-
PDF
- Split View
-
Views
-
Cite
Cite
Jacopo Novelli, Antony P Page, Jonathan Hodgkin, The C Terminus of Collagen SQT-3 Has Complex and Essential Functions in Nematode Collagen Assembly, Genetics, Volume 172, Issue 4, 1 April 2006, Pages 2253–2267, https://doi.org/10.1534/genetics.105.053637
Close - Share Icon Share
Abstract
The nematode exoskeleton is a multilayered structure secreted by the underlying hypodermal cells and mainly composed of small collagens, which are encoded by a large gene family. In previous work, we reported analysis of the C. elegans dpy-31 locus, encoding a hypodermally expressed zinc-metalloprotease of the BMP-1/TOLLOID family essential for viability and cuticle deposition. We have generated a large set of extragenic suppressors of dpy-31 lethality, most of which we show here to be allelic to the cuticle collagen genes sqt-3 and dpy-17. We analyzed the interaction among dpy-31, sqt-3, and dpy-17 using a SQT-3-specific antiserum, which was employed in immunofluorescence experiments. Our results support a role for DPY-31 in SQT-3 extracellular processing and suggest that the SQT-3 C-terminal nontrimeric region serves multiple roles during SQT-3 assembly. Different missense mutations of this region have diverse phenotypic consequences, including cold-sensitive lethality. Furthermore, the biochemical and genetic data indicate that the extracellular assemblies of DPY-17 and SQT-3 are interdependent, most likely because the collagens are incorporated into the same cuticular substructure. We find that absence of DPY-17 causes extensive intracellular retention of SQT-3, indicating that formation of the SQT-3–DPY-17 polymer could begin in the intracellular environment before secretion.
NEMATODE worms are ensheathed in a proteinaceous exoskeleton secreted by the underlying hypodermal cells. This exoskeleton, the cuticle, is a complex multilayered structure predominantly composed of small collagen-like molecules. Like their vertebrate counterparts, nematode collagens form trimers via repeats of Gly–X–Y triplets, where Gly is an obligate glycine and X and Y can be any amino acid (aa), but are more frequently represented by proline and hydroxyproline residues. Nematode collagens differ considerably from the collagens of vertebrates or other phyla, in structure and size, and are regarded as a nematode-specific evolutionary specialization (Hutter et al. 2000). Cuticle collagens are encoded by a large gene family, which in the free-living species Caenorhabditis elegans includes ∼170 members scattered across the genome. Cuticle collagens can be grouped into subfamily on the basis of spacing of cysteine residues and sequence homology of the nontrimeric regions (Kramer 1994; Johnstone 2000). In comparison to vertebrate fibrillar collagens, nematode cuticle collagens are significantly smaller and structurally simpler, being composed of 30–50 interrupted Gly–X–Y repeats flanked by nontrimeric N- and C-terminal extensions, which are generally ∼100 aa and ∼20–30 aa long, respectively (see Johnstone 2000 for review). The assembly of nematode collagens also sets them apart from their vertebrate counterparts. Although some post-translational modifications appear conserved between vertebrate and nematode collagens, such as the hydroxylation of proline residues by the prolyl 4-hydroxylase complex, other events, such as N-terminal proteolytic processing and the mode of extracellular crosslinking, are entirely nonhomologous (see Page and Winter 2003 and Myllyharju and Kivirikko 2004 for recent reviews).
Despite the fact that collagens are the most abundant structural components of the nematode cuticle, we still know remarkably little about the types of extracellular assemblies formed by collagens within the exoskeleton. The small size and highly crosslinked state of assembled nematode cuticle collagens have hampered biochemical and structural studies. The most significant insights into the mechanisms of cuticle formation and collagen assembly have come from genetic analysis. Many loci required for normal body shape and size, collectively termed morphogenetic genes, have been determined to encode cuticle collagens and/or enzymatic components of the collagen biosynthetic pathway (reviewed in Page and Winter 2003; Myllyharju and Kivirikko 2004). Hence, analysis of morphogenetic loci can highlight collagen genes required for cuticle function as well as the molecular machinery responsible for collagen processing. Since the nematode cuticle is essential for viability, and in parasitic species represents the interface with the host's defense system, elucidation of its assembly can pave the way to the development of new vaccines or drug therapies targeting cuticle synthesis and immune evasion (see Burglin et al. 1998 for a review discussing the usefulness of C. elegans as a model to study parasitic nematodes).
Processing of procollagens by removal of N-terminal and C-terminal peptides is likely to be critical for nematode collagen maturation. N-terminal processing of SQT-1 collagen has been directly demonstrated (Yang and Kramer 1999) and is likely to be carried out by the subtilisin-like protease BLI-4 (Thacker et al. 1995) but little is known thus far about C-terminal processing. Previously, we reported the characterization of dpy-31, a C. elegans morphogenetic locus encoding a hypodermally expressed zinc-metalloprotease homologous to vertebrate BMP-1 and Drosophila TOLLOID. C. elegans possesses 40 BMP-1 orthologs (Mohrlen et al. 2003), including nas-36 and nas-37, which have been demonstrated to mediate molting (Davis et al. 2004; Suzuki et al. 2004; Frand et al. 2005) while a third BMP-1 homolog, hch-1, is required for eggshell hatching (Hishida et al. 1996). Unlike these genes, dpy-31 is essential for viability and cuticle deposition (Novelli et al. 2004). dpy-31 mutants exhibit a Dumpy (Dpy; shorter than normal) morphological phenotype and temperature-sensitive (ts) lethality (i.e., worms are viable at 15° but lethal at 25°). A Dpy phenotype is also seen in several cuticle collagen gene mutants, suggesting that dpy-31 is involved in collagen processing (see Kenning et al. 2004 for a recent list of cloned C. elegans morphogenetic loci). In mutagenesis screens, extragenic suppressors of dpy-31 have been isolated, some of which were shown to carry distinct mutations in the collagen gene sqt-3 (Novelli et al. 2004). sqt-3 is thought to be essential for viability and to play a crucial role in embryonic morphogenesis (Priess and Hirsh 1986; van der Keyl et al. 1994). The sqt-3 suppressor alleles, termed sqt-3(sup), allow survival of dpy-31 worms at restrictive temperature, but do not rescue the Dpy phenotype. At the sequence level, all sqt-3(sup) carry missense lesions mapping to the SQT-3 C terminus. These lesions act on dpy-31 in a dominant and allele-nonspecific fashion, suggesting that they lead to bypass of dpy-31 function. Other mutations in sqt-3 fail to suppress dpy-31. The structural homology of DPY-31 to vertebrate BMP-1, which has procollagen C-proteinase activity (Kessler et al. 1996; Li et al. 1996), together with the complex genetic interaction between dpy-31 and sqt-3, suggests that DPY-31 is involved in C-terminal proteolytic maturation of SQT-3 and that substitutions altering the structure of the SQT-3 C terminus allow collagen function in the absence of cleavage by DPY-31.
