-
PDF
- Split View
-
Views
-
Cite
Cite
Namboori B Raju, Robert L Metzenberg, Patrick K T Shiu, Neurospora Spore Killers Sk-2 and Sk-3 Suppress Meiotic Silencing by Unpaired DNA, Genetics, Volume 176, Issue 1, 1 May 2007, Pages 43–52, https://doi.org/10.1534/genetics.106.069161
Close - Share Icon Share
Abstract
In Neurospora crassa, pairing of homologous DNA segments is monitored during meiotic prophase I. Any genes not paired with a homolog, as well as any paired homologs of that gene, are silenced during the sexual phase by a mechanism known as meiotic silencing by unpaired DNA (MSUD). Two genes required for MSUD have been described previously: sad-1 (suppressor of ascus dominance), encoding an RNA-directed RNA polymerase, and sad-2, encoding a protein that controls the perinuclear localization of SAD-1. Inactivation of either sad-1 or sad-2 suppresses MSUD. We have now shown that MSUD is also suppressed by either of two Spore killer strains, Sk-2 and Sk-3. These were both known to contain a haplotype segment that behaves as a meiotic drive element in heterozygous crosses of killer × sensitive. Progeny ascospores not carrying the killer element fail to mature and are inviable. Crosses homozygous for either of the killer haplotypes suppress MSUD even though ascospores are not killed. The killer activity maps to the same 30-unit-long region within which recombination is suppressed in killer × sensitive crosses. We suggest that the region contains a suppressor of MSUD.
SEXUAL reproduction in Neurospora crassa is mediated by the fusion of two haploid nuclei that carry compatible mating-type genes (mat A and mat a). The haploid nuclei proliferate in specialized premeiotic ascogenous tissue (precroziers and three-celled croziers) within the fertilized fruiting body (perithecium). Two nuclei of opposite mating type that are sequestered in the subapical cell of the crozier then migrate into a tubular sac (ascus) and fuse to create the zygote, which immediately undergoes two meiotic divisions and a postmeiotic mitosis. The resulting eight haploid nuclei reside in the same cytoplasm until they are sequestered into eight linearly ordered ascospores. The mating-type locus and other segregating markers show 1:1 segregation (Raju 1992; Davis 2000).
Meiotic drive is a phenomenon in which certain chromosomal loci show a progeny ratio different from 1:1 and one allele is favored over the other. Examples of meiotic drive in animals include the segregation distorter (SD) system of Drosophila melanogaster and the t haplotype in the mouse (Lyttle 1991a,b; Schimenti 2000; Kusano et al. 2003). In heterozygous males, the meiotic products that do not carry the drive element (SD chromosome or t haplotype) fail to differentiate into functional sperm. Although meiotic drive is widespread in insects, mammals, plants, and fungi, very few cases have been dissected at the molecular level (Pennisi 2003; Burt and Trivers 2006).
In Neurospora, Spore killers are chromosomal elements that cause the death of ascospores that do not contain the killer element (Turner and Perkins 1979, 1991). For example, in a cross of Spore killer × wild type (Sk sensitive), every mature ascus contains four normal-sized, black, viable ascospores and four tiny, undeveloped ascospores that are inviable (see Figure 2A). All survivors carry the killer element. When both killer and sensitive nuclei are enclosed in the same ascospore (as occurs in the giant-spore N. crassa mutant Banana and in the four-spored species N. tetrasperma), the sensitive nuclei are sheltered (Raju 1979, 1994, 2002; Raju and Perkins 1991; van der Gaag et al. 2000). There is little or no ascospore death in homozygous killer × killer or sensitive × sensitive crosses. It was inferred that the killer has a resistance factor that protects not only its own nucleus from getting killed, but also other nonkiller nuclei in the same ascospore.
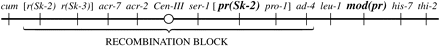
Three Spore killers have been studied extensively. Spore killer-1 (Sk-1) was found in N. sitophila, while Spore killer-2 (Sk-2) and Spore killer-3 (Sk-3) were found in N. intermedia (Turner and Perkins 1979). Sk-2 and Sk-3 have been introgressed into N. crassa for detailed genetic analysis. Both have been mapped to the same general region on linkage group (LG) III. When either one is heterozygous, crossing over is blocked in a 30-MU region that spans the centromere (Figure 1) (Campbell and Turner 1987; Turner 2003).
Loci conferring resistance to Spore killers have been found in wild populations (Turner and Perkins 1979, 1993; Campbell and Turner 1987). For example, the Spore-killer-resistant gene r(Sk-2) prevents killing of ascospores that do not contain the Sk-2 haplotype. The recombination block observed in a heterozygous Spore killer cross is not suppressed by the resistant allele, however. An allele conferring partial resistance to Sk-2 has also been reported (Turner 2003). pr(Sk-2) gives only partial resistance to Sk-2 killing when acting alone. However, when paired with a modifier allele, mod(pr), pr(Sk-2) confers a high level of resistance. In a wild-type cross (i.e., in the absence of a Spore killer), the resistant alleles do not affect crossover frequencies and can therefore be mapped precisely. Both r(Sk-2) and pr(Sk-2) are located within the recombination block region, while mod(pr) is located just outside of it (Figure 1).
We are interested in another seemingly unrelated ascus-dominant phenomenon called meiotic silencing by unpaired DNA (MSUD). If a copy of a gene is not properly paired with its homolog during prophase I, the MSUD mechanism creates a sequence-specific signal, which transiently silences all copies of that gene during meiosis and the subsequent mitosis (Shiu et al. 2001). For example, if an ectopic copy of the β-tubulin gene is inserted into one mating partner but not the other, all three copies (including those paired canonically) will be silenced. The resulting ascus is devoid of microtubules and will be arrested before metaphase I. Evidently, the presence of an unpaired copy of a gene is a red flag for mischief, which will alert the silencing system to destroy all transcripts similar to it (Shiu et al. 2001).
The MSUD mechanism suggests an explanation for the nature of many long-known ascus-dominant mutants, such as Round spore, Peak, and Banana. For example, the Round spore gene is formally categorized as ascus dominant because, in a heterozygous cross, all nuclei of the tetrad give rise to round ascospores; even those nuclei that contain a wild-type allele are round. The cause of dominance is probably rearrangements and/or deletions that prevent the wild-type allele from pairing with a homolog. MSUD is implicated in these ascus-dominant phenotypes of these mutants by virtue of the fact that a suppressor of meiotic silencing can eliminate the dominance. Although any gene can be subject to the action of MSUD, only those that encode a meiotic function will give an abnormal sexual phenotype. Genes that are responsible for nonmeiosis-specific products (such as amino acids) can be silenced but may not show a mutant sexual phenotype.
MSUD is likely to involve the production of double-stranded (ds)RNA and homologous mRNA degradation, since an RNA-directed RNA polymerase (RdRP), as encoded by sad-1 (“suppressor of ascus dominance”), is involved (Shiu and Metzenberg 2002). RdRP is known to be important in other RNA interference (RNAi) and post-transcriptional silencing systems (Ahlquist 2002). For example, quelling, an RNA-silencing mechanism that targets hyperhaploid transgenes during the vegetative phase of N. crassa, requires an RdRP (QDE-1) (Cogoni and Macino 1999). Other genes involved in the meiotic silencing process include sad-2, which encodes a novel protein that controls the perinuclear localization of SAD-1 (Shiu et al. 2006), and sms-2, which encodes an argonaute-like protein (Lee et al. 2003). We report here that the Spore killer strains Sk-2 and Sk-3 contain a previously unidentified element that suppresses MSUD. Both Sk-2 and Sk-3, like Sad-1Δ and Sad-2RIP mutants, allow the meiotic gene expression of many unpaired loci that otherwise would result in silencing by MSUD. The mechanism of suppression by the Spore killers, however, is different from those of Sad-1 and Sad-2.
MATERIALS AND METHODS
Strains and crosses:
Genotypes of the Neurospora strains used in this study are listed in Table 1. Individual genes are described in Perkins et al. (2001) or RADFORD'S e-Compendium (http://www.bioinf.leeds.ac.uk/∼gen6ar/newgenelist/genes/gene_list.htm). Various crosses between Spore killer strains and strains containing ectopically inserted genes (e.g., ∷act+, ∷asm-1+) and two dominant mutants (Dip-1, Round spore) are shown in Table 2. Although multiple strains in both mating types were often used for various crosses, only a single representative cross of each mutant with wild type, with Sad, and with Sk is shown in Table 2. Culturing, crossing media, and genetic techniques were utilized as described in Davis and De Serres (1970), and Davis (2000).
