | Oxidoreductase, FAD/FMN-binding family protein |
| 2911.m00759 | Hypothetical protein |
| 2911.m00760 | Sterol 4-α-methyl-oxidase-related |
| 2911.m00761 | Hypothetical protein |
| 2911.m00762 | Major facilitator superfamily protein |
| 2911.m00763 | Hypothetical protein |
| 2911.m00764 | Hypothetical protein |
| 2911.m00765 | ATPase, AAA family protein |
| 2911.m00766 | Hypothetical protein |
| 2911.m00767 | ATPase, AAA family protein |
| 2911.m00768 | Major facilitator superfamily protein |
| 2911.m00769 | Ser/Thr protein phosphatase family protein |
| 2911.m00770 | Transcriptional regulator, putative |
| 2911.m00771 | α-glucosidase/α-amylase, putative |
| 2911.m00772 | Hexose transporter, putative |
| 2911.m00774 | Oxidoreductase, FAD/FMN-binding family protein |
| 2911.m00775 | aflY HypA, identical |
| 2911.m00776 | aflX toxin biosynthesis protein, putative |
| 2911.m00777 | aflW toxin biosynthesis monooxygenase MoxY-like, putative |
| 2911.m00778 | aflV aflatoxin biosynthesis cytochrome P450 monooxygenase CypX |
| 2911.m00779 | aflK aflatoxin biosynthesis versicolorin B synthase/GMC oxidoreductase Vbs |
| 2911.m00780 | Aflatoxin biosynthesis cytochrome P450 O-methylsterigmatocystin oxidoreductase aflQ OrdA |
| 2911.m00781 | aflP aflatoxin biosynthesis O-methyltransferase OmtA |
| 2911.m00782 | aflO aflatoxin biosynthesis O-methyltransferase OmtB |
| 2911.m00783 | aflI aflatoxin biosynthesis averufin dehydrogenase AvfA |
| 2911.m00784 | Hypothetical protein |
| 2911.m00785 | aflL aflatoxin biosynthesis P450 monooxygenase VerB |
| 2911.m00786 | aflG aflatoxin biosynthesis P450 monooxygenase AvnA |
| 2911.m00787 | Hypothetical protein |
| 2911.m00788 | aflN sterigmatocystin biosynthesis P450 monooxygenase VerA |
| 2911.m00789 | Aflatoxin biosyntheses short-chain alcohol dehydrogenase/versicolorin reductase aflM Ver1 |
| 2911.m00790 | aflE aflatoxin biosynthesis norsolorinic acid reductase NorA |
| 2911.m00791 | aflJ esterase, EstA |
| 2911.m00792 | aflH aflatoxin biosyntheses short-chain alcohol dehydrogenases AdhA |
| 2911.m00793 | aflS AflJ, identical |
| 2911.m00794 | aflR C6 transcription factor AFLR |
| 2911.m00795 | aflB aflatoxin biosynthesis hexanoate synthase/fatty acid synthase HexB |
| 2911.m00796 | aflA aflatoxin biosynthesis hexanoate synthase/fatty acid synthase HexA |
| 2911.m00797 | aflD aflatoxin biosynthesis norsolorinic acid ketoreductase Nor-1 |
| 2911.m00798 | Hypothetical protein |
| 2911.m00800 | aflC polyketide synthetase PksP, putative |
| 2911.m00801 | aflT aflatoxin MFS-type transporter AflT |
| 2911.m00804 | aflF aflatoxin biosynthesis oxidoreductase NorB |
| 2911.m00805 | Major facilitator superfamily protein |
| 2911.m00806 | Hypothetical protein |
| 2911.m00807 | Dimethylallyl tryptophan synthase, putative |
| 2911.m00808 | NAD-dependent epimerase/dehydratase family protein |
| 2911.m00809 | Fungal-specific transcription factor domain containing protein |
| 2911.m00810 | Hypothetical protein |
| 2911.m00811 | Serine protease prots related |
| 2911.m00812 | Amidohydrolase family protein |
| 2911.m00813 | Hypothetical protein |
| 2911.m00814 | Lignostilbene dioxygenase family protein |
| 2911.m00815 | Fungal-specific transcription factor domain containing protein |
| 2911.m00816 | Hypothetical protein |
| 2911.m00817 | Gluconolactone oxidase, putative |
| 2911.m00818 | Hypothetical protein |
| 2911.m00819 | Hypothetical protein |
| 2911.m00820 | Conserved hypothetical protein |
| 2911.m00821 | Amino acid permease family protein |
| 2911.m00822 | ABC multidrug transporter, putative |
| 2911.m00823 | Hypothetical protein |
| 2911.m00825 | Hypothetical protein |
| 2911.m00826 | Non-ribosomal peptide synthase, putative |
Protein ID . | TIGR gene prediction . |
|---|---|
| 2911.m00707 | Cytochrome P450 family protein |
| 2911.m00708 | Fungal-specific transcription factor domain containing protein |
| 2911.m00709 | glnA4-related |
| 2911.m00710 | Glutamine synthetase, catalytic domain containing protein |
| 2911.m00711 | Hypothetical protein |
| 2911.m00712 | Hypothetical protein |
| 2911.m00713 | Hypothetical protein |
| 2911.m00714 | Hypothetical protein |
| 2911.m00715 | Hypothetical protein |
| 2911.m00716 | Emopamil binding protein |
| 2911.m00717 | Alkaline serine protease AorO, putative |
| 2911.m00718 | G protein-coupled receptor α-related |
| 2911.m00719 | Cysteine-type peptidase, putative |
| 2911.m00720 | Enoyl-CoA hydratase/isomerase family protein |
| 2911.m00721 | Hypothetical protein |
| 2911.m00722 | Hypothetical protein |
| 2911.m00723 | Major facilitator superfamily protein |
| 2911.m00724 | Amino acid permease family protein |
| 2911.m00725 | Hypothetical protein |
| 2911.m00726 | Hypothetical protein |
| 2911.m00727 | MIP family channel proteins containing protein |
| 2911.m00728 | FAD-dependent oxidoreductase family protein |
| 2911.m00729 | Hypothetical protein |
| 2911.m00730 | Hypothetical protein |
| 2911.m00731 | Amino acid permease family protein |
| 2911.m00732 | AMP-binding enzyme family protein |
| 2911.m00733 | GMC oxidoreductase family protein |
| 2911.m00734 | Hydantoinase/oxoprolinase N-terminal region family protein |
| 2911.m00735 | Thioredoxin family protein |