Herein, we extend the analysis of the roles of sqt-3 and dpy-31 in cuticle formation. We cloned additional extragenic suppressors of dpy-31, most of which represent further sqt-3 alleles. Several novel sqt-3(sup) alleles display a striking cold-sensitive (cs)-lethal mutant phenotype. cs-lethal animals are viable at 25°, while lethal at 15°, which is the reverse of the pattern shown by dpy-31 and sqt-3 loss-of-function alleles. We have generated a null allele of sqt-3 and show that the amorphic phenotype of the gene is ts lethal, indicating that the cs-lethal effect reflects a non-null phenotype. Furthermore, three extragenic suppressors of dpy-31 were found to carry unusual missense mutations in an uncharacterized collagen gene, which we demonstrate corresponds to the morphogenetic locus dpy-17. To investigate the role of DPY-31 and the effects of the sqt-3 and dpy-17 suppressor alleles on SQT-3 assembly, we made use of a SQT-3-specific antiserum for immunofluorescence (IF) staining experiments. Our results support the hypothesis that DPY-31 is responsible for SQT-3 extracellular processing and suggest an additional intracellular role for the SQT-3 C terminus in the course of assembly. Furthermore, we present evidence indicating that the intracellular and extracellular assemblies of SQT-3 and DPY-17 are interdependent processes and that the two collagens are likely to participate in formation of a common matrix structure within the embryonic exoskeleton.
MATERIALS AND METHODS
Nematode strains:
Mutant strains were derived from the C. elegans Bristol N2, which was used as wild type. The Hawaiian isolate CB4856 was employed in snip–SNP mapping experiments. Unless otherwise stated, mutant strains are described by Hodgkin (1997):
Linkage group (LG) II: dpy-10(e128);
LGIII: unc-93(e1500), dpy-17(e164, e586, e1345), dpy-17(e2898, e2899, e2910) (this study), unc-32(e189), dpy-31(e2770) (Novelli et al. 2004), unc-119(ed3);
LGIV: fem-1(hc17); him-8(e1489), dpy-13(e184);
LGV: sqt-3(sc8, sc63, e2117), sqt-3(e2889, e2901, e2906, e2911) (Novelli et al. 2004), sqt-3(e2810, e2893, e2897, e2902, e2908, e2909, e2924, e2810e2944, e2908e2945, e2909e2943) (this study); lon-3(e2175);
LGX: xol-1(y9), dpy-7(e88);
Integrant strains: eIs101[pU119+D17GF] (this study).
Assignment of further extragenic suppressors of dpy-31 to sqt-3:
Generation of suppressors of dpy-31 and assignment of nine of them to sqt-3 were reported elsewhere (Novelli et al. 2004). To determine whether any of the unidentified suppressors were sqt-3 alleles, dpy-31 worms carrying the suppressor were mated to him-8 IV; lon-3 V males. Presumptive dpy-31/+ III; him-8/+ IV; sqt-3(sup)/lon-3 V lines were identified by progeny testing and putative sqt-3(sup) homozygotes were isolated. These were complementation tested against known sqt-3 alleles. The presence of Sqt-3 phenotypes in the cross-progeny suggested allelism to sqt-3. Genomic DNA was then extracted by lysis from the putative sqt-3(sup) homozygotes, and sqt-3 was amplified by PCR using the lysates as templates and subjected to DNA sequencing as described previously (Novelli et al. 2004).
Mutagenesis screens for suppressors of cs-lethal sqt-3(sup) alleles:
Ethyl methanesulfonate (EMS) mutagenesis was performed as in Sulston and Hodgkin (1988). sqt-3 (e2810, e2908, e2909) worms were raised at 25°, mutagenized en masse, incubated at 25° for 24 hr, and then downshifted to 15°. Approximately 105 genomes were screened. L1 viable F2–F3 progeny were picked and selfed at 15°. sqt-3 intragenic suppressors were identified on the basis of phenotypic features. sqt-3 was analyzed by sequencing in three intragenic suppressors, e2810e2944, e2908e2945, and e2909e2943.
Isolation of a sqt-3(0) allele:
To obtain a sqt-3(0) allele, a mutagenesis screen for suppressors of the dominant left-handed roller (LRol) phenotype of sqt-3(sc63) mutants (Cox et al. 1980) was conducted as follows. Animals of genotype fem-1 IV; sqt-3(sc63) V; xol-1 X were raised at 22.5° for 2 days before being mutagenized with a 100-mm dose of EMS. Except for the difference in the final EMS concentration, mutagenesis was performed as in Sulston and Hodgkin (1988). Mutagenized females were mated to him-8 IV; lon-3 V males at 22.5°. Approximately 6000 cross-progeny were inspected for the presence of non-LRol animals, which were cloned. One candidate line was analyzed further. The sqt-3 genomic region was amplified by PCR and subjected to DNA sequencing, which revealed the presence in the mutant line of a deletion removing most of sqt-3. The line was retained and the mutation designated e2924.