Strains used in this study and their genotypes
Strain name or symbola . | Genotype . | Strain nos.b . |
|---|---|---|
| ORS a | Oak Ridge wild-type mat a | FGSC 2490 |
| OR23-1V A | Oak Ridge wild-type mat A | FGSC 2489 |
| Houma-1 a | Wild-type mat a | FGSC 2221 |
| fluffy(OR) A | fl mat A | FGSC 4317 |
| fluffy(RL) a | fl mat a | FGSC 6683 |
| ∷act+A | mep his-3+∷act+; pan-2 mat A | 95-20 |
| ∷act+ a | mep his-3+∷act+; pan-2 mat a | 95-21 |
| ∷asm-1+ A | mep his-3+∷asm-1+; pan-2 mat A | 01-39, 80-21, 80-22 |
| ∷asm-1+ a | mep his-3+∷asm-1+; pan-2 mat a | 95-07 |
| ∷BmlR A (β-tubulin gene) | mep his-3+∷BmlR; pan-2 mat A | 95-24 |
| ∷BmlR a (β-tubulin gene) | mep his-3+∷BmlR; pan-2 mat a | 95-25 |
| ∷Bml+-GFP A | rid his-3+∷Bml+-GFP mat A | FGSC 9520, N2527 |
| ∷Bml+-GFP a | rid his-3+∷Bml+-GFP mat a | N2505 |
| Diploid ascospores-1 A | Dip-1 mat A | 24-40, 84-02 |
| Diploid ascospores-1 a | Dip-1 mat a | 20-39, 25-39 |
| ∷hH1-GFP A | his-3+∷hH1-GFP mat A | FGSC 9518 |
| ∷hH1-GFP a | rid his-3+∷hH1-GFP mat a | FGSC 9517 |
| ∷hH3hH4-1 A | mep his-3+∷hH3hH4-1+; pan-2 mat A | 95-22 |
| Δ mat (mating-type deletion) | fl; nic-3; Δ mat | 92-40 |
| mat A(IL → VR) | Δ mat; am∷mat A | 89-27 |
| Dp(VIIR → IL)5936 A | fl; Dp(VIIR → IL)5936 mat A | 18-13 |
| ∷mei-3+ A | mep his-3+∷mei-3+; pan-2 mat A | 15-39 |
| ∷mei-3+ a | mep his-3+∷mei-3+; pan-2 mat a | 15-30 |
| Round spore A | R; fl; inl mat A | 92-38 |
| Round spore a | R; fl; inl mat a | 92-39 |
| Sad-1Δ A | Sad-1Δ mat A | 96-02, 48-25 |
| Sad-1Δ a | Sad-1Δ mat a | 96-01 |
| Sad-2RIP A | Sad-2RIP mat A | 30-03 |
| Sad-2RIP a | Sad-2RIP mat a | 30-04, 33-12 |
| Sk-2 A | Sk-2 mat A | 30-38, 60-38, FGSC 6648 |
| Sk-2 a | Sk-2 mat a | FGSC 6647, DDP 3120 |
| Sk-2;∷hH1-GFP a | Sk-2; his-3+∷hH1-GFP mat a | 24-37 |
| Resistant to Sk- 2 a | r(Sk-2) acr-7 mat a | 88-01 |
| Partially resistant to Sk-2 a | pr(Sk-2) mod(pr) mat a | 97-40 |
| Sk-3 A | Sk-3 mat A | 24-16, FGSC 7076 |
| Sk-3 a | Sk-3 acr-2 mat a | FGSC 7077 |
| Sk-3;∷hH1-GFP a | Sk-3; his-3+∷hH1-GFP mat a | 46-02 |
Strain name or symbola . | Genotype . | Strain nos.b . |
|---|---|---|
| ORS a | Oak Ridge wild-type mat a | FGSC 2490 |
| OR23-1V A | Oak Ridge wild-type mat A | FGSC 2489 |
| Houma-1 a | Wild-type mat a | FGSC 2221 |
| fluffy(OR) A | fl mat A | FGSC 4317 |
| fluffy(RL) a | fl mat a | FGSC 6683 |
| ∷act+A | mep his-3+∷act+; pan-2 mat A | 95-20 |
| ∷act+ a | mep his-3+∷act+; pan-2 mat a | 95-21 |
| ∷asm-1+ A | mep his-3+∷asm-1+; pan-2 mat A | 01-39, 80-21, 80-22 |
| ∷asm-1+ a | mep his-3+∷asm-1+; pan-2 mat a | 95-07 |
| ∷BmlR A (β-tubulin gene) | mep his-3+∷BmlR; pan-2 mat A | 95-24 |
| ∷BmlR a (β-tubulin gene) | mep his-3+∷BmlR; pan-2 mat a | 95-25 |
| ∷Bml+-GFP A | rid his-3+∷Bml+-GFP mat A | FGSC 9520, N2527 |
| ∷Bml+-GFP a | rid his-3+∷Bml+-GFP mat a | N2505 |
| Diploid ascospores-1 A | Dip-1 mat A | 24-40, 84-02 |
| Diploid ascospores-1 a | Dip-1 mat a | 20-39, 25-39 |
| ∷hH1-GFP A | his-3+∷hH1-GFP mat A | FGSC 9518 |
| ∷hH1-GFP a | rid his-3+∷hH1-GFP mat a | FGSC 9517 |
| ∷hH3hH4-1 A | mep his-3+∷hH3hH4-1+; pan-2 mat A | 95-22 |
| Δ mat (mating-type deletion) | fl; nic-3; Δ mat | 92-40 |
| mat A(IL → VR) | Δ mat; am∷mat A | 89-27 |
| Dp(VIIR → IL)5936 A | fl; Dp(VIIR → IL)5936 mat A | 18-13 |
| ∷mei-3+ A | mep his-3+∷mei-3+; pan-2 mat A | 15-39 |
| ∷mei-3+ a | mep his-3+∷mei-3+; pan-2 mat a | 15-30 |
| Round spore A | R; fl; inl mat A | 92-38 |
| Round spore a | R; fl; inl mat a | 92-39 |
| Sad-1Δ A | Sad-1Δ mat A | 96-02, 48-25 |
| Sad-1Δ a | Sad-1Δ mat a | 96-01 |
| Sad-2RIP A | Sad-2RIP mat A | 30-03 |
| Sad-2RIP a | Sad-2RIP mat a | 30-04, 33-12 |
| Sk-2 A | Sk-2 mat A | 30-38, 60-38, FGSC 6648 |
| Sk-2 a | Sk-2 mat a | FGSC 6647, DDP 3120 |
| Sk-2;∷hH1-GFP a | Sk-2; his-3+∷hH1-GFP mat a | 24-37 |
| Resistant to Sk- 2 a | r(Sk-2) acr-7 mat a | 88-01 |
| Partially resistant to Sk-2 a | pr(Sk-2) mod(pr) mat a | 97-40 |
| Sk-3 A | Sk-3 mat A | 24-16, FGSC 7076 |
| Sk-3 a | Sk-3 acr-2 mat a | FGSC 7077 |
| Sk-3;∷hH1-GFP a | Sk-3; his-3+∷hH1-GFP mat a | 46-02 |
“∷” indicates that an extra copy of a wild-type gene is inserted ectopically at the his-3 locus on LG IR or at the am locus on LG VR.
The four-digit strain numbers (e.g., 95-20, 95-21) are from R. L. Metzenberg's collection. N2505 and N2527 are from M. Freitag, Oregon State University, Corvallis. The remaining strains are from either the Fungal Genetics Stock Center (FGSC) or the Perkins collection (DDP 3120) at Stanford University.