| 2911.m00736 | Permease, cytosine/purines, uracil, thiamine, allantoin family protein |
| 2911.m00737 | C6 transcription factor, putative |
| 2911.m00738 | Sterigmatocystin biosynthesis peroxidase stcC precursor-related |
| 2911.m00739 | Hypothetical protein |
| 2911.m00740 | Hypothetical protein |
| 2911.m00741 | Hypothetical protein |
| 2911.m00742 | Hypothetical protein |
| 2911.m00743 | Hypothetical protein |
| 2911.m00744 | Sugar transporter, putative |
| 2911.m00745 | Glycosyl hydrolases family 16 protein |
| 2911.m00746 | Conserved hypothetical protein |
| 2911.m00747 | Protein kinase domain containing protein |
| 2911.m00748 | Hypothetical protein |
| 2911.m00749 | Major facilitator superfamily protein |
| 2911.m00750 | Metallo-beta-lactamase superfamily protein |
| 2911.m00751 | Flavin-binding monooxygenase-like family protein |
| 2911.m00752 | Fungal-specific transcription factor domain containing protein |
| 2911.m00753 | Flavin-binding monooxygenase-like family protein |
| 2911.m00754 | Oxidoreductase, zinc-binding dehydrogenase family protein |
| 2911.m00755 | Oxidoreductase, short chain dehydrogenase/reductase family protein |
| 2911.m00756 | Hypothetical protein |
| 2911.m00757 | Oxidoreductase, FAD/FMN-binding family protein |
| 2911.m00759 | Hypothetical protein |
| 2911.m00760 | Sterol 4-α-methyl-oxidase-related |
| 2911.m00761 | Hypothetical protein |
| 2911.m00762 | Major facilitator superfamily protein |
| 2911.m00763 | Hypothetical protein |
| 2911.m00764 | Hypothetical protein |
| 2911.m00765 | ATPase, AAA family protein |
| 2911.m00766 | Hypothetical protein |
| 2911.m00767 | ATPase, AAA family protein |
| 2911.m00768 | Major facilitator superfamily protein |
| 2911.m00769 | Ser/Thr protein phosphatase family protein |
| 2911.m00770 | Transcriptional regulator, putative |
| 2911.m00771 | α-glucosidase/α-amylase, putative |
| 2911.m00772 | Hexose transporter, putative |
| 2911.m00774 | Oxidoreductase, FAD/FMN-binding family protein |
| 2911.m00775 | aflY HypA, identical |
| 2911.m00776 | aflX toxin biosynthesis protein, putative |
| 2911.m00777 | aflW toxin biosynthesis monooxygenase MoxY-like, putative |
| 2911.m00778 | aflV aflatoxin biosynthesis cytochrome P450 monooxygenase CypX |
| 2911.m00779 | aflK aflatoxin biosynthesis versicolorin B synthase/GMC oxidoreductase Vbs |
| 2911.m00780 | Aflatoxin biosynthesis cytochrome P450 O-methylsterigmatocystin oxidoreductase aflQ OrdA |
| 2911.m00781 | aflP aflatoxin biosynthesis O-methyltransferase OmtA |
| 2911.m00782 | aflO aflatoxin biosynthesis O-methyltransferase OmtB |
| 2911.m00783 | aflI aflatoxin biosynthesis averufin dehydrogenase AvfA |
| 2911.m00784 | Hypothetical protein |
| 2911.m00785 | aflL aflatoxin biosynthesis P450 monooxygenase VerB |
| 2911.m00786 | aflG aflatoxin biosynthesis P450 monooxygenase AvnA |
| 2911.m00787 | Hypothetical protein |
| 2911.m00788 | aflN sterigmatocystin biosynthesis P450 monooxygenase VerA |
| 2911.m00789 | Aflatoxin biosyntheses short-chain alcohol dehydrogenase/versicolorin reductase aflM Ver1 |
| 2911.m00790 | aflE aflatoxin biosynthesis norsolorinic acid reductase NorA |
| 2911.m00791 | aflJ esterase, EstA |
| 2911.m00792 | aflH aflatoxin biosyntheses short-chain alcohol dehydrogenases AdhA |
| 2911.m00793 | aflS AflJ, identical |
| 2911.m00794 | aflR C6 transcription factor AFLR |
| 2911.m00795 | aflB aflatoxin biosynthesis hexanoate synthase/fatty acid synthase HexB |
| 2911.m00796 | aflA aflatoxin biosynthesis hexanoate synthase/fatty acid synthase HexA |
| 2911.m00797 | aflD aflatoxin biosynthesis norsolorinic acid ketoreductase Nor-1 |
| 2911.m00798 | Hypothetical protein |
| 2911.m00800 | aflC polyketide synthetase PksP, putative |
| 2911.m00801 | aflT aflatoxin MFS-type transporter AflT |
| 2911.m00804 | aflF aflatoxin biosynthesis oxidoreductase NorB |
| 2911.m00805 | Major facilitator superfamily protein |
| 2911.m00806 | Hypothetical protein |
| 2911.m00807 | Dimethylallyl tryptophan synthase, putative |
| 2911.m00808 | NAD-dependent epimerase/dehydratase family protein |
| 2911.m00809 | Fungal-specific transcription factor domain containing protein |
| 2911.m00810 | Hypothetical protein |
| 2911.m00811 | Serine protease prots related |
| 2911.m00812 | Amidohydrolase family protein |
| 2911.m00813 | Hypothetical protein |
| 2911.m00814 | Lignostilbene dioxygenase family protein |
| 2911.m00815 | Fungal-specific transcription factor domain containing protein |
| 2911.m00816 | Hypothetical protein |
| 2911.m00817 | Gluconolactone oxidase, putative |
| 2911.m00818 | Hypothetical protein |
| 2911.m00819 | Hypothetical protein |
| 2911.m00820 | Conserved hypothetical protein |
| 2911.m00821 | Amino acid permease family protein |
| 2911.m00822 | ABC multidrug transporter, putative |
| 2911.m00823 | Hypothetical protein |
| 2911.m00825 | Hypothetical protein |
| 2911.m00826 | Non-ribosomal peptide synthase, putative |
Deleted genes in strain 649
Protein ID . | TIGR gene prediction . |
|---|---|
| 2911.m00707 | Cytochrome P450 family protein |
| 2911.m00708 | Fungal-specific transcription factor domain containing protein |
| 2911.m00709 | glnA4-related |
| 2911.m00710 | Glutamine synthetase, catalytic domain containing protein |
| 2911.m00711 | Hypothetical protein |