Mapping of the dpy-17(sup) allele e2898:
Preliminary three-factor crosses placed e2898 between unc-93 and dpy-31 on LGIII. The triply marked strain unc-93(e1500) sup(e2898) dpy-31(e2770) was employed in snip–SNP mapping experiments as described by Wicks et al. (2001). Thirty-one non-Unc non-ts Dpy recombinants were isolated among the progeny of e1500 e2898 e2770/CB4856 cis-heterozygotes raised at 25°. Since e2898 acts on dpy-31 both dominantly and recessively, non-Unc non-ts Dpy recombinants could have two genotypes: e1500 e2898 e2770/ + + e2770 and e1500 e2898 e2770/+ e2898 e2770. The genotype was determined by driving the recombinants to homozygosity. Of 31 recombinants, 29 belonged to the non-ts Dpy class and 2 to the lethal Dpy class. A panel of validated LGIII snip–SNP markers was used for mapping (details available on request). The mapping results are reported below, starting from the left-most marker. The snip–SNP designation refers to the corresponding genomic cosmid clones. Map position is in parentheses. The fraction of N2 signal observed is indicated: non-ts Dpy recombinants—marker C32A3 (−5.3), 0/29 N2; marker ZK1058 (−4.2), 20/29 N2; marker F26A1 (−2.5), 27/29 N2; marker Y54H54A (−2.0), 29/29 N2; marker W03A5 (−1.7), 29/29 N2; marker T20B12 (−0.74), 29/29 N2; lethal Dpy recombinants—marker C32A3 (−5.3) 0/2 N2; marker ZK1058 (−4.2), 0/2 N2; marker F26A1 (−2.5), 0/29 N2; marker Y54H54A (−2.0), 0/2 N2; marker W03A5 (−1.7), 1/2 N2; marker T20B12 (−0.74), 2/2 N2.
Extraction of the e2898 suppressor by EMS mutagenesis and cloning of dpy-17:
To extract e2898 from the e2770 background, a population of sup(e2898) dpy-31(e2770) worms was mutagenized using a 30-mm dose of EMS. Except for the difference in the mutagen concentration, EMS mutagenesis was performed as in Sulston and Hodgkin (1988). Mutagenized animals were incubated at 25° for 10 days, at which point wild-type revertants of dpy-31 were sought among the F2–F3 progeny. Approximately 105 genomes were screened. Several wild-type-like lines were isolated. EMS mutagenesis results in a high frequency of intragenic revertants of dpy-31(e2770), approximately half of which carry exact back-mutations (Novelli et al. 2004). These were identified as previously described (Novelli et al. 2004). One line, carrying a wild-type codon at the position affected by e2770, was retained as a putative e2898 homozygous strain. The candidate collagen open reading frame (ORF) F54D8.1 was amplified by PCR from the three linked sup alleles e2898, e2899, and e2910 as well as from the dpy-17 alleles e164, e586, and e1345 and sequenced using gene-specific primers (primer details are available on request).
Generation of the unc-119(+) dpy-17(sup) construct and biolistic transformation:
A 2423-bp fragment encompassing dpy-17 and the intergenic regions between dpy-17 and flanking ORFs was amplified by PCR from lysates of dpy-17(e2898) mutants using primers D17H3F and VR (sequences available on request). D17H3F incorporates a HindIII recognition site, while a unique HindIII site is present 199 bp downstream of the termination codon of dpy-17. To generate plasmid pU119 + D17GF, the resultant PCR product was digested with HindIII (coordinates of the fragment on cosmid clone F54D8: 31015–32913) and cloned into the unc-119 rescue construct pDP#MM016β (Maduro and Pilgrim 1995) using standard techniques (Sambrook et al. 1989). pU119 + D17GF was used in biolistic transformation experiments of unc-119(ed3) animals as described by Praitis et al. (2001), resulting in the recovery of the integration event eIs101[pU119+D17GF].
Generation of a SQT-3-specific rabbit polyclonal antiserum:
The R584 polyclonal serum was generated by immunizing rabbits with a mixture of the following synthetic peptides conjugated to keyhole limpet haemocyanin (CALDGGVFFEDGTRR; CPVAPCEPTTPPP). These peptides correspond to most of the SQT-3 nontrimeric C-terminal domain and to the longest nontrimeric interruption in the Gly–X–Y repeats, respectively.
IF staining of C.elegans embryos:
The general method used for fixation of C. elegans embryos before immunolocalization was freeze-fracture on slides followed by methanol and acetone fixation at −20°, as described by (Miller and Shakes (1995). The R584 serum was used as a primary antibody at a 1/100 dilution. Alexa Fluor 488 chicken anti-rabbit IgG (Molecular Probes, Eugene, OR) was used as a secondary antibody at a 1/100 dilution. Incubations were performed in 1× phosphate-buffered saline (PBS) containing 0.1% Tween 20 and 1% dried semiskimmed milk. Washes were carried out in 1× PBS containing 0.1% Tween 20. Slowfade Light antifade (Molecular Probes) was used as a mount solution. Slides were examined with a Zeiss Axioplan 2 microscope equipped for epifluorescence according to standard protocols (Sulston and Hodgkin 1988).