Strains used in this study and their genotypes
Strain name or symbola . | Genotype . | Strain nos.b . |
|---|---|---|
| ORS a | Oak Ridge wild-type mat a | FGSC 2490 |
| OR23-1V A | Oak Ridge wild-type mat A | FGSC 2489 |
| Houma-1 a | Wild-type mat a | FGSC 2221 |
| fluffy(OR) A | fl mat A | FGSC 4317 |
| fluffy(RL) a | fl mat a | FGSC 6683 |
| ∷act+A | mep his-3+∷act+; pan-2 mat A | 95-20 |
| ∷act+ a | mep his-3+∷act+; pan-2 mat a | 95-21 |
| ∷asm-1+ A | mep his-3+∷asm-1+; pan-2 mat A | 01-39, 80-21, 80-22 |
| ∷asm-1+ a | mep his-3+∷asm-1+; pan-2 mat a | 95-07 |
| ∷BmlR A (β-tubulin gene) | mep his-3+∷BmlR; pan-2 mat A | 95-24 |
| ∷BmlR a (β-tubulin gene) | mep his-3+∷BmlR; pan-2 mat a | 95-25 |
| ∷Bml+-GFP A | rid his-3+∷Bml+-GFP mat A | FGSC 9520, N2527 |
| ∷Bml+-GFP a | rid his-3+∷Bml+-GFP mat a | N2505 |
| Diploid ascospores-1 A | Dip-1 mat A | 24-40, 84-02 |
| Diploid ascospores-1 a | Dip-1 mat a | 20-39, 25-39 |
| ∷hH1-GFP A | his-3+∷hH1-GFP mat A | FGSC 9518 |
| ∷hH1-GFP a | rid his-3+∷hH1-GFP mat a | FGSC 9517 |
| ∷hH3hH4-1 A | mep his-3+∷hH3hH4-1+; pan-2 mat A | 95-22 |
| Δ mat (mating-type deletion) | fl; nic-3; Δ mat | 92-40 |
| mat A(IL → VR) | Δ mat; am∷mat A | 89-27 |
| Dp(VIIR → IL)5936 A | fl; Dp(VIIR → IL)5936 mat A | 18-13 |
| ∷mei-3+ A | mep his-3+∷mei-3+; pan-2 mat A | 15-39 |
| ∷mei-3+ a | mep his-3+∷mei-3+; pan-2 mat a | 15-30 |
| Round spore A | R; fl; inl mat A | 92-38 |
| Round spore a | R; fl; inl mat a | 92-39 |
| Sad-1Δ A | Sad-1Δ mat A | 96-02, 48-25 |
| Sad-1Δ a | Sad-1Δ mat a | 96-01 |
| Sad-2RIP A | Sad-2RIP mat A | 30-03 |
| Sad-2RIP a | Sad-2RIP mat a | 30-04, 33-12 |
| Sk-2 A | Sk-2 mat A | 30-38, 60-38, FGSC 6648 |
| Sk-2 a | Sk-2 mat a | FGSC 6647, DDP 3120 |
| Sk-2;∷hH1-GFP a | Sk-2; his-3+∷hH1-GFP mat a | 24-37 |
| Resistant to Sk- 2 a | r(Sk-2) acr-7 mat a | 88-01 |
| Partially resistant to Sk-2 a | pr(Sk-2) mod(pr) mat a | 97-40 |
| Sk-3 A | Sk-3 mat A | 24-16, FGSC 7076 |
| Sk-3 a | Sk-3 acr-2 mat a | FGSC 7077 |
| Sk-3;∷hH1-GFP a | Sk-3; his-3+∷hH1-GFP mat a | 46-02 |
Strain name or symbola . | Genotype . | Strain nos.b . |
|---|---|---|
| ORS a | Oak Ridge wild-type mat a | FGSC 2490 |
| OR23-1V A | Oak Ridge wild-type mat A | FGSC 2489 |
| Houma-1 a | Wild-type mat a | FGSC 2221 |
| fluffy(OR) A | fl mat A | FGSC 4317 |
| fluffy(RL) a | fl mat a | FGSC 6683 |
| ∷act+A | mep his-3+∷act+; pan-2 mat A | 95-20 |
| ∷act+ a | mep his-3+∷act+; pan-2 mat a | 95-21 |
| ∷asm-1+ A | mep his-3+∷asm-1+; pan-2 mat A | 01-39, 80-21, 80-22 |
| ∷asm-1+ a | mep his-3+∷asm-1+; pan-2 mat a | 95-07 |
| ∷BmlR A (β-tubulin gene) | mep his-3+∷BmlR; pan-2 mat A | 95-24 |
| ∷BmlR a (β-tubulin gene) | mep his-3+∷BmlR; pan-2 mat a | 95-25 |
| ∷Bml+-GFP A | rid his-3+∷Bml+-GFP mat A | FGSC 9520, N2527 |
| ∷Bml+-GFP a | rid his-3+∷Bml+-GFP mat a | N2505 |
| Diploid ascospores-1 A | Dip-1 mat A | 24-40, 84-02 |
| Diploid ascospores-1 a | Dip-1 mat a | 20-39, 25-39 |
| ∷hH1-GFP A | his-3+∷hH1-GFP mat A | FGSC 9518 |
| ∷hH1-GFP a | rid his-3+∷hH1-GFP mat a | FGSC 9517 |
| ∷hH3hH4-1 A | mep his-3+∷hH3hH4-1+; pan-2 mat A | 95-22 |
| Δ mat (mating-type deletion) | fl; nic-3; Δ mat | 92-40 |
| mat A(IL → VR) | Δ mat; am∷mat A | 89-27 |
| Dp(VIIR → IL)5936 A | fl; Dp(VIIR → IL)5936 mat A | 18-13 |
| ∷mei-3+ A | mep his-3+∷mei-3+; pan-2 mat A | 15-39 |
| ∷mei-3+ a | mep his-3+∷mei-3+; pan-2 mat a | 15-30 |
| Round spore A | R; fl; inl mat A | 92-38 |
| Round spore a | R; fl; inl mat a | 92-39 |
| Sad-1Δ A | Sad-1Δ mat A | 96-02, 48-25 |
| Sad-1Δ a | Sad-1Δ mat a | 96-01 |
| Sad-2RIP A | Sad-2RIP mat A | 30-03 |
| Sad-2RIP a | Sad-2RIP mat a | 30-04, 33-12 |
| Sk-2 A | Sk-2 mat A | 30-38, 60-38, FGSC 6648 |
| Sk-2 a | Sk-2 mat a | FGSC 6647, DDP 3120 |
| Sk-2;∷hH1-GFP a | Sk-2; his-3+∷hH1-GFP mat a | 24-37 |
| Resistant to Sk- 2 a | r(Sk-2) acr-7 mat a | 88-01 |
| Partially resistant to Sk-2 a | pr(Sk-2) mod(pr) mat a | 97-40 |
| Sk-3 A | Sk-3 mat A | 24-16, FGSC 7076 |
| Sk-3 a | Sk-3 acr-2 mat a | FGSC 7077 |
| Sk-3;∷hH1-GFP a | Sk-3; his-3+∷hH1-GFP mat a | 46-02 |
“∷” indicates that an extra copy of a wild-type gene is inserted ectopically at the his-3 locus on LG IR or at the am locus on LG VR.
The four-digit strain numbers (e.g., 95-20, 95-21) are from R. L. Metzenberg's collection. N2505 and N2527 are from M. Freitag, Oregon State University, Corvallis. The remaining strains are from either the Fungal Genetics Stock Center (FGSC) or the Perkins collection (DDP 3120) at Stanford University.