| 2911.m00712 | Hypothetical protein |
| 2911.m00713 | Hypothetical protein |
| 2911.m00714 | Hypothetical protein |
| 2911.m00715 | Hypothetical protein |
| 2911.m00716 | Emopamil binding protein |
| 2911.m00717 | Alkaline serine protease AorO, putative |
| 2911.m00718 | G protein-coupled receptor α-related |
| 2911.m00719 | Cysteine-type peptidase, putative |
| 2911.m00720 | Enoyl-CoA hydratase/isomerase family protein |
| 2911.m00721 | Hypothetical protein |
| 2911.m00722 | Hypothetical protein |
| 2911.m00723 | Major facilitator superfamily protein |
| 2911.m00724 | Amino acid permease family protein |
| 2911.m00725 | Hypothetical protein |
| 2911.m00726 | Hypothetical protein |
| 2911.m00727 | MIP family channel proteins containing protein |
| 2911.m00728 | FAD-dependent oxidoreductase family protein |
| 2911.m00729 | Hypothetical protein |
| 2911.m00730 | Hypothetical protein |
| 2911.m00731 | Amino acid permease family protein |
| 2911.m00732 | AMP-binding enzyme family protein |
| 2911.m00733 | GMC oxidoreductase family protein |
| 2911.m00734 | Hydantoinase/oxoprolinase N-terminal region family protein |
| 2911.m00735 | Thioredoxin family protein |
| 2911.m00736 | Permease, cytosine/purines, uracil, thiamine, allantoin family protein |
| 2911.m00737 | C6 transcription factor, putative |
| 2911.m00738 | Sterigmatocystin biosynthesis peroxidase stcC precursor-related |
| 2911.m00739 | Hypothetical protein |
| 2911.m00740 | Hypothetical protein |
| 2911.m00741 | Hypothetical protein |
| 2911.m00742 | Hypothetical protein |
| 2911.m00743 | Hypothetical protein |
| 2911.m00744 | Sugar transporter, putative |
| 2911.m00745 | Glycosyl hydrolases family 16 protein |
| 2911.m00746 | Conserved hypothetical protein |
| 2911.m00747 | Protein kinase domain containing protein |
| 2911.m00748 | Hypothetical protein |
| 2911.m00749 | Major facilitator superfamily protein |
| 2911.m00750 | Metallo-beta-lactamase superfamily protein |
| 2911.m00751 | Flavin-binding monooxygenase-like family protein |
| 2911.m00752 | Fungal-specific transcription factor domain containing protein |
| 2911.m00753 | Flavin-binding monooxygenase-like family protein |
| 2911.m00754 | Oxidoreductase, zinc-binding dehydrogenase family protein |
| 2911.m00755 | Oxidoreductase, short chain dehydrogenase/reductase family protein |
| 2911.m00756 | Hypothetical protein |
| 2911.m00757 | Oxidoreductase, FAD/FMN-binding family protein |
| 2911.m00759 | Hypothetical protein |
| 2911.m00760 | Sterol 4-α-methyl-oxidase-related |
| 2911.m00761 | Hypothetical protein |
| 2911.m00762 | Major facilitator superfamily protein |
| 2911.m00763 | Hypothetical protein |
| 2911.m00764 | Hypothetical protein |
| 2911.m00765 | ATPase, AAA family protein |
| 2911.m00766 | Hypothetical protein |
| 2911.m00767 | ATPase, AAA family protein |
| 2911.m00768 | Major facilitator superfamily protein |
| 2911.m00769 | Ser/Thr protein phosphatase family protein |
| 2911.m00770 | Transcriptional regulator, putative |
| 2911.m00771 | α-glucosidase/α-amylase, putative |
| 2911.m00772 | Hexose transporter, putative |
| 2911.m00774 | Oxidoreductase, FAD/FMN-binding family protein |
| 2911.m00775 | aflY HypA, identical |
| 2911.m00776 | aflX toxin biosynthesis protein, putative |
| 2911.m00777 | aflW toxin biosynthesis monooxygenase MoxY-like, putative |
| 2911.m00778 | aflV aflatoxin biosynthesis cytochrome P450 monooxygenase CypX |
| 2911.m00779 | aflK aflatoxin biosynthesis versicolorin B synthase/GMC oxidoreductase Vbs |
| 2911.m00780 | Aflatoxin biosynthesis cytochrome P450 O-methylsterigmatocystin oxidoreductase aflQ OrdA |
| 2911.m00781 | aflP aflatoxin biosynthesis O-methyltransferase OmtA |
| 2911.m00782 | aflO aflatoxin biosynthesis O-methyltransferase OmtB |
| 2911.m00783 | aflI aflatoxin biosynthesis averufin dehydrogenase AvfA |
| 2911.m00784 | Hypothetical protein |
| 2911.m00785 | aflL aflatoxin biosynthesis P450 monooxygenase VerB |
| 2911.m00786 | aflG aflatoxin biosynthesis P450 monooxygenase AvnA |
| 2911.m00787 | Hypothetical protein |
| 2911.m00788 | aflN sterigmatocystin biosynthesis P450 monooxygenase VerA |
| 2911.m00789 | Aflatoxin biosyntheses short-chain alcohol dehydrogenase/versicolorin reductase aflM Ver1 |
| 2911.m00790 | aflE aflatoxin biosynthesis norsolorinic acid reductase NorA |
| 2911.m00791 | aflJ esterase, EstA |
| 2911.m00792 | aflH aflatoxin biosyntheses short-chain alcohol dehydrogenases AdhA |
| 2911.m00793 | aflS AflJ, identical |
| 2911.m00794 | aflR C6 transcription factor AFLR |
| 2911.m00795 | aflB aflatoxin biosynthesis hexanoate synthase/fatty acid synthase HexB |
| 2911.m00796 | aflA aflatoxin biosynthesis hexanoate synthase/fatty acid synthase HexA |
| 2911.m00797 | aflD aflatoxin biosynthesis norsolorinic acid ketoreductase Nor-1 |
| 2911.m00798 | Hypothetical protein |
| 2911.m00800 | aflC polyketide synthetase PksP, putative |
| 2911.m00801 | aflT aflatoxin MFS-type transporter AflT |
| 2911.m00804 | aflF aflatoxin biosynthesis oxidoreductase NorB |
| 2911.m00805 | Major facilitator superfamily protein |
| 2911.m00806 | Hypothetical protein |
| 2911.m00807 | Dimethylallyl tryptophan synthase, putative |
| 2911.m00808 | NAD-dependent epimerase/dehydratase family protein |