RESULTS
Identification of more sqt-3(sup) alleles:
In the initial characterization of extragenic suppressors of dpy-31, 9 of 23 suppressors were found to be sqt-3 alleles. Further complementation tests and DNA sequence analyses revealed that a total of 18 suppressors are allelic to sqt-3. Of the remaining 5 extragenic suppressors of dpy-31 lethality, 3 (e2898, e2899, and e2910) were determined to be linked to dpy-31, while 2 (e2894 and e2895) have been tentatively assigned to LGI.
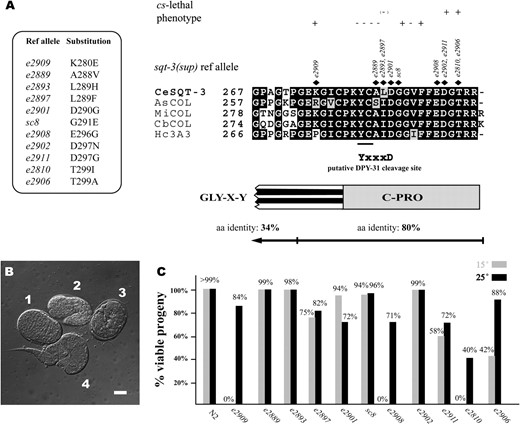
The lesions identified in the additional sqt-3(sup) alleles correspond to more missense alterations mapping to the C-terminal end of the protein (Figure 1A). In all, the 18 alleles represent 11 distinct lesions affecting eight residues in SQT-3 (Figure 1A).
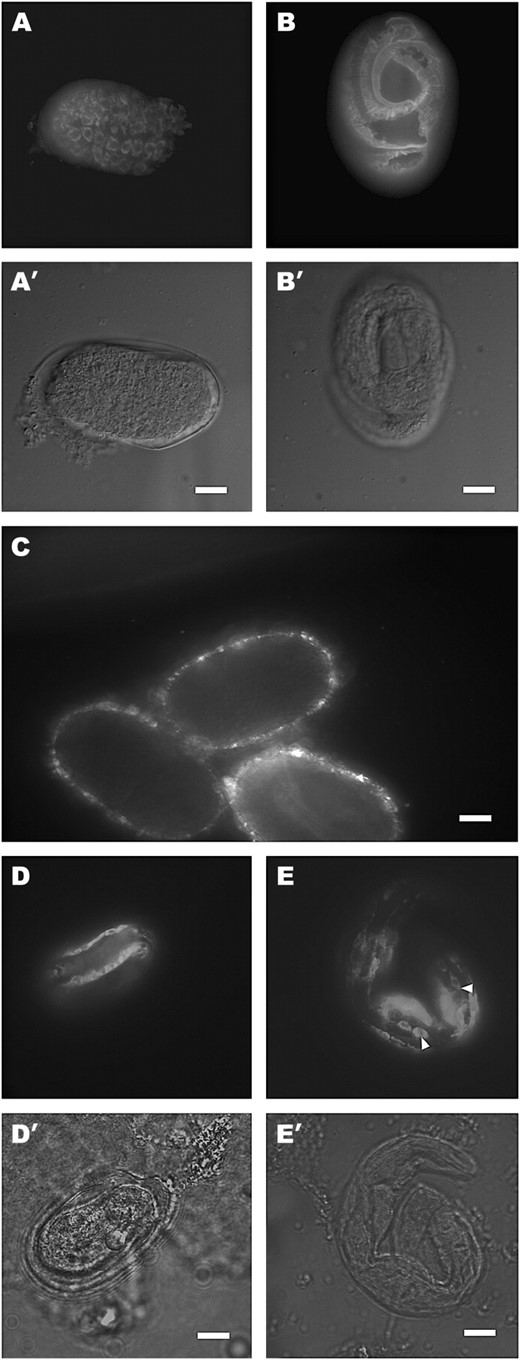
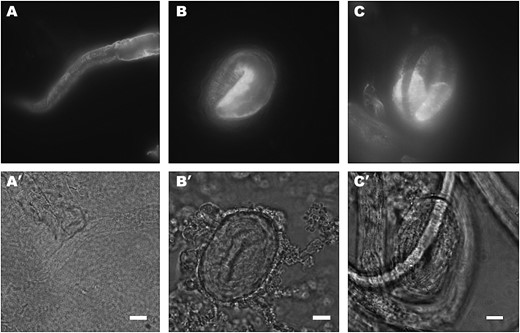
sqt-3(sup) alleles are associated with cs lethality. (A) Sequence alignment of the C-terminal regions of five SQT-3-like collagens from various nematode species. CeSQT-3, C. elegans SQT-3 (GenPept accession no. CAA98942); AsCOL, Ascaris suum collagen (found as Ascaris EST cluster no. ASC00066 at http://nema.cap.ed.ac.uk); MiCOL, Meloidogyne incognita collagen (GenPept accession no. AAC48358); CbCOL, C. briggsae collagen (GenPept accession no. CAE75463); Hc3A3, Haemonchus contortus 3A3 collagen (GenPept accession no. AAA29173). The residues altered by sqt-3(sup) lesions are indicated above the alignment along with the reference allele designation. The encoded substitutions are indicated on the left. The YC motif underlined may be required for crosslinking. The putative DPY-31 site in SQT-3 is shown (YxxxD). A schematic of the SQT-3 domain boundaries is shown below the alignment (compare with Figure 2). The conservation of the C-terminal prodomain and the last two Gly–X–Y repeats (overall aa identity 80%) is compared to that of the rest of the protein sequence (overall aa identity 34%). (B) cs-lethal phenotype of sqt-3(e2909) mutants. When raised at 15°, e2909 embryos undergo ventral enclosure (1, dorsal view showing hypodermal cells during intercalation) and elongate (2, 1.5-stage embryo) normally. However, at the threefold stage (3) they arrest, displaying cuticle abnormalities. Escapers (4) arrest at L1 stage with morphological abnormalities. (C) Progeny scoring of the sqt-3(sup) alleles. The percentage of viable progeny at 15° and 25° is indicated. A total of 700–1000 progeny were counted for each allele at each temperature. Parental animals were placed at the desired temperature as L3 larvae. Progeny were scored as viable if they reached the L3 stage. The results of the progeny-scoring experiments are summarized above the alignment in A. Bar, 10 μm.