Suppression of meiotic silencing of unpaired loci by Sad-1/Sad-2, Sk-2, and Sk-3
. | Parent 2 . | |||||
|---|---|---|---|---|---|---|
| Parent 1a . | × Wild type . | × Sad-1Δ or Sad-2RIP . | × Spore killer-2 . | × Spore killer-3 . | × r(Sk-2) . | × pr(Sk-2) mod(pr) . |
| ∷act+ | Silenced, lollipop asci arrested in meiosis I | Not silenced, normal asci | Not silenced, 4:4 asci | Partially silenced, 4:4 asci | Silenced (no suppression) | Silenced (no suppression) |
| ∷asm-1+ | Silenced, mostly white ascospores | Not silenced, 8-spored asci | Partially silenced, 50% 4:4 asci | Partially silenced, 25% 4:4 asci | Silenced | Silenced |
| ∷BmlR (mutant β-tubulin) | Silenced, all asci blocked in meiosis I | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷Bml+-GFP | Silenced, asci blocked in meiosis I | Not silenced, 8-spored asci | Not silenced, 4:4 asci for fluorescence and Sk | Not silenced, 4:4 asci for fluorescence and Sk | ND | ND |
| Dip-1 (Diploid ascospores) | Silenced, asci mostly 2-4 spored | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Silenced, asci mostly 2-4 spored | Silenced | Silenced |
| Dp(VIIR → IL)5936 | Silenced, barren perithecia | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷hH1-GFP | Silenced, no fluorescent nuclei in meiosis | Not silenced, all nuclei fluoresce | Not silenced, nuclei fluoresce, 4:4 asci | Not silenced, nuclei fluoresce, 4:4 asci | ND | ND |
| ∷hH3hH4-1+ | Silenced, asci showed meiotic defects | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| mat A(IL → VR) | Silenced, reduced fertility | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷mei-3+ | Silenced, meiotic defects, aborted asci | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Partially silenced, meiotic defects | Silenced | Silenced |
| Round spore | Silenced, round ascospores | Not silenced, normal ascospores | Silenced, round ascospores, 4:4 asci | Silenced, round ascospores, 4:4 asci | Silenced | Silenced |
| Sk-2 (Spore killer-2) | 4K:4S ascospores in all asci | 4K:4S ascospores in all asci | No killing, all 8 ascospores viable | All ascospores killed (mutual killing) | Partial killing, mostly 8:0, some 4:4 asci | Partial resistance, mostly 4:4 asci |
| Sk-3 (Spore killer-3) | 4K:4S ascospores in all asci | 4K:4S ascospores in all asci | All ascospores killed (mutual killing) | No killing, all 8 ascospores viable | ND | ND |
. | Parent 2 . | |||||
|---|---|---|---|---|---|---|
| Parent 1a . | × Wild type . | × Sad-1Δ or Sad-2RIP . | × Spore killer-2 . | × Spore killer-3 . | × r(Sk-2) . | × pr(Sk-2) mod(pr) . |
| ∷act+ | Silenced, lollipop asci arrested in meiosis I | Not silenced, normal asci | Not silenced, 4:4 asci | Partially silenced, 4:4 asci | Silenced (no suppression) | Silenced (no suppression) |
| ∷asm-1+ | Silenced, mostly white ascospores | Not silenced, 8-spored asci | Partially silenced, 50% 4:4 asci | Partially silenced, 25% 4:4 asci | Silenced | Silenced |
| ∷BmlR (mutant β-tubulin) | Silenced, all asci blocked in meiosis I | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷Bml+-GFP | Silenced, asci blocked in meiosis I | Not silenced, 8-spored asci | Not silenced, 4:4 asci for fluorescence and Sk | Not silenced, 4:4 asci for fluorescence and Sk | ND | ND |
| Dip-1 (Diploid ascospores) | Silenced, asci mostly 2-4 spored | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Silenced, asci mostly 2-4 spored | Silenced | Silenced |
| Dp(VIIR → IL)5936 | Silenced, barren perithecia | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷hH1-GFP | Silenced, no fluorescent nuclei in meiosis | Not silenced, all nuclei fluoresce | Not silenced, nuclei fluoresce, 4:4 asci | Not silenced, nuclei fluoresce, 4:4 asci | ND | ND |
| ∷hH3hH4-1+ | Silenced, asci showed meiotic defects | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| mat A(IL → VR) | Silenced, reduced fertility | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷mei-3+ | Silenced, meiotic defects, aborted asci | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Partially silenced, meiotic defects | Silenced | Silenced |
| Round spore | Silenced, round ascospores | Not silenced, normal ascospores | Silenced, round ascospores, 4:4 asci | Silenced, round ascospores, 4:4 asci | Silenced | Silenced |
| Sk-2 (Spore killer-2) | 4K:4S ascospores in all asci | 4K:4S ascospores in all asci | No killing, all 8 ascospores viable | All ascospores killed (mutual killing) | Partial killing, mostly 8:0, some 4:4 asci | Partial resistance, mostly 4:4 asci |
| Sk-3 (Spore killer-3) | 4K:4S ascospores in all asci | 4K:4S ascospores in all asci | All ascospores killed (mutual killing) | No killing, all 8 ascospores viable | ND | ND |
“Silenced” indicates that the mutation or the inserted gene failed to function during ascus development because of meiotic silencing; “Not silenced” indicates that the mutation or the inserted gene functioned normally because of suppression of meiotic silencing of unpaired loci by Sad-1Δ/Sad-2RIP, Sk-2, or Sk-3; “ND” (no data) indicates that the cross was not examined.
“∷” indicates that an extra copy of a wild-type gene is inserted ectopically at the his-3 locus on LG IR or at the am locus on LG VR.
Suppression of meiotic silencing of unpaired loci by Sad-1/Sad-2, Sk-2, and Sk-3
. | Parent 2 . | |||||
|---|---|---|---|---|---|---|
| Parent 1a . | × Wild type . | × Sad-1Δ or Sad-2RIP . | × Spore killer-2 . | × Spore killer-3 . | × r(Sk-2) . | × pr(Sk-2) mod(pr) . |
| ∷act+ | Silenced, lollipop asci arrested in meiosis I | Not silenced, normal asci | Not silenced, 4:4 asci | Partially silenced, 4:4 asci | Silenced (no suppression) | Silenced (no suppression) |
| ∷asm-1+ | Silenced, mostly white ascospores | Not silenced, 8-spored asci | Partially silenced, 50% 4:4 asci | Partially silenced, 25% 4:4 asci | Silenced | Silenced |
| ∷BmlR (mutant β-tubulin) | Silenced, all asci blocked in meiosis I | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷Bml+-GFP | Silenced, asci blocked in meiosis I | Not silenced, 8-spored asci | Not silenced, 4:4 asci for fluorescence and Sk | Not silenced, 4:4 asci for fluorescence and Sk | ND | ND |
| Dip-1 (Diploid ascospores) | Silenced, asci mostly 2-4 spored | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Silenced, asci mostly 2-4 spored | Silenced | Silenced |
| Dp(VIIR → IL)5936 | Silenced, barren perithecia | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷hH1-GFP | Silenced, no fluorescent nuclei in meiosis | Not silenced, all nuclei fluoresce | Not silenced, nuclei fluoresce, 4:4 asci | Not silenced, nuclei fluoresce, 4:4 asci | ND | ND |
| ∷hH3hH4-1+ | Silenced, asci showed meiotic defects | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| mat A(IL → VR) | Silenced, reduced fertility | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷mei-3+ | Silenced, meiotic defects, aborted asci | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Partially silenced, meiotic defects | Silenced | Silenced |
| Round spore | Silenced, round ascospores | Not silenced, normal ascospores | Silenced, round ascospores, 4:4 asci | Silenced, round ascospores, 4:4 asci | Silenced | Silenced |
| Sk-2 (Spore killer-2) | 4K:4S ascospores in all asci | 4K:4S ascospores in all asci | No killing, all 8 ascospores viable | All ascospores killed (mutual killing) | Partial killing, mostly 8:0, some 4:4 asci | Partial resistance, mostly 4:4 asci |
| Sk-3 (Spore killer-3) | 4K:4S ascospores in all asci | 4K:4S ascospores in all asci | All ascospores killed (mutual killing) | No killing, all 8 ascospores viable | ND | ND |
. | Parent 2 . | |||||
|---|---|---|---|---|---|---|
| Parent 1a . | × Wild type . | × Sad-1Δ or Sad-2RIP . | × Spore killer-2 . | × Spore killer-3 . | × r(Sk-2) . | × pr(Sk-2) mod(pr) . |
| ∷act+ | Silenced, lollipop asci arrested in meiosis I | Not silenced, normal asci | Not silenced, 4:4 asci | Partially silenced, 4:4 asci | Silenced (no suppression) | Silenced (no suppression) |
| ∷asm-1+ | Silenced, mostly white ascospores | Not silenced, 8-spored asci | Partially silenced, 50% 4:4 asci | Partially silenced, 25% 4:4 asci | Silenced | Silenced |
| ∷BmlR (mutant β-tubulin) | Silenced, all asci blocked in meiosis I | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷Bml+-GFP | Silenced, asci blocked in meiosis I | Not silenced, 8-spored asci | Not silenced, 4:4 asci for fluorescence and Sk | Not silenced, 4:4 asci for fluorescence and Sk | ND | ND |
| Dip-1 (Diploid ascospores) | Silenced, asci mostly 2-4 spored | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Silenced, asci mostly 2-4 spored | Silenced | Silenced |
| Dp(VIIR → IL)5936 | Silenced, barren perithecia | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷hH1-GFP | Silenced, no fluorescent nuclei in meiosis | Not silenced, all nuclei fluoresce | Not silenced, nuclei fluoresce, 4:4 asci | Not silenced, nuclei fluoresce, 4:4 asci | ND | ND |
| ∷hH3hH4-1+ | Silenced, asci showed meiotic defects | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| mat A(IL → VR) | Silenced, reduced fertility | Not silenced | Not silenced, 4:4 asci | Not silenced, 4:4 asci | Silenced | Silenced |
| ∷mei-3+ | Silenced, meiotic defects, aborted asci | Not silenced, 8-spored asci | Not silenced, 4:4 asci | Partially silenced, meiotic defects | Silenced | Silenced |
| Round spore | Silenced, round ascospores | Not silenced, normal ascospores | Silenced, round ascospores, 4:4 asci | Silenced, round ascospores, 4:4 asci | Silenced | Silenced |
| Sk-2 (Spore killer-2) | 4K:4S ascospores in all asci | 4K:4S ascospores in all asci | No killing, all 8 ascospores viable | All ascospores killed (mutual killing) | Partial killing, mostly 8:0, some 4:4 asci | Partial resistance, mostly 4:4 asci |
| Sk-3 (Spore killer-3) | 4K:4S ascospores in all asci | 4K:4S ascospores in all asci | All ascospores killed (mutual killing) | No killing, all 8 ascospores viable | ND | ND |
“Silenced” indicates that the mutation or the inserted gene failed to function during ascus development because of meiotic silencing; “Not silenced” indicates that the mutation or the inserted gene functioned normally because of suppression of meiotic silencing of unpaired loci by Sad-1Δ/Sad-2RIP, Sk-2, or Sk-3; “ND” (no data) indicates that the cross was not examined.