| 2911.m00809 | Fungal-specific transcription factor domain containing protein |
| 2911.m00810 | Hypothetical protein |
| 2911.m00811 | Serine protease prots related |
| 2911.m00812 | Amidohydrolase family protein |
| 2911.m00813 | Hypothetical protein |
| 2911.m00814 | Lignostilbene dioxygenase family protein |
| 2911.m00815 | Fungal-specific transcription factor domain containing protein |
| 2911.m00816 | Hypothetical protein |
| 2911.m00817 | Gluconolactone oxidase, putative |
| 2911.m00818 | Hypothetical protein |
| 2911.m00819 | Hypothetical protein |
| 2911.m00820 | Conserved hypothetical protein |
| 2911.m00821 | Amino acid permease family protein |
| 2911.m00822 | ABC multidrug transporter, putative |
| 2911.m00823 | Hypothetical protein |
| 2911.m00825 | Hypothetical protein |
| 2911.m00826 | Non-ribosomal peptide synthase, putative |
Protein ID . | TIGR gene prediction . |
|---|---|
| 2911.m00707 | Cytochrome P450 family protein |
| 2911.m00708 | Fungal-specific transcription factor domain containing protein |
| 2911.m00709 | glnA4-related |
| 2911.m00710 | Glutamine synthetase, catalytic domain containing protein |
| 2911.m00711 | Hypothetical protein |
| 2911.m00712 | Hypothetical protein |
| 2911.m00713 | Hypothetical protein |
| 2911.m00714 | Hypothetical protein |
| 2911.m00715 | Hypothetical protein |
| 2911.m00716 | Emopamil binding protein |
| 2911.m00717 | Alkaline serine protease AorO, putative |
| 2911.m00718 | G protein-coupled receptor α-related |
| 2911.m00719 | Cysteine-type peptidase, putative |
| 2911.m00720 | Enoyl-CoA hydratase/isomerase family protein |
| 2911.m00721 | Hypothetical protein |
| 2911.m00722 | Hypothetical protein |
| 2911.m00723 | Major facilitator superfamily protein |
| 2911.m00724 | Amino acid permease family protein |
| 2911.m00725 | Hypothetical protein |
| 2911.m00726 | Hypothetical protein |
| 2911.m00727 | MIP family channel proteins containing protein |
| 2911.m00728 | FAD-dependent oxidoreductase family protein |
| 2911.m00729 | Hypothetical protein |
| 2911.m00730 | Hypothetical protein |
| 2911.m00731 | Amino acid permease family protein |
| 2911.m00732 | AMP-binding enzyme family protein |
| 2911.m00733 | GMC oxidoreductase family protein |
| 2911.m00734 | Hydantoinase/oxoprolinase N-terminal region family protein |
| 2911.m00735 | Thioredoxin family protein |
| 2911.m00736 | Permease, cytosine/purines, uracil, thiamine, allantoin family protein |
| 2911.m00737 | C6 transcription factor, putative |
| 2911.m00738 | Sterigmatocystin biosynthesis peroxidase stcC precursor-related |
| 2911.m00739 | Hypothetical protein |
| 2911.m00740 | Hypothetical protein |
| 2911.m00741 | Hypothetical protein |
| 2911.m00742 | Hypothetical protein |
| 2911.m00743 | Hypothetical protein |
| 2911.m00744 | Sugar transporter, putative |
| 2911.m00745 | Glycosyl hydrolases family 16 protein |
| 2911.m00746 | Conserved hypothetical protein |
| 2911.m00747 | Protein kinase domain containing protein |
| 2911.m00748 | Hypothetical protein |
| 2911.m00749 | Major facilitator superfamily protein |
| 2911.m00750 | Metallo-beta-lactamase superfamily protein |
| 2911.m00751 | Flavin-binding monooxygenase-like family protein |
| 2911.m00752 | Fungal-specific transcription factor domain containing protein |
| 2911.m00753 | Flavin-binding monooxygenase-like family protein |
| 2911.m00754 | Oxidoreductase, zinc-binding dehydrogenase family protein |
| 2911.m00755 | Oxidoreductase, short chain dehydrogenase/reductase family protein |
| 2911.m00756 | Hypothetical protein |
| 2911.m00757 | Oxidoreductase, FAD/FMN-binding family protein |
| 2911.m00759 | Hypothetical protein |
| 2911.m00760 | Sterol 4-α-methyl-oxidase-related |
| 2911.m00761 | Hypothetical protein |
| 2911.m00762 | Major facilitator superfamily protein |
| 2911.m00763 | Hypothetical protein |
| 2911.m00764 | Hypothetical protein |
| 2911.m00765 | ATPase, AAA family protein |
| 2911.m00766 | Hypothetical protein |
| 2911.m00767 | ATPase, AAA family protein |
| 2911.m00768 | Major facilitator superfamily protein |
| 2911.m00769 | Ser/Thr protein phosphatase family protein |
| 2911.m00770 | Transcriptional regulator, putative |
| 2911.m00771 | α-glucosidase/α-amylase, putative |
| 2911.m00772 | Hexose transporter, putative |
| 2911.m00774 | Oxidoreductase, FAD/FMN-binding family protein |
| 2911.m00775 | aflY HypA, identical |
| 2911.m00776 | aflX toxin biosynthesis protein, putative |
| 2911.m00777 | aflW toxin biosynthesis monooxygenase MoxY-like, putative |
| 2911.m00778 | aflV aflatoxin biosynthesis cytochrome P450 monooxygenase CypX |
| 2911.m00779 | aflK aflatoxin biosynthesis versicolorin B synthase/GMC oxidoreductase Vbs |
| 2911.m00780 | Aflatoxin biosynthesis cytochrome P450 O-methylsterigmatocystin oxidoreductase aflQ OrdA |
| 2911.m00781 | aflP aflatoxin biosynthesis O-methyltransferase OmtA |
| 2911.m00782 | aflO aflatoxin biosynthesis O-methyltransferase OmtB |
| 2911.m00783 | aflI aflatoxin biosynthesis averufin dehydrogenase AvfA |
| 2911.m00784 | Hypothetical protein |
| 2911.m00785 | aflL aflatoxin biosynthesis P450 monooxygenase VerB |
| 2911.m00786 | aflG aflatoxin biosynthesis P450 monooxygenase AvnA |
| 2911.m00787 | Hypothetical protein |