The C-terminal domain of SQT-3 is a 20-aa-long non-Gly–X–Y, presumably globular motif. SQT-3-like collagens, which have been detected in all nematode species examined, have invariant C-terminal domains (Cox et al. 1990). In Figure 1A, an alignment of the C termini of SQT-3-like collagens from five nematode species is shown (refer to Figure 2 for a diagram of SQT-3 domain organization). While upstream of the C-terminal domain the average aa identity between these collagens is 34%, the C termini are >80% identical. The last two trimeric repeats of the collagens are also highly conserved (Figure 1A).
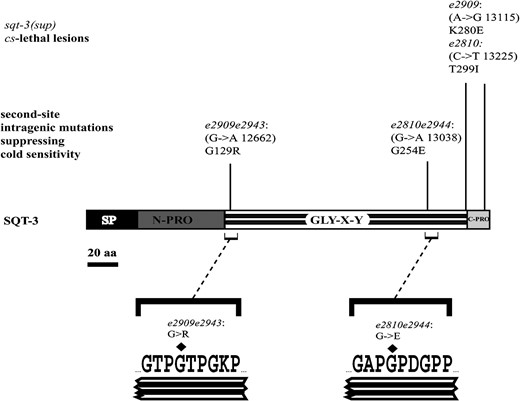
Intragenic suppressors of cold-sensitive sqt-3(sup) alleles have Gly substitutions. Schematic representing the domain organization of the predicted SQT-3 protein. Position of the e2810, e2908, and e2909 cs-lethal lesions is reported (see also Figure 1). The second-site missense mutations identified in e2810e2944, e2908e2945, and e2909e2943 are indicated along with the encoded Gly substitutions. Numbers in parentheses represent the coordinates on genomic clone F23H12 (GenBank accession no. Z74472). SP, signal peptide; N-PRO, N-terminal prodomain; GLY–X–Y, Gly–X–Y interrupted repeats (for clarity, the interruptions are not shown); C-PRO, C-terminal prodomain.
Despite its tight conservation, little is known about the functions of this short stretch of residues. Studies with the collagen gene sqt-1 showed that a tyrosine–cysteine motif present in the C-terminal region of the encoded protein is required for formation of tyrosine-derived crosslinks (Yang and Kramer 1999). The same motif is present in SQT-3-like collagens (underlined, Figure 1A) and in one case its involvement in Tyr crosslinks has been directly demonstrated by biochemical analysis (Kramer 1994).
The sqt-3(sup) lesions are associated with various recessive phenotypes, which become evident in a dpy-31(+) background. As reported before, e2889, e2901, and sc8 exhibit a LRol morphological abnormality whereas e2906 displays cs lethality (Novelli et al. 2004). Among the new alleles, e2810, e2908, and e2909 exhibit weak Dpy and hermaphrodite Tail abnormal (Tal) phenotypes while e2893, e2897, and e2902 show no obvious morphological defects. In addition, e2810, e2908, and e2909 also display a severe cs-lethal phenotype similar to but stronger than that seen in e2906 animals. sqt-3 cs-lethal mutants have zero or low viability at 15° and progressively higher survival rates correlating with the temperature increase. As with sqt-3 ts alleles (van der Keyl et al. 1994), the lethality is particularly pronounced in embryos and appears to coincide with the time of cuticle synthesis. sqt-3 cs-lethal mutants develop normally up to the pretzel stage, at which point they arrest, displaying cuticle abnormalities (Figure 1B). Escapers are arrested at the first larval stage (L1) and exhibit distorted morphologies (Figure 1B).
To determine the penetrance of the cs-lethal effect in each allele, the progeny of single sqt-3(sup) homozygous mutants reared at 15° and 25° were scored for viability. This revealed that the cs-lethal phenotype is totally penetrant in e2810, e2908, and e2909 worms and partially penetrant in e2906 and e2911 (Figure 1C). cs lethality is also observed at low penetrance in e2897. Intriguingly, mutations affecting the same residues in the encoded SQT-3 can result in both cs-lethal and non-cs-lethal phenotypes, as illustrated by e2902 (D297N) and e2911 (D297G; Figure 1). Also, lesions mapping to the same residue can be associated with different degrees of cs lethality, as in e2810 (T299I) and e2906 (T299A; Figure 1).
Mutagenesis screens for suppressors of cold sensitivity:
To explore the molecular basis of cs lethality, we conducted mutagenesis screens for suppressors of the fully penetrant cs-lethal sqt-3(sup) alleles e2810, e2908, and e2909 (see materials and methods for experimental procedure). The screens led to the isolation of several dominant suppressors of cold sensitivity, most of which were identified as probable intragenic sqt-3 second-site mutations because they behaved as strong sqt-3 loss-of-function alleles and exhibited LRol, Dpy, and Tal phenotypes. sqt-3 was analyzed by sequencing in three intragenic suppressor strains, e2810e2944, e2908e2945, and e2909e2943, in each of which a second-site intragenic lesion was identified, resulting in the substitution of an obligate glycine in the Gly–X–Y repeats (Figure 2). This common type of collagen mutation hereafter will be referred to as Gly substitutions. All Gly substitutions isolated as intragenic suppressors of cold sensitivity are also associated with ts lethality (i.e., worms are viable at 15° but lethal at 25°), as reported before for Gly substitution alleles e24 and e2117 (van der Keyl et al. 1994).