“∷” indicates that an extra copy of a wild-type gene is inserted ectopically at the his-3 locus on LG IR or at the am locus on LG VR.
Generally each of these duplication strains was crossed with a wild-type strain and with Sk-2 and Sk-3. Many of the same duplication strains had previously been examined in homozygous and heterozygous crosses and in crosses with Sad-1Δ and Sad-2RIP (Shiu et al. 2001, 2006; Shiu and Metzenberg 2002).
For scoring fertility, crosses were made by simultaneous inoculation of the two mating types at opposite sides of petri plates containing crossing medium. Ejected ascospores were examined on the petri plate lid 3 weeks after simultaneous inoculation of the parents.
Cytological methods:
Crosses for examining ascus development were made in petri plates. The protoperithecial parent was grown for 5 days (25°) and then fertilized by spreading conidia of the male parent. The scoring of fertility of each cross was initially based on ejected ascospores. Perithecia were opened for detailed observations on developing asci and for photographing rosettes of asci. Four to 6-day-old perithecia containing asci at all stages of meiosis and ascospore delimitation were stained using iron–hematoxylin or acriflavin (Raju and Newmeyer 1977; Raju 1986). Rosettes of maturing asci from 7- to 10-day-old perithecia were lightly stained using 10- to 20-fold dilutions of ferric acetate and hematoxylin solutions. Developing asci from crosses of GFP-tagged strains were examined using an epifluorescence microscope (Freitag et al. 2004). Each perithecium typically contains 200–400 asci. Over a thousand asci were scored for each cross.
RESULTS
Characteristics of Sk-2 and Sk-3:
The map locations of Sk-2 and Sk-3 and several linked genes in linkage group III are shown in Figure 1. The behavior of Sk-2 and Sk-3 in heterozygous crosses with wild type and in homozygous crosses (Sk × Sk) is shown in Figure 2A and described in the Introduction.
Partial map of linkage group III showing the recombination block (RB) in crosses heterozygous for Sk-2 or Sk-3. The RB contains the locus or loci responsible for spore killing, but more precise mapping of Sk is impossible because the Spore killer element does not recombine with loci in the RB. Intervals in the diagram are not proportional to genetic map distances. (The figure is from Turner 2003 with permission of the author.)
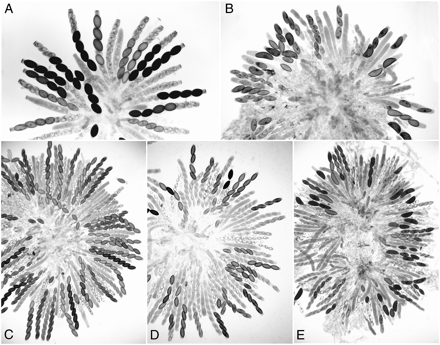
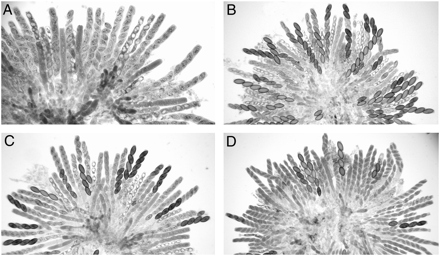
Rosettes of maturing asci showing the phenotype of Dip-1 and its suppression by Sk-2 and Sad-1Δ 8 days after fertilization. (A) Sk-2 × wild type. Maturing asci show four normal (larger) spores and four small aborted spores. All the mature ascospores carry the Sk-2 killer. The first-division segregation (4K:4S) in all asci indicates that there is no crossing over between Sk-2 and the centromere (from Raju 1979). (B) Dip-1 × wild type. Asci showing two to four abnormally large ascospores because of MSUD. (C) Dip-1 × Sad-1Δ. A rosette of asci, all showing eight normal-size ascospores. The Dip-1 phenotype is completely suppressed by Sad-1Δ. (D) Dip-1 × Sk-2. A rosette of eight-spored asci, where the Dip-1 phenotype is completely suppressed by Sk-2. The asci show the characteristic 4:4 Sk phenotype. (E) Dip-1 × Sk-3. Asci showing mostly large ascospores. The infrequent eight-spored asci show a 4:4 Sk phenotype. The Dip-1 phenotype is not suppressed by Sk-3.
Sk-2 suppresses the ascus-dominant mutant Diploid ascospores:
In crosses of Diploid ascospores (Dip-1) × wild type, asci frequently contain two to four large ascospores instead of the normal eight (Figure 2B). Dip-1 is thus ascus dominant. The large ascospores are expected to be either heterozygous diploids or heterokaryons, and upon germination they produce one or more heterokaryons. A cross between Sad-1Δ and Dip-1 yields mostly normal eight-spored asci (Figure 2C). Expression of Dip-1, which presumably contains a deletion or a chromosome rearrangement, is suppressed by Sad-1Δ.
When a cross is heterozygous for Spore killer, the Sk-sensitive nuclei are sheltered from killing in a giant ascospore when the ascospore also contains one or more killer nuclei (Raju 1979, 2002; Raju and Perkins 1991). While we were gathering evidence that Dip-1 indeed causes diploidy or heterozygosity, we crossed Dip-1 with Sk-2 to determine whether the spore killer death is expressed in the large-spored asci. We predicted that the sensitive chromosome would be protected because it would be in the same large ascospore as the killer chromosome; i.e., we expected to observe four black ascospores in most asci. Surprisingly, the cross produced almost entirely eight-spored asci, and these showed 4:4 ascospore patterns because of spore killing (Figure 2D). The eight-spored condition resembles that in a cross of Dip-1 × Sad-1Δ. This suggests that Sk-2 might contain a dominant suppressor of meiotic silencing, and it prompted us to test the behavior of Sk-2 and Sk-3 in crosses with several loci previously shown to experience meiotic silencing (Shiu et al. 2001). Sk-2 suppressed the Dip-1 phenotype but Sk-3 did not; the asci from Dip-1 × Sk-3 resembled those in Dip-1 × wild type (Figure 2E).
Sk-2 and Sk-3 suppress the meiotic silencing of several unpaired loci:
Strains having a gene-sized duplication due to the insertion of an extra copy at an ectopic location (∷act+, ∷asm-1+, ∷mei-3+, ∷BmlR, and ∷hH3/hH4-1), a gene relocated to an ectopic site [mat A(IL → VR)], or a deletion allele, as is believed to be the case for Round spore and Dip-1, will each have unpaired DNA that will be silenced throughout meiosis when crossed to a wild-type strain. The result is a dramatic reduction of ascospore production and/or a mutant ascus phenotype. The mutant phenotypes, for example, include ascospore maturation defects (∷asm-1+), swollen lollipop asci (∷act+), and round ascospores (Round spore). To determine whether Sk-2 and Sk-3 carry a dominant suppressor of meiotic silencing, we made crosses heterozygous for unpaired MSUD-sensitive loci containing Sk-2 and Sk-3, respectively.