| 2911.m00788 | aflN sterigmatocystin biosynthesis P450 monooxygenase VerA |
| 2911.m00789 | Aflatoxin biosyntheses short-chain alcohol dehydrogenase/versicolorin reductase aflM Ver1 |
| 2911.m00790 | aflE aflatoxin biosynthesis norsolorinic acid reductase NorA |
| 2911.m00791 | aflJ esterase, EstA |
| 2911.m00792 | aflH aflatoxin biosyntheses short-chain alcohol dehydrogenases AdhA |
| 2911.m00793 | aflS AflJ, identical |
| 2911.m00794 | aflR C6 transcription factor AFLR |
| 2911.m00795 | aflB aflatoxin biosynthesis hexanoate synthase/fatty acid synthase HexB |
| 2911.m00796 | aflA aflatoxin biosynthesis hexanoate synthase/fatty acid synthase HexA |
| 2911.m00797 | aflD aflatoxin biosynthesis norsolorinic acid ketoreductase Nor-1 |
| 2911.m00798 | Hypothetical protein |
| 2911.m00800 | aflC polyketide synthetase PksP, putative |
| 2911.m00801 | aflT aflatoxin MFS-type transporter AflT |
| 2911.m00804 | aflF aflatoxin biosynthesis oxidoreductase NorB |
| 2911.m00805 | Major facilitator superfamily protein |
| 2911.m00806 | Hypothetical protein |
| 2911.m00807 | Dimethylallyl tryptophan synthase, putative |
| 2911.m00808 | NAD-dependent epimerase/dehydratase family protein |
| 2911.m00809 | Fungal-specific transcription factor domain containing protein |
| 2911.m00810 | Hypothetical protein |
| 2911.m00811 | Serine protease prots related |
| 2911.m00812 | Amidohydrolase family protein |
| 2911.m00813 | Hypothetical protein |
| 2911.m00814 | Lignostilbene dioxygenase family protein |
| 2911.m00815 | Fungal-specific transcription factor domain containing protein |
| 2911.m00816 | Hypothetical protein |
| 2911.m00817 | Gluconolactone oxidase, putative |
| 2911.m00818 | Hypothetical protein |
| 2911.m00819 | Hypothetical protein |
| 2911.m00820 | Conserved hypothetical protein |
| 2911.m00821 | Amino acid permease family protein |
| 2911.m00822 | ABC multidrug transporter, putative |
| 2911.m00823 | Hypothetical protein |
| 2911.m00825 | Hypothetical protein |
| 2911.m00826 | Non-ribosomal peptide synthase, putative |
Strain 649 also contains an addition:
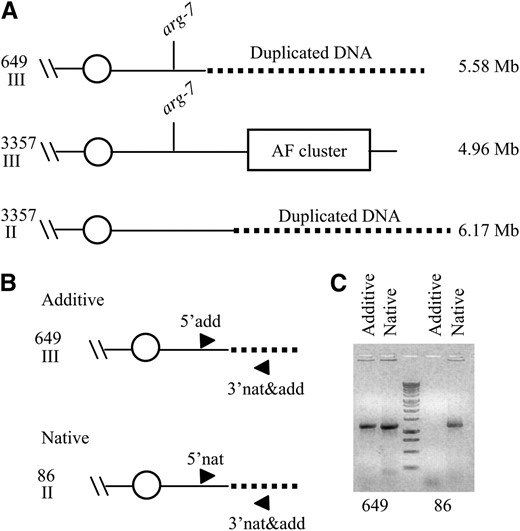
Previous studies showed that the wild-type A. flavus linkage group VII is located on a 4.9-Mb chromosome (Foutz et al. 1995). Strain 649 lacks a chromosome of this size, but contains instead a unique ∼6 Mb chromosome, which contains the leu-7 locus (Woloshuk et al. 1995). These data suggest that strain 649 also carries an addition of DNA as well as a deletion. These data were confirmed by the Genome Walker results, which identified a 672-bp region adjacent to the break point that did not align with scaffold 1047283863273 (Figure 1B). Blastn analysis with this sequence against A. flavus genomic DNA identified a region of alignment on scaffold 1047283863271, which is part of chromosome II. Since the deletion in 649 included the region adjacent to the telomere, it is probable that the telomere was lost during a rearrangement that created the mutation. To maintain structural stability, chromosomes require telomeres. It is therefore highly likely that the addition in strain 649 includes the sequence obtained from Genome Walker and extends to the telomeric end of chromosome II, represented in scaffold 1047283863271. This would result in an addition of 939 kb of DNA (Figure 2A), which agrees with previous reports regarding the chromosome sizes in strain 649 (Woloshuk et al. 1995). There are 320 predicted genes within the 939-kb region (supplemental Table S2 at http://www.genetics.org/supplemental/). To determine if the rearranged DNA in strain 649 was the result of a duplication or a translocation event, two sets of primers were designed (Figure 2B). Since both sets of primers amplified a product from 649 genomic DNA, it indicates that the additional DNA in strain 649 also resides in its native genomic location (Figure 2C). Therefore, the genome of strain 649 appears to have undergone a deletion of 317 kb of DNA from the end of chromosome III. The subsequent attachment of 939 kb of DNA, duplicated from chromosome II, resulted in a 5.58-Mb chromosome.
Identification and characterization of the addition in strain 649. (A) Schematic of deletion and addition in strain 649. The dotted line represents the DNA duplicated in 649. Chromosomes II and III from the wild-type strain NRRL 3357 are shown. Chromosome III in 649 contains a region from the wild-type chromosome II. Chromosome size is indicated on the right in Mb. (B) The rearrangement of DNA in strain 649 is the result of an addition and not a translocation. 5′add, 3′nad&add, and 5′nat are DNA oligos used to amplify the PCR products. The primers 5′add and 3′nat&add were designed to amplify a 1200-bp fragment spanning the chromosome II/chromosome III unique junction sequence in 649 to confirm the Genome Walker results. Primers 5′nat and 3′nat&add were designed to amplify a 1159-bp fragment from chromosome II in the wild-type strain, which contains a portion of the sequence obtained by Genome Walker and extends toward the centromere. This region of DNA would not be present if a translocation event had occurred. (C) Ethidium bromide-stained agarose gel (1% w/v) is shown. The 1-kb ladder from Promega was used as a size standard.