A null allele of sqt-3:
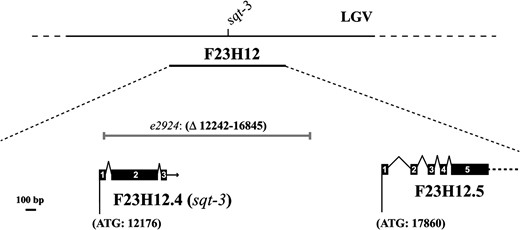
To further clarify the role of sqt-3 in cuticle formation, a mutagenesis screen aimed at isolating a sqt-3 amorph was performed (refer to materials and methods for the experimental procedure), resulting in the recovery of the novel sqt-3 allele e2924. Sequence analysis showed that e2924 carries a 4603-bp deletion, removing 95% of the sqt-3 coding region (Figure 3). In e2924, only the initial 66 bp of the gene are retained, specifying a 22-aa-long fragment of the signal peptide. Since all Gly–X–Y repeats of sqt-3 are deleted, it can be assumed that the gene is entirely nonfunctional in e2924 worms. The mutation is unlikely to affect the downstream gene F23H12.5, which starts ∼1 kb to the right of the deletion endpoint (Figure 3).
The e2924 allele is a putative sqt-3 molecular null. Schematic illustrating the extent of the e2924 rearrangement deleting sqt-3. Numbers in parentheses refer to the coordinates on cosmid clone F23H12 (GenBank accession no. Z74472). The solid boxes represent exons.
Inspection of the mutant phenotype of e2924 worms revealed that the null state of sqt-3 is ts lethal. e2924 worms are ∼80% viable at 15° and totally inviable at 25°. At 25°, e2924 embryos arrest at the threefold stage, showing the retracted Dpy phenotype typical of sqt-3 and dpy-31 loss of function (van der Keyl et al. 1994; Novelli et al. 2004).
Linked extragenic suppressors of dpy-31 are alleles of the collagen gene dpy-17:
As mentioned above, three extragenic suppressors of dpy-31 were found to be linked to the gene but separable from it. Worms carrying e2898, e2899, and e2910 together with dpy-31 are viable Dpy at 25°. The three suppressors were found to act both dominantly and recessively to suppress dpy-31 lethality. However, one phenotypic feature set them apart from the sqt-3(sup) alleles. While capable of suppressing the embryonic and larval ts lethality associated with dpy-31 (Table 1), the suppressors are not fully effective at relieving ts lethality in adults. When raised at 25°, most suppressed animals fail to survive the L4-to-adult molt (Table 2). These findings suggest that suppression of dpy-31 lethality by e2898, e2899, and e2910 is stage specific. Moreover, the identification of a shared suppression property for linked events is a strong indication of allelism.
Suppression efficiency of two dpy-17(sup) alleles
dpy-17(sup) allele . | Genotype of the parental and broods counted . | Viable phenotypes . | Viable Dumpies expected if rescue of the lethality conferred by the dpy-17(sup) is fully penetrant . |
|---|---|---|---|
| None (control) | e2770/e189 (5) | 586 wild type | |
| 283 Unc | |||
| 0 Dpy | 0 | ||
| e2898 | e2898 e2770/e189 (5) | 455 wild type | |
| 241 Unc | |||
| 165 Dpy | 227 | ||
| e2899 | e2899 e2770/e189 (6) | 488 wild type | |
| 251 Unc | |||
| 118 Dpy | 244 |
dpy-17(sup) allele . | Genotype of the parental and broods counted . | Viable phenotypes . | Viable Dumpies expected if rescue of the lethality conferred by the dpy-17(sup) is fully penetrant . |
|---|---|---|---|
| None (control) | e2770/e189 (5) | 586 wild type | |
| 283 Unc | |||
| 0 Dpy | 0 | ||
| e2898 | e2898 e2770/e189 (5) | 455 wild type | |
| 241 Unc | |||
| 165 Dpy | 227 | ||
| e2899 | e2899 e2770/e189 (6) | 488 wild type | |
| 251 Unc | |||
| 118 Dpy | 244 |
The progeny segregated by dpy-17(sup) dpy-31(e2770)/unc-32(e189) trans-heterozygotes were counted and summed. The progeny segregated by dpy-31(e2770)/unc-32(e189) animals were counted as controls. Since e2898 and e2910 represent re-isolates of the same event only e2898 was analyzed. The total progeny expected were calculated on the basis of the wild-type scores. In column 2, the number of broods counted is reported in parentheses. The number of observed and expected viable Dpy is in italics in columns 3 and 4. Expected Dpy's were also calculated on the basis of the wild-type score. Note that Dpy animals were scored as viable even if they did not survive the L4-to-adult molt.
Suppression efficiency of two dpy-17(sup) alleles
dpy-17(sup) allele . | Genotype of the parental and broods counted . | Viable phenotypes . | Viable Dumpies expected if rescue of the lethality conferred by the dpy-17(sup) is fully penetrant . |
|---|---|---|---|
| None (control) | e2770/e189 (5) | 586 wild type | |
| 283 Unc | |||
| 0 Dpy | 0 | ||
| e2898 | e2898 e2770/e189 (5) | 455 wild type | |
| 241 Unc | |||
| 165 Dpy | 227 | ||
| e2899 | e2899 e2770/e189 (6) | 488 wild type | |
| 251 Unc | |||
| 118 Dpy | 244 |
dpy-17(sup) allele . | Genotype of the parental and broods counted . | Viable phenotypes . | Viable Dumpies expected if rescue of the lethality conferred by the dpy-17(sup) is fully penetrant . |
|---|---|---|---|
| None (control) | e2770/e189 (5) | 586 wild type | |
| 283 Unc | |||
| 0 Dpy | 0 | ||
| e2898 | e2898 e2770/e189 (5) | 455 wild type | |
| 241 Unc | |||
| 165 Dpy | 227 | ||
| e2899 | e2899 e2770/e189 (6) | 488 wild type | |
| 251 Unc | |||
| 118 Dpy | 244 |
The progeny segregated by dpy-17(sup) dpy-31(e2770)/unc-32(e189) trans-heterozygotes were counted and summed. The progeny segregated by dpy-31(e2770)/unc-32(e189) animals were counted as controls. Since e2898 and e2910 represent re-isolates of the same event only e2898 was analyzed. The total progeny expected were calculated on the basis of the wild-type scores. In column 2, the number of broods counted is reported in parentheses. The number of observed and expected viable Dpy is in italics in columns 3 and 4. Expected Dpy's were also calculated on the basis of the wild-type score. Note that Dpy animals were scored as viable even if they did not survive the L4-to-adult molt.