Cytological observations on the suppression of meiotic silencing by Sk-2 and Sk-3 are given here for several partial duplication strains that contain an ectopically inserted gene at the his-3 locus on linkage group I.
actin:
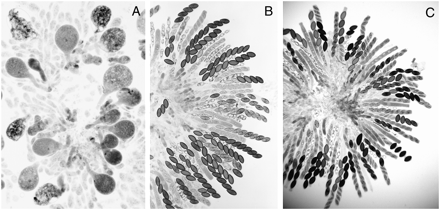
Partial duplication strains for the wild-type actin (act+) gene grew normally in the vegetative phase, but they showed dramatic deleterious effects in the sexual phase. In crosses of wild type × ∷act+, >90% of young asci became abnormally swollen like lollipops (Figure 3A). These asci, which usually contained a single nucleus, were arrested in meiotic prophase and subsequently degenerated. Apparently, both the unpaired ∷act+ and its native paired homologs were silenced during meiosis. The remaining 5–10% of asci that were less severely swollen progressed through meiosis and a postmeiotic mitosis. These often delimited one or two large ascospores and less frequently up to eight normal-sized ascospores (Shiu et al. 2001). In contrast, crosses of ∷act+ × Sad-1Δ and ∷act+ × Sk-2 showed completely normal ascus development through the stages of ascospore delimitation and formation of linearly ordered ascospores (Figure 3, B and C). The maturing asci of ∷act+ × Sk-2 contained four viable black ascospores and four inviable unpigmented ascospores (4K:4S) because of the Spore killer. Thus Sad-1Δ and Sk-2 completely suppressed the meiotic silencing of the unpaired ∷act+ gene.
Rosettes of asci showing the consequences of the meiotic silencing of actin and the suppression of silencing by Sad-1Δ and Sk-2. (A) ∷act+ × wild type 6 days after fertiliztion. Abnormally swollen asci resulting from silencing of actin genes. Karyogamy is normal in the swollen asci but almost all asci abort and degenerate without completing meiosis I. (B) ∷act+ × Sad-1Δ 8 days after fertilization. Meiosis and postmeiotic mitoses are completely normal; the silencing of actin is suppressed by Sad-1Δ. (C) ∷act+ × Sk-2 8 days after fertilization. As in the case of Sad-1Δ, the meiotic silencing of actin is suppressed by Sk-2. The 4:4 ascospore patterns result from the death of the Sk-sensitive ascospores. The consequence of the suppression of MSUD by Sk-2 is identical to that of Sad-1Δ, except that there is no ascospore death in the Sad-1Δ cross. Sk-3 also suppresses the MSUD of actin but to a lesser degree than with Sk-2.
Sk-3 also suppressed the meiotic silencing of ∷act+, but to a lesser degree. Eight-spored asci were produced, but many young asci were slightly swollen, suggesting that actin levels were less than normal. Nevertheless, the asci progressed through meiosis, postmeiotic mitoses, and ascospore development.
β-tubulin:
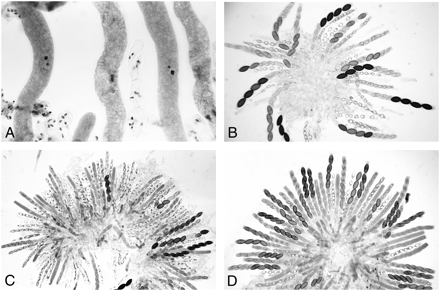
As with ∷act+, the mycelia of duplication strains grew normally if they carried an ectopic ∷BmlR gene [a mutant β-tubulin (Bml) gene, which confers resistance to benomyl]. However, in crosses of ∷BmlR × wild type, asci became elongated but failed to undergo meiosis or delimit ascospores. The asci were arrested and contained a single, condensed nucleus, and no division spindle was formed (Figure 4A). Apparently, the β-tubulin genes were meiotically silenced when an unpaired copy was present in the cross (Shiu et al. 2001). In Sk-2 × ∷BmlR, meiotic silencing of Bml was completely suppressed by Sk-2: asci developed normally and produced 4:4 ascospore patterns for Sk (Figure 4B). Sk-3 also suppressed the meiotic silencing of Bml. However, nuclear divisions were delayed in up to 25% of asci. The tardy asci produced fewer than eight ascospores and also showed defects in ascospore maturation.
Abnormal ascus development resulting from the meiotic silencing of Bml and mei-3 genes and the suppression of silencing by Sk-2. (A) ∷BmlR × wild type 5 days after fertilization. The β-tubulin genes are silenced, and ascus development is arrested prior to metaphase I. (B) ∷BmlR × Sk-2 8 days after fertilization. Meiotic silencing of β-tubulin is suppressed by Sk-2, and asci continue to develop through ascospore maturation. The maturing asci show Sk-caused 4:4 ascospore abortion patterns. (C) ∷mei-3+ × wild type 8 days after fertilization. A rosette of mostly aborted asci; <5–10% of asci contain mature ascospores. Nuclear divisions do occur in the aborted asci, albeit abnormally, and most of the asci delimit eight ascospores, which become vacuolated and degenerate. (D) ∷mei-3+ × Sk-2 7 days after fertilization. Meiotic silencing is completely suppressed by Sk-2, and the ascus development through spore delimitation is normal. The maturing asci show 4:4 patterns for Sk.
meiotic-3, an ortholog of recA and RAD51:
Heterozygous crosses of ∷mei-3+ with wild type resulted in the meiotic silencing of the meiotic-3 (mei-3) genes, as expected. Asci showed perturbations during meiosis and almost all asci aborted at or shortly after spore delimitation; <5% of asci contained any maturing ascospores (Figure 4C). In Sk-2 × ∷mei-3+, ascus development was fully restored, and all maturing asci showed normal 4:4 ascospore patterns for Sk (Figure 4D). Thus, the suppression of meiotic silencing of mei-3 genes by Sk-2 is complete. Sk-3 also suppressed the silencing of mei-3, but only partially. In Sk-3 × ∷mei-3+, asci underwent meiosis and a postmeiotic mitosis, and they delimited ascospores. However, ascospore development was abnormal and all eight ascospores were aborted in ∼50% of the asci. The remaining asci contained only one to four maturing ascospores.
asm-1 (APSES transcription factor):
A mutant of the ascospore maturation-1 gene (Asm-1Δ) showed dominant effects in crosses with a wild type, where all eight ascospores failed to mature in >95% of asci (Figure 5A; Aramayo and Metzenberg 1996). This result was shown to be due to MSUD, and the abnormality could be suppressed by Sad-1Δ, restoring near-normal fertility (Figure 5B; Shiu et al. 2001). We have tested∷asm-1+ in crosses with Sk-2 and Sk-3. In Sk-2 × ∷asm-1+, ascus development was completely normal through meiosis and a postmeiotic mitosis. Ascospore delimitation and the first mitosis in young ascospores were also normal. In ∼50% of asci, further development of all eight ascospores was arrested soon after the first mitosis, just as in wild type × ∷asm-1+(or Asm-1Δ). The two nuclei in the arrested ascospores were much condensed. In the remaining 50% of asci, up to four ascospores developed to full size and maturity (Figure 5C). In Sk-3 × ∷asm-1+, early ascus development was the same as that in Sk-2 × ∷asm-1+, except that <20% of asci showed any maturing ascospores (Figure 5D). Thus Sk-2 suppresses MSUD of ∷asm-1+ more efficiently than does Sk-3.
Rosettes of asci showing the consequences of the meiotic silencing of asm-1, and the suppression of meiotic silencing by Sad-1Δ, Sk-2, and Sk-3 8 days after fertilization. (A) ∷asm-1+ × wild type. Early ascus development is normal through spore delimitation and the first mitosis in young ascospores. All eight ascospores are subsequently arrested and fail to mature (in >95% of asci) because of the meiotic silencing of asm-1 genes. (B) ∷asm-1+ × Sad-1Δ. The meiotic silencing of asm-1 is suppressed by Sad-1Δ and the ascospores develop and mature in the majority of asci. (C) ∷asm-1+ × Sk-2. A rosette of maturing asci at 8 days. Meiotic silencing is suppressed by Sk-2 in ∼50% of asci. These asci show normal ascospore development (4:4 ascospore abortion patterns). (D) ∷asm-1+ × Sk-3. A rosette of maturing asci at 8 days. Suppression of asm-1 by Sk-3 occurs in <25% of asci. These minority asci show 4:4 ascospore abortion patterns, and there is no suppression in the majority of asci, where all eight ascospores are aborted, just as in ∷asm-1+ × wild type.
hH3hH4-1+, mat A, and Dp(VIIR → IL)5936:
Three additional strains, ∷hH3hH4-1+ (ectopic insert of histone H3/H4-1), mat A(IL → VR) (relocation of mating-type A), and Dp(VIIR → IL)5936 (a long segmental duplication of VIIR inserted into IL), were tested. Crosses of these three strains to wild type, Sad-1Δ, Sad-2RIP, Sk-2, and Sk-3 were scored for fertility and for the numbers of black ascospores ejected; they were not examined cytologically, however. In crosses with wild types, all three strains produced very few ascospores (silenced). Sad-1Δ, Sad-2RIP, Sk-2, and Sk-3 restored the fertility of all three strains to near-normal levels (i.e., silencing was suppressed) (Table 2).