An ectopic copy of aflR in strain 649 pyr restores aflatoxin production in a T25 × 86 diploid:
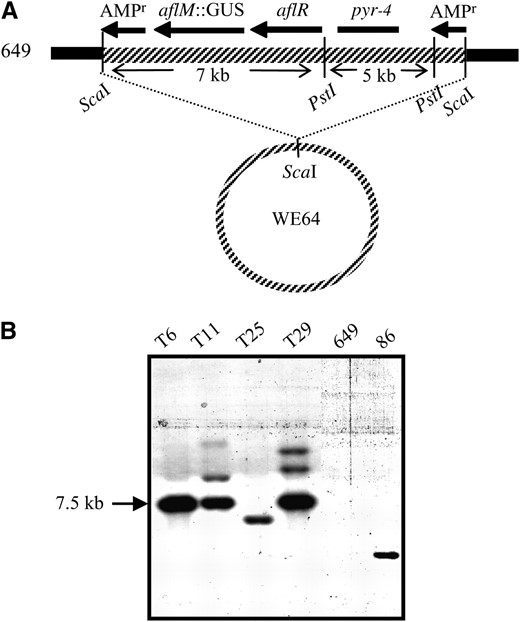
To further investigate the mechanism of repression of aflatoxin biosynthesis observed in diploids derived from strain 649 and aflatoxin-producing strains, we addressed the hypothesis that strain 649 produces a repressor of AflR activity. Previous studies indicated that expression of aflR is reduced in these diploids using Northern blot analysis. One hundred ten stable colonies were recovered after transforming strain 649 pyr with WE64, a vector containing aflR and a aflM:GUS reporter fusion (Figure 3A). Because AflR transcriptionally controls aflM, GUS will be expressed only in transformants in which aflR is transcribed and translated properly. All transformants were positive for GUS histochemical staining when grown on medium that supports aflatoxin production (data not shown). Dot-blots of genomic DNA from the transformants were probed with aflR to identify those containing low copy numbers of WE64 (data not shown). From the initial screen, four appeared to have low copies of aflR. Southern blot indicated that three of the transformants (T6, T11, and T29) had multiple tandem copies of aflR, whereas T25 contains a single-copy insertion as indicated by the 7-kb band that had similar intensity to the band in strain 86 (Figure 3B). Because it is single copy, T25 was used in further crosses.
Transformation of 649 pyr with a single copy of aflR. (A) WE64 carrying aflM∷GUS and aflR was linearized with ScaI within the ampicillin resistance gene and transformed into strain 649 pyr. (B) Southern blot of genomic DNA from transformants (T6, T11, T25, and T29), strain 649, and strain 86 probed with radiolabeled aflR. Transformants containing multiple copies in tandem resulted in a 7.5-kb PstI band of hybridization (T6, T11, and T29). Transformants containing more than one copy of WE64 at different locations in the genome produce multiple bands on the blot (T11 and T29). T25 contains a single copy of aflR and the intensity of the band is similar to the band produced with strain 86.
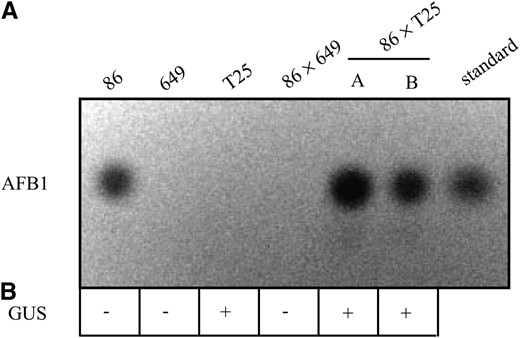
Two diploids were obtained from a parasexual cross between T25 and strain 86, designated T25 × 86A and T25 × 86B. Both diploids retained GUS activity indicating that the ectopically expressed AflR was still able to function in the diploid background (Figure 4B). Surprisingly, these diploids produced aflatoxin when grown on coconut medium (Figure 4A). A 649 × 86 diploid described previously (Woloshuk et al. 1995) did not produce aflatoxin under these same conditions. Together, these data demonstrate that strain 649 does not synthesize a repressor that interferes with the activity of AflR.
The effects of the dominant mutation in strain 649 are relieved when an ectopic copy of aflR is expressed. (A) Aflatoxin production is restored in 86 × T25 diploids. Chloroform extractions from transformant T25, strains 86 and 649, 86 × 649, and two independent diploids of 86 × T25 (A and B) grown on coconut medium separated by TLC. The dark circles indicate the presence of aflatoxin B1 when exposed to UV light. (B) GUS histochemical activity indicates a functional ectopic AflR. +, positive GUS staining; −, no detectable GUS staining.
The silencing phenomenon in 649 × 86 diploids does not affect genes outside of the aflatoxin gene cluster:
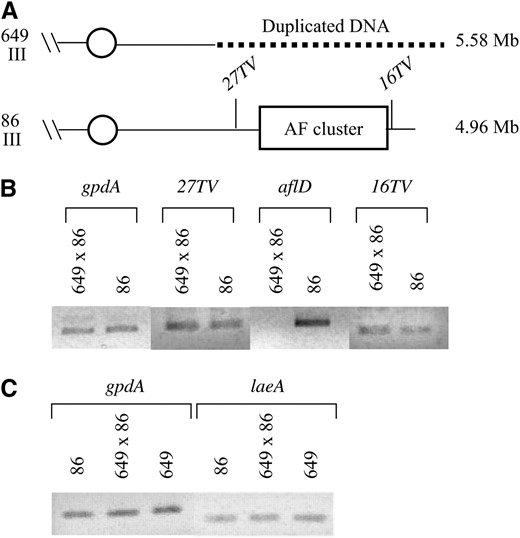
To determine if the other genes deleted in 649 were expressed in the 649 × 86 diploid, two genes outside of the aflatoxin gene cluster were selected for investigation (Figure 5A). Genes 27TV and 16TV were selected because they were expressed under aflatoxin-conducive conditions in a separate microarray study (data not shown); 27TV encodes an amino acid permease family protein and 16TV encodes a putative dimethylallyltryptophan synthase. The gene with homology to the A. nidulans glyceraldehyde 3-phosphate dehydrogenase (gpdA) was used as a positive control and the aflatoxin biosynthetic gene aflD was used as a negative control for gene expression in 649 × 86. A cDNA reaction (3 μl) was used for each PCR reaction with 30 cycles. To ensure complete removal of any genomic DNA contamination, cDNA reactions that did not contain reverse transcriptase were also used for PCR reactions (data not shown). Because expression of 27TV and 16TV was too low to see in our experiment, nested PCR primers were designed to be able to detect expression. The primary PCR reaction (1 μl) was used with the nested primers for an additional 10 cycles (Figure 5B). Genes gpdA, 27TV, and 16TV are expressed in both 86 and 649 × 86, while aflD is expressed only in the wild-type 86.