The e2898 suppressor of dpy-31 was mapped using snip–SNP markers (see materials and methods). This confirmed that e2898 is an extragenic event and placed e2898 to the left of dpy-31 (map position −0.77 on LGIII) between map positions −2.5 and −1.7.
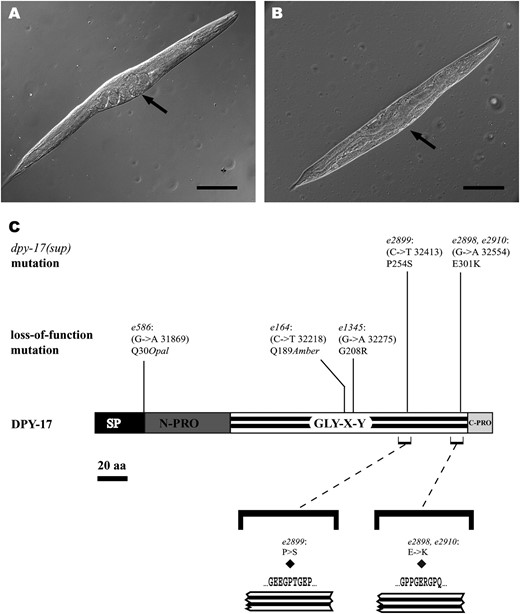
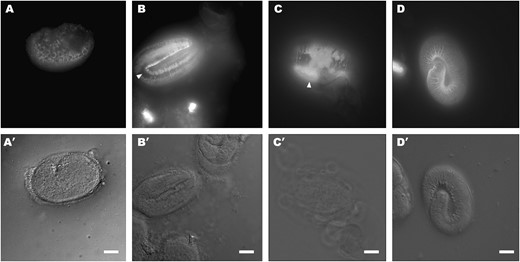
Given the tight linkage to dpy-31, extraction of e2898 by recombination was not experimentally straightforward. To place e2898 on a dpy-31(+) background, we took advantage of the ease with which exact intragenic revertants of dpy-31 can be isolated after EMS mutagenesis (Novelli et al. 2004). A line of genotype sup(e2898) dpy-31(+) was obtained. e2898 homozygotes were inspected for mutant phenotypes. While these animals appear grossly normal and display no significant lethality at any temperature, 5% of e2898 homozygotes (n = 690) express a spindle-like Dpy morphological abnormality at 25°. In spindle-like Dpy animals, the midbody region is notably enlarged in comparison to the rest of the body, conferring a distinct spindle shape on the animals (Figure 4A). A similar phenotype is also seen in dpy-17 mutants (Figure 4B). dpy-17 is a hitherto uncloned morphogenetic gene mapping within the e2898 interval. Several lines of evidence pointed to dpy-17 as ORF F54D8.1, encoding a cuticle collagen. To ascertain whether dpy-17 is synonymous with F54D8.1, the gene was amplified and sequenced with gene-specific primers in three allelic loss-of-function mutants, e164, e586, and e1345. In each case, a GC → AT transition was identified in F54D8.1. Like most C. elegans collagens, the predicted DPY-17 protein is small (352 aa) and contains a signal peptide for secretion, an N-terminal prodomain, Gly–X–Y interrupted repeats, and a C-terminal prodomain (Figure 4C). However, DPY-17 does not belong to the SQT-3 subfamily of collagens, being most closely related to SQT-1, ROL-6, and LON-3 (overall primary sequence identity: 43.3, 43, and 40.3%, respectively). e164 and e586 are nonsense lesions (Q189Amber and Q30Opal, respectively, Figure 4C). These alleles are equally strong and probably represent the null state of dpy-17. In e1345, a missense lesion that would cause a Gly substitution at position 208 in the repeats (G208R; Figure 4C) was found. Consistent with the nature of the mutation, e1345 is a ts allele, suggesting that partial dpy-17 function could be retained in this allele. In summary, these results confirmed that dpy-17 corresponds to ORF F54D8.1.
Morphological phenotypes seen in dpy-17(e2898, e586) mutants and DNA lesions identified in sequenced dpy-17 alleles. (A) e2898 homozygous adult expressing the spindle Dpy phenotype. This specimen is only weakly Dpy, but the spindle-shaped body morphology is very pronounced (arrow). Occasional specimens resemble more closely dpy-17 loss-of-function mutants. (B) dpy-17(e586) homozygous adult displaying the characteristic spindle Dpy phenotype. Note the enlargement of the midbody region in comparison to the rest of the body (arrow). (C) Schematic representing the domain organization of DPY-17. For simplicity, nontrimeric interruptions in the GLY–X–Y domain are not shown. Position of lesions identified in dpy-17 is indicated above the protein structure. The numbers in parentheses indicate the location of the mutation relative to the cosmid clone F54D8 coordinate (GenBank accession no. U12966). The aa replacements caused by the dpy-17(sup) alleles are also shown below the protein structure together with flanking sequences. Note that both e2899 and e2898-e2910 cause replacements of residues occupying the X position in a Gly–X–Y repeat. Bars, 100 μm.