Use of GFP-tagged histone H1 and β-tubulin gene inserts for visualizing the suppression of MSUD by Sk-2 and Sk-3:
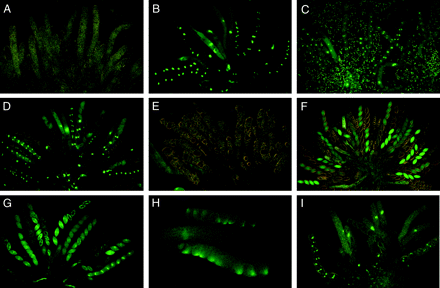
In wild type × ∷hH1-GFP, an unpaired copy of an ectopically integrated histone H1-GFP gene and its homologs were silenced throughout meiosis and until after ascospore delimitation (Figure 6A); the silenced hH1-GFP was expressed in the developing ascospores ∼20–24 hr after spore delimitation (Freitag et al. 2004). In contrast, in a cross of Sad-2RIP (or Sad-1Δ) × ∷hH1-GFP, the hH1-GFP is expressed throughout meiosis owing to the suppression of MSUD. As a result, the nuclei fluoresced throughout meiosis, a postmeiotic mitosis, and the early development of the young ascospores (Figure 6B). To determine whether Sk-2 and Sk-3 gave the same chronological suppression pattern as Sad-1Δ and Sad-2RIP, we crossed ∷hH1-GFP with Sk-2 and Sk-3. In both crosses, nuclei fluoresced throughout meiosis, a postmeiotic mitosis, and ascospore delimitation, just as in Sad-1Δ (or Sad-2RIP) × ∷hH1-GFP (Figure 6, C and D). Initially, all eight young ascospores (both hH1-GFP and non-hH1-GFP) showed the nuclear fluorescence. As the ascospores developed and matured, the nuclear fluorescence gradually faded away in the four non-hH1-GFP ascospores. Subsequently, four of the eight ascospores that carried the Sk-sensitive nuclei stopped developing, and only the four ascospores that contained the killer nuclei matured. Because ∷hH1-GFP and Sk are unlinked, the four maturing ascospores showed fluorescence in only 50% of the asci. Just as with ∷hH1-GFP × Sk, crosses of ∷Bml-GFP × Sk-2 or Sk-3 (or Sad-1Δ) showed suppression of meiotic silencing, and asci showed normal development through meiosis and spore delimitation (Figure 6, E–H). β-Tubulin–GFP fluorescence could be seen in the developing asci and in four of the eight ascospores (Figure 6F). Thus, the suppression of MSUD by Sk-2 and Sk-3 is qualitatively and chronologically similar to that of conventional Sad mutants.
The use of GFP-tagged hH1 and β-tubulin genes for visualizing meiotic silencing and its suppression by Sad-2RIP, Sk-2, and Sk-3. (A) Wild type × ∷hH1-GFP 4 days after fertilization. In the heterozygous asci, the unpaired hH1-GFP is completely silenced in young asci throughout meiosis, a postmeiotic mitosis, and spore delimitation. (B) Sad-2RIP × ∷hH1-GFP 5–6 days after fertilization. Meiotic silencing of hH1-GFP is suppressed by Sad-2RIP, and the nuclei fluoresce throughout meiosis, a postmeiotic mitosis, and in the young ascospores. As the ascospores develop, the nuclear hH1-GFP fluorescence gradually fades away in four of the eight non-hH1-GFP ascospores. (C) ∷hH1-GFP (female) × Sk-2 (male) 5–6 days after fertilization. Meiotic silencing of hH1-GFP is suppressed by Sk-2, and the nuclei fluoresce throughout meiosis and in the young ascospores. The background fluorescent nuclei are in the nonascogenous paraphyseal cells of the female parent. (D) Sk-3 × ∷hH1-GFP 6 days after fertilization. Meiotic silencing of hH1-GFP is suppressed by Sk-3, just as in Sad-2RIP × ∷hH1-GFP (6B). (E–H) Meiotic silencing of β-tubulin and its suppression by Sad-1Δ, Sk-2, and Sk-3. (E) Wild type × ∷Bml-GFP 6 days after fertilization. The expression of β-tubulin is silenced, and the asci are arrested early in meiosis. (F) Sad-1Δ × ∷Bml-GFP 8 days after fertilization. Meiotic silencing of β-tubulin is suppressed by Sad-1Δ. Developing asci and four of the eight ascospores of each ascus show β-tubulin–GFP. (G) Sk-2 × ∷Bml-GFP 6 days after fertilization. Meiotic silencing of β-tubulin is suppressed by Sk-2, resulting in normal ascus and ascospore delimitation. The far right ascus shows a bright spindle in each ascospore at the first mitosis. Sk-caused death of four of the eight ascospores is just visible in two asci. (H) Sk-3 × ∷Bml-GFP 5 days after fertilization. Meiotic silencing of β-tubulin is suppressed by Sk-3. Two asci at spore delimitation (top and bottom) show fluorescent spindle pole bodies at one end of each young ascospore. (I) Sk-2 × Sk-2;∷hH1-GFP 5 days after fertilization. Meiotic silencing of hH1-GFP is suppressed by Sk-2 even when Sk-2 is homozygous, and there is no ascospore killing. Thus suppression of MSUD is independent of ascospore killing. (A and B are from Shiu et al. 2006.)
Heterozygosity for Sk-2 or Sk-3 is not required for the suppression of meiotic silencing:
We tested whether meiotic silencing is defective in crosses that are homozygous (and hence completely paired) for Sk-2 or for Sk-3 and heterozygous for either ∷hH1-GFP or ∷Bml-GFP. Our results demonstrate that MSUD was suppressed in these crosses. For example, in Sk-2 × Sk-2;∷hH1-GFP, nuclei fluoresced throughout meiosis and ascospore development, indicating that meiotic silencing of ∷hH1-GFP was suppressed (Figure 6I). Similar suppression of MSUD was observed in Sk-3 × Sk-3; ∷hH1-GFP, Sk-2 × Sk-2; ∷Bml-GFP, and Sk-3 × Sk-3; ∷Bml-GFP. These observations confirm that MSUD is inoperative (i.e., suppressed) when Sk-2 or Sk-3 is homozygous, as well as when it is heterozygous, and that the unpaired ∷hH1-GFP and ∷Bml-GFP genes are expressed normally. Thus, unlike spore killing, where Sk-2 or Sk-3 must be heterozygous, heterozygosity for the Sk complex is not required for the suppression of meiotic silencing by either of the Sk haplotypes.
Loci that confer resistance to Sk-2 killing do not suppress MSUD:
To determine whether the resistant alleles can suppress MSUD, we crossed strains containing r(Sk-2) and pr(Sk-2) mod(pr), respectively, to strains containing different unpaired loci. Results show that these Spore-killer-resistant alleles do not suppress meiotic silencing in any of the unpaired loci tested (Table 2). These data suggest that, unlike the Sk-2 and Sk-3 complexes, the Sk-2-resistant strains do not contain a dominant suppressor of MSUD.
Interactions of Sk-2 and Sk-3 with Sad-1Δ and Sad-2RIP:
We have seen that the two Spore killers suppress the meiotic silencing of several gene inserts nearly as efficiently as do the two previously described Sad mutants. Clearly, the Sk and sad genes are not allelic: Sk-2 and Sk-3 are on linkage group III (tight centromere linkage), sad-1 is on linkage group IL, and sad-2 is on linkage group VR. We have examined the interactions of Sad-1Δ and Sad-2RIPwith Sk-2 and Sk-3 in various intercrosses. In Sk-2 × Sad-2RIP, for example, ascus development was completely normal through ascospore delimitation, and four of the eight ascospores failed to mature, just as in Sk-2 × wild type. Similarly, Sk-3 × Sad-1Δ also produced 4:4 ascospore patterns, indicating that Sad mutants do not prevent the death of Sk-sensitive ascospores.