Genes that are outside of the aflatoxin gene cluster in the 649 deletion are not silenced in 649 × 86 diploids. (A) Gene locations in 649 and 86 chromosomes. 27TV and 16TV are located outside of the aflatoxin gene cluster; 27TV is located between the aflatoxin gene cluster and the break point and 16TV is located between the aflatoxin gene cluster and the telomere. (B) The gpdA gene was used as a positive control. aflD is a gene within the aflatoxin gene cluster and is silenced in the 649 × 86 diploid while genes outside of the aflatoxin gene cluster are expressed at equivalent levels in both the 649 × 86 diploid and the wild-type 86. (C) The gpdA gene was used as a positive control. laeA is expressed at equivalent levels in the 649 × 86 diploid and the two parental strains 649 and 86.
When laeA, a methyltransferase that may be involved in remodeling the chromatin state of several secondary metabolite clusters in Aspergillus species, is deleted there is a loss of sterigmatocystin production in A. nidulans (Bok and Keller 2004). With the addition of a single ectopic copy of aflR, sterigmatocystin is restored in the mutant background (Bok et al. 2006). Expression of laeA was tested in 86, 649, and 649 × 86. The gpdA gene was used as a positive control and 3 μl of a cDNA reaction was used for each PCR reaction with 30 cycles. laeA expression is not altered in 649 × 86 or in 649.
DISCUSSION
The aflatoxin biosynthetic pathway is well characterized and is a model for understanding the regulation of secondary metabolism in filamentous fungi. Our current understanding of the pathway and its regulation is largely the result of mutant complementation. K. E. Papa generated >23 mutants for aflatoxin biosynthesis and complementation of these mutants was used to identify biosynthetic as well as regulatory genes. Thus complementation and characterization of these mutants have aided our understanding of aflatoxin biosynthesis as well as secondary metabolism in general.
Our labs have focused on characterizing the afl-1 mutation. This mutation is of interest because it is the only dominant mutation for aflatoxin biosynthesis known. In an earlier study (Woloshuk et al. 1995), we showed that the entire aflatoxin gene cluster was absent in strain 649, which carries the afl-1 mutation. While this defect explains the inability of this strain to produce aflatoxin, it does not provide insight into the dominant effect observed in diploids. The focus of this study was to use newly available genomic sequence as well as genetic reporter constructs to better characterize this mutant. By comparing the mutant sequence to the wild-type sequence of the NRRL 3357 strain, we determined that 317 kb of chromosome III was deleted and replaced by 939 kb of chromosome II. This explained the null phenotype for aflatoxin production but did not explain why the aflatoxin production was still inhibited in diploids containing a wild-type gene cluster for aflatoxin.
One hypothesis for the dominant effect of afl-1 in diploids was that the genomic rearrangement in strain 649 created a novel or altered open reading frame (ORF) at the deletion/addition junction. This could lead to the production of a new repressor or the activation of an existing repressor of AflR. We showed that this hypothesis is unlikely for two reasons. First, examination of the genomic sequence of 649 around the break junction did not reveal sequence for a predicted repressor. Second, we ruled out the possibility of an AflR repressor by transforming strain 649 with an additional copy of aflR on a cassette containing the aflM∷GUS reporter. AflM is an aflatoxin pathway gene that is regulated by the binding of AflR to its promoter. The transformed strain expressed aflM∷GUS, indicating that AflR activity was not inhibited in the 649 genetic background. On the basis of these results, the hypothesis that a trans-sensing mechanism is responsible for the silencing of the aflatoxin gene cluster in 649 × 86 diploids is more likely.
Many forms of silencing exist in filamentous fungi. The best-characterized mechanism is repeat-induced point mutation (RIP), which has been studied in the model fungus N. crassa. RIP is a mechanism that causes C:G to T:A mutations within duplicated sequences during the sexual cycle (Selker 1990; Galagan et al. 2003). The mutations accumulate and become associated with DNA methylation, which results in the inactivation of the duplicated genes (Rountree and Selker 1997; Freitag et al. 2002). To date there have been no reports of the RIP mechanism in Aspergillus. Even if RIP were active in A. flavus the aflatoxin gene cluster could not be silenced by this mechanism because it is present only in one copy. Furthermore, the DNA alterations caused by RIP are irreversible and therefore are not likely to be responsible for the silencing seen in 649 × 86 diploids since the silencing phenotype can be reversed.
Likewise, silencing of the aflatoxin gene cluster cannot be easily explained by quelling. Also known as RNAi, quelling is a form of posttranscriptional gene silencing that operates in many species, including A. flavus (Pickford et al. 2002; McDonald et al. 2005). Often when too many copies of a gene are present in an organism posttranscriptional gene silencing is triggered. In quelling, small interfering RNAs (siRNAs) homologous to the duplicated genes are produced and all copies of the duplicated genes are silenced (Catalanotto et al. 2002). This mechanism could silence the duplicated genes, but it could not be responsible for silencing of the aflatoxin gene cluster, which is present only in one copy in the diploid.
Another form of silencing, originally known as transvection, was first discovered in fungi while observing the ascus-dominant mutation Asm-1− (Aramayo and Metzenberg 1996). Now referred to as meiotic silencing, this mechanism silences genes that are not paired with a homolog in prophase I of meiosis (Shiu et al. 2001). The genome is scanned by a trans-sensing mechanism that requires communication or interaction between chromosomal homologs to detect unpaired alleles. Once a misalignment is detected the meiotic silencing pathway is triggered and the unpaired alleles are silenced. Despite the lack of a sexual stage, chromosomes can still interact in diploids of A. flavus during mitosis as seen by crossover events during the parasexual cycle (Papa 1973). This provides evidence that the duplicated region from strain 649 and the aflatoxin cluster from wild-type strain 86 could physically interact in the somatic diploid on the basis of upon their relative location. However, if a similar mechanism was responsible for the silencing of the aflatoxin gene cluster in 649 × wild type diploids, then other genes in the 649 deletion should also be silenced in the diploids and this was not the case (Figure 5). Still it is possible that the aflatoxin gene cluster is regulated differently and is more sensitive to chromosomal misalignment.