Next, we sequenced dpy-17 in the three suppressor mutants. In each case, a missense lesion was identified in the repeat region of dpy-17. e2899 causes a proline-to-serine substitution at position 254 of the encoded protein (P254S; Figure 4C). e2898 and e2910 represent independent isolates of the same mutation and lead to the replacement of a glutamic acid residue with lysine at position 301 (E301K; Figure 4C). These results demonstrate that the linked intragenic suppressors of dpy-31 are allelic to the collagen gene dpy-17. For clarity, we will refer to these as dpy-17(sup).
The e2898 and e2899 mutations are unusual in that, although they affect the DPY-17 Gly–X–Y repeats, they do not cause Gly substitutions but rather result in alteration of two X position residues in the repeat (Figure 4C). Complementation tests showed that dpy-17(e586) dpy-31(+)/dpy-17(e2898) dpy-31(+) and dpy-17(e586) dpy-31(+)/dpy-17(e2899) dpy-31(e2770) trans-heterozygotes do not express a Dpy-17 phenotype. Furthermore, animals of genotype dpy-17(e2898) +/dpy-17(e2899) dpy-31(e2770) are also not Dpy. Hence, the dpy-17(sup) are not loss- or reduction-of-function alleles. The genetic behavior of e2898 and e2899 is more suggestive of hypermorphic or gain-of-function alleles (Park and Horvitz 1986).
The nature of the lesions points to e164 and e586 as null alleles of dpy-17. Significantly, dpy-17(0) worms share several features with sqt-3(0) animals. In both cases, viable adults are Tal; furthermore, dpy-17(0) L1 larvae display the characteristic retracted Dpy phenotype also seen in sqt-3(0) and dpy-31(0) mutants. Unlike sqt-3, dpy-17 does not, however, appear to be essential for viability: both e164 and e586 mutants do not exhibit significant lethality at any temperature.
To determine whether the dpy-17(sup) alleles can act to suppress a sqt-3 null mutation, a dpy-17(e2898) III; sqt-3(e2924) V doubly mutant strain was constructed and tested for viability at 25°. These animals are as ts lethal as e2924 single mutants, indicating that the dpy-17(sup) alleles are not capable of suppressing a null allele of sqt-3.
To confirm that the lesion identified in the dpy-17(sup) alleles acts as a suppressor of dpy-31 lethality, we attempted to achieve transgenic rescue of dpy-31 lethality with dpy-17(e2898). The gene was amplified in dpy-17(e2898) mutants and the resulting DNA was injected into dpy-31 mutants together with a sur-5∷GFP co-injection marker. However, GFP-expressing F1 transformants invariably died during embryogenesis at any temperature (data not shown). This effect suggests that the gene cannot be tolerated in multicopy arrays. We therefore resorted to introducing the dpy-17(e2898) allele into worms by biolistic transformation, which tends to result in low-copy-number integration events (Praitis et al. 2001). Briefly, the dpy-17(e2898) gene was amplified and cloned into an unc-119(+) construct to yield pU119 + D17GF (see materials and methods). unc-119(ed3) worms were then subjected to biolistic transformation, and non-Unc transformants were sought among the F2–F3 progeny. A putative integrant line was retained and the integration event was designated eIs101. The eIs101 transgene was placed into an unc-119(ed3) dpy-31(e2770) genetic background by crossing. As expected, unc-119 dpy-31; eIs101 worms are viable Dpy at 25°. In addition, progeny testing revealed that the strain exhibited the same pattern of late-onset lethality as that displayed by dpy-31(e2898) dpy-31(e2770) homozygotes (Table 2). The eIs101 transgene was also confirmed to rescue unc-119(ed3) dpy-17(e586) double mutants.
Penetrance of the suppression of dpy-31 adult lethality conferred by the dpy-17(sup) alleles and by the eIs101 transgene
Allele . | Viable adults/total worms counted (n) . | Viable adults (%) . |
|---|---|---|
| e2898 | 111/523 | 21 |
| e2899 | 72/447 | 19 |
| e2910 | 128/515 | 25 |
| eIs101 | 101/633 | 16 |
Allele . | Viable adults/total worms counted (n) . | Viable adults (%) . |
|---|---|---|
| e2898 | 111/523 | 21 |
| e2899 | 72/447 | 19 |
| e2910 | 128/515 | 25 |
| eIs101 | 101/633 | 16 |
Single dpy-31 worms carrying the suppressors in homozygous combination were raised at 25° and their full broods were scored. Worms that reached adulthood were scored as viable, while worms that died as L4 were scored as lethal. The genotype of the strain carrying the integration event is unc-119(ed3) dpy-31(e2770); eIs101.
Penetrance of the suppression of dpy-31 adult lethality conferred by the dpy-17(sup) alleles and by the eIs101 transgene
Allele . | Viable adults/total worms counted (n) . | Viable adults (%) . |
|---|---|---|
| e2898 | 111/523 | 21 |
| e2899 | 72/447 | 19 |
| e2910 | 128/515 | 25 |
| eIs101 | 101/633 | 16 |
Allele . | Viable adults/total worms counted (n) . | Viable adults (%) . |
|---|---|---|
| e2898 | 111/523 | 21 |
| e2899 | 72/447 | 19 |
| e2910 | 128/515 | 25 |
| eIs101 | 101/633 | 16 |
Single dpy-31 worms carrying the suppressors in homozygous combination were raised at 25° and their full broods were scored. Worms that reached adulthood were scored as viable, while worms that died as L4 were scored as lethal