DISCUSSION
Meiotic silencing is a phenomenon in which a gene that is unpaired in meiosis generates a signal that silences all copies of that gene. Since presence of a sequence found in one mating partner but not in the other could result from a virus on the move, targeting unpaired DNA for silencing could represent a defense mechanism that protects the organism during times of vulnerability. In other words, the MSUD mechanism could prevent the invasion of a partner genome by an insertion element during a period when other premeiotic silencing mechanisms such as quelling and repeat-induced point (RIP) mutation are dormant (Galagan and Selker 2004). Furthermore, Shiu et al. (2001) have shown that a dominant suppressor of meiotic silencing can confer fertility (albeit marginal) on otherwise barren interspecific crosses in the genus Neurospora. This suggests that MSUD not only could provide surveillance against invading sequences but also might play a role in reproductive isolation.
Meiotic silencing works by examining the pairing between homologous sequences during prophase I. One possible model is that the MSUD scanning machinery can sense the presence of an unpaired DNA sequence and subsequently generate a single-stranded aberrant (a)RNA from it. The aRNA is then duplicated into a dsRNA species by the action of SAD-1 (an RNA-directed RNA polymerase). SAD-2 is required to recruit SAD-1 to the perinuclear region, which could be the central location for RNAi activity. The dsRNA, through the action of dicers, is then processed into short-interfering RNAs, which subsequently direct the specific destruction of mRNA similar in sequence to the unpaired DNA.
Previously, we identified two dominant suppressors of MSUD (Sad-1Δ and Sad-2RIP) via the selection of mutants that can bypass the pairing requirement of genes coding for wild-type spore production (Shiu and Metzenberg 2002; Shiu et al. 2006). Initially, we expected the dominant Sad-1 mutant to contain a hypermorphic allele that was able to interfere with the normal function of a wild-type SAD-1 protein. As it turned out, the original Sad-1 mutant is dominant over wild type because it has a truncation at its 3′-end (Shiu et al. 2001; Shiu and Metzenberg 2002). This deletion deprives the wild-type allele of a pairing partner in a Sad-1Δ × wild-type cross and therefore remains under the radar of the MSUD mechanism. Consequently, the silencer will be silencing itself to some low level via a negative feedback mechanism, thus creating a dominant phenotype for the Sad-1Δ allele. Similarly, the original Sad-2 mutant contains numerous RIP mutations that leave the wild-type allele unpaired in a heterozygous Sad-2 cross and therefore subject to self-silencing.
This article describes the finding that two meiotic drive elements in Neurospora (Sk-2 and Sk-3) act as dominant suppressors of meiotic silencing in a cross. One may wonder what could be the nature of their sequences that make them dominant over wild type. The Sk-2 and Sk-3 loci, which originated in N. intermedia, are likely to have a gene order in linkage group III different from that of wild-type N. intermedia and N. crassa. One possible explanation for our findings is that an Sk chromosome contains a deletion allele of a sad gene, which, unlike the two known sad genes, is not needed for the completion of meiosis. More plausibly, the suppression of MSUD could be due to a suppressor protein that interferes with the gene-silencing pathway. The gene coding for the suppressor protein, however, is unlikely to be represented by the r(Sk-2)- and pr(Sk-2)-resistant loci, since they themselves do not suppress MSUD.
Results presented here show that Sk-2 and Sk-3 indeed suppress most of the unpaired loci tested (Table 2), suggesting that Sk-2 and Sk-3 contain a dominant suppressor of MSUD and superficially resemble Sad-1Δ and Sad-2RIP in suppressing meiotic silencing. Sk-2 suppresses the meiotic silencing of ∷act+, ∷asm-1+, ∷BmlR, and ∷mei-3+ more efficiently than does Sk-3. However, any differences between the suppression by Sk-2 and Sk-3 are quantitative, not qualitative, except in the case of Dip-1. Spore killers differ from the two Sad mutants in several respects: (1) spore killers are meiotic drive elements that distort genetic ratios of Sk-linked genes; (2) spore killers are not simple mutant alleles, but rather putative complex rearrangements that show a recombination block in a 30-MU area (and are thus haplotypes); (3) both Sk-2 and Sk-3 are homozygous fertile, whereas Sad-1Δ and Sad-2RIP are barren when homozygous in a cross (Raju 2002; Shiu et al. 2006); and (4) neither Sk-2 nor Sk-3 suppresses the MSUD associated with the unpairing of the Round spore locus.
It remains unclear whether there is an evolutionary benefit of having a dominant suppressor of meiotic silencing in the Sk region. At first glance, it could increase the mating potential of fungi carrying an Sk haplotype, since they can breed with strains that have a different genome arrangement. Normally, a cross lacking perfect DNA pairing may have some of its meiotic genes silenced, thus affecting overall fertility. The wider selection of potential mating partners could help the Sk elements to transmit themselves into a population. It will be of interest to determine whether elimination of the putative MSUD suppressor would affect the mating potential of strains carrying an Sk haplotype.
The studies of SD, het-s, Sk, and other examples described by Lyttle (1991a,b), Dalstra et al. (2003, 2005), and Burt and Trivers (2006) show that there are several diverse cellular mechanisms that can trigger meiotic drive. However, the Podospora [Het-s] prion, the Drosophila SD locus, and the mouse t locus are the only three meiotic drive elements that have been characterized at the molecular level (Schimenti 2000; Kusano et al. 2001, 2003; Maddelein et al. 2002; Dalstra et al. 2003, 2005). For example, SD in Drosophila causes preferential transmission of SD chromosomes because SD+-bearing spermatids fail to mature in SD/SD+ males. Sd encodes a mutant form of RanGap protein, which is an important player in the Ran-signaling pathway required for nuclear transport, spindle assembly, and other nuclear functions that ultimately affect spermatid elongation and maturation (Merrill et al. 1999; Kusano et al. 2001).
Neurospora Spore killers provide yet another example of the diverse themes of segregation distortion. Sk-2 and Sk-3 behave like the two previously characterized dominant suppressors of meiotic silencing (Sad-1 and Sad-2), yet little is known about their mode of action in killing spores or in overcoming meiotic silencing. Future studies concerning the Spore killer loci and molecular identification of the killing and resistance factors, as well as their suppressor function, will be fascinating for those who are interested in meiotic silencing, sexual selection, and how evolutionary accidents can lead to the creation of selfish DNA elements.
Footnotes
We dedicate this article to the memory of David Perkins for his enthusiastic support of our work on Spore killers and meiotic silencing.
Footnotes
Communicating editor: M. S. Sachs
Acknowledgement
N.B.R., R.L.M., and P.K.T.S. were supported by National Science Foundation grants MCB0417282 (to D. Perkins), MCB0234421 (to R.L.M.), and MCB0544237 (to P.K.T.S.).
References
Ahlquist, P.,
Aramayo, R., and R. L. Metzenberg,
Burt, A., and R. Trivers,
Campbell, J. L., and B. C. Turner,
Cogoni, C., and G. Macino,
Dalstra, H. J., K. Swart, A. J. Debets, S. J. Saupe and R. F. Hoekstra,
Dalstra, H. J., R. Van Der Zee, L. Swart, R. F. Hoekstra, S. J. Saupe et al.,
Davis, R. H.,
Davis, R. H., and F. J. De Serres,
Freitag, M., P. C. Hickey, N. B. Raju, E. U. Selker and N. D. Read,
Galagan, J. E., and E. U. Selker,
Kusano, A., C. Staber and B. Ganetzky,
Kusano, A., C. Staber, H. Y. Chan and B. Ganetzky,
Lee, D. W., R. J. Pratt, M. Mclaughlin and R. Aramayo,
Lyttle, T. W. (Editor),
Maddelein, M. L., S. Dos Reis, S. Duvezin-Caubet, B. Coulary-Salin and S. J. Saupe,
Merrill, C., L. Bayraktaroglu, A. Kusano and B. Ganetzky,
Pennisi, E.,
Perkins, D. D., A. Radford and M. S. Sachs,
Raju, N. B.,
Raju, N. B.,
Raju, N. B.,
Raju, N. B.,
Raju, N. B.,
Raju, N. B., and D. Newmeyer,
Raju, N. B., and D. D. Perkins,
Schimenti, J.,
Shiu, P. K. T., and R. L. Metzenberg,
Shiu, P. K. T., N. B. Raju, D. Zickler and R. L. Metzenberg,
Shiu, P. K. T., D. Zickler, N. B. Raju, G. Ruprich-Robert and R. L. Metzenberg,
Turner, B. C.,
Turner, B. C., and D. D. Perkins,
Turner, B. C., and D. D. Perkins,
Turner, B. C., and D. D. Perkins,
van der Gaag, M., A. J. M. Debets, J. Oosterhof, M. Slakhorst, J. A. G. M. Thijssen et al.,