The silencing of the wild-type aflatoxin gene cluster in the 649 × 86 diploid is a phenotype that can be reversed with the addition of an ectopic copy of aflR into strain 649 as seen in the T25 × 86 diploids. Thus, a single ectopic copy of the transcriptional regulator in the mutant strain prevented the dominant action of the afl-1 mutation in the diploid and the expression of the formerly silenced genes required for aflatoxin biosynthesis in a 649 × 86 diploid is restored. Bok et al. (2006) obtained similar results in a ΔlaeA strain of A. nidulans with the addition of a single ectopic copy of aflR. Thus our data support an altered regulation of the aflatoxin gene cluster upstream of aflR in a 649 × 86 diploid. However, laeA is expressed at similar levels in strains 86, 649, and 649 × 86, which suggests that the silencing mechanism in the diploid is not caused by a lack of laeA expression. It remains unclear as to why the large deletion and addition in strain 649 can cause such an effect. The importance of this silencing mechanism in natural populations of A. flavus is not known. It is clear, however, that large deletions in the aflatoxin gene cluster occur in native strains of fungus (Chang et al. 2005).
This study has successfully characterized the complete structure of the afl-1 mutation in strain 649, which has remained unresolved for a number of years. With a large deletion and duplication, 649 is a unique strain with a readily scorable phenotype that can provide information in several different areas. In addition to the aflatoxin gene cluster, 87 putative genes are missing, suggesting that 649 could be used for studies of a number of gene products. The restoration of aflatoxin production in the T25 × 86 diploid may allow us to further characterize the activity of the transcriptional regulator AflR and its ability to activate silenced genes. Future studies examining the silencing phenomenon observed in the 649 × 86 diploids may provide insight as to how the aflatoxin gene cluster is regulated and why it shows a unique silencing response compared to other deleted genes in 649.
Footnotes
Communicating editor: J. J. Loros
Acknowledgement
The authors thank Bethan Pritchard for her assistance with genomic analysis and Ignazzio Carbone for the use of the AAU1F and AAU1R primers. We also thank Marilee Ramesh, Gyung-Hye Huh, Joseph Flaherty, and Burt Bluhm for their assistance. Support for this project was provided in part by the United States Department of Agriculture/National Research Initiative Competitive Grants Program award no. 94-37201-1160 to C.P.W. and award no. 2002-35201-12562 to G.A.P. C.A.S. was supported by the United States Department of Agriculture/Initiative for Future Agriculture and Food Systems award no. 2001-52101-11507.
References
Aramayo, R., and R. L. Metzenberg,
Bennett, J. W., and M. Klich,
Bennett, J. W., and K. E. Papa,
Bok, J. W., and N. P. Keller,
Bok, J. W., D. Noordermeer, S. P. Kale and N. P. Keller,
Catalanotto, C., G. Azzalin, G. Macino and C. Cogoni,
Chang, P. K., C. D. Skory and J. E. Linz,
Chang, P. K., J. W. Cary, J. J. Yu, D. Bhatnagar and T. E. Cleveland,
Chang, P. K., J. J. Yu, K. C. Ehrlich, S. M. Boue, B. G. Montalbano et al.,
Chang, P. K., B. W. Horn and J. W. Dorner,
Davis, N. D., S. K. Iyer and U. L. Diener,
Fakhoury, A. M., and C. P. Woloshuk,
Flaherty, J. E., M. A. Weaver, G. A. Payne and C. P. Woloshuk,
Foutz, K. R., C. P. Woloshuk and G. A. Payne,
Freitag, M., R. L. Williams, G. O. Kothe and E. U. Selker,
Galagan, J. E., S. E. Calvo, K. A. Borkovich, E. U. Selker, N. D. Read et al.,
Jefferson, R. A., T. A. Kavanagh and M. W. Bevan,
Kelkar, H. S., T. W. Skloss, J. F. Haw, N. P. Keller and T. H. Adams,
Keller, N. P., G. Turner and J. W. Bennett,
Leaich, L. L., and K. E. Papa,
Mahanti, N., D. Bhatnagar, J. W. Cary, J. Joubran and J. E. Linz,
McDonald, T., D. Brown, N. P. Keller and T. M. Hammond,
McGuire, S. M., J. C. Silva, E. G. Casillas and C. A. Townsend,
Meyers, D. M., G. O Brian, W. L. Du, D. Bhatnagar and G. A. Payne,
Motomura, M., N. Chihaya, T. Shinozawa, T. Hamasaki and K. Yabe,
Papa, K. E.,
Papa, K. E.,
Papa, K. E.,
Papa, K. E.,
Papa, K. E.,
Payne, G. A., G. J. Nystrom, D. Bhatnagar, T. E. Cleveland and C. P. Woloshuk,
Payne, G. A., W. C. Nierman, J. R. Wortman, B. L. Pritchard, D. Brown et al.,
Pickford, A. S., C. Catalanotto, C. Cogoni and G. Macino,
Pontecorvo, G., and J. A. Roper,
Prieto, R., G. L. Yousibova and C. P. Woloshuk,
Rountree, M. R., and E. U. Selker,
Rozen, S., and H. J. Skaletsky,
Sambrook, J., and D. W. Russell,
Selker, E. U.,
Shiu, P. K. T., N. B. Raju, D. Zickler and R. L. Metzenberg,
Silva, J. C., and C. A. Townsend,
Silva, J. C., R. E. Minto, C. E. Barry, K. A. Holland and C. A. Townsend,
Skory, C. D., P. K. Chang, J. Cary and J. E. Linz,
Tartof, K. D., and S. Henikoff,
Woloshuk, C. P., E. R. Seip, G. A. Payne and C. R. Adkins,
Woloshuk, C. P., K. R. Foutz, J. F. Brewer, D. Bhatnagar, T. E. Cleveland et al.,
Woloshuk, C. P., G. L. Yousibova, J. A. Rollins, D. Bhatnagar and G. A. Payne,
Yu, J. H., and N. Keller,
Yu, J. J., J. W. Cary, D. Bhatnagar, T. E. Cleveland, N. P. Keller et al.,
Yu, J. J., P. K. Chang, J. W. Cary, D. Bhatnagar and T. E. Cleveland,
Yu, J. J., P. K. Chang, K. C. Ehrlich, J. W. Cary, B. Montalbano et al.,
Yu, J. J., C. P. Woloshuk, D. Bhatnagar and T. E. Cleveland,
Yu, J. J., P. K. Chang, D. Bhatnagar and T. E. Cleveland,
Yu, J. J., P. K. Chang, K. C. Ehrlich, J. W. Cary, D. Bhatnagar et al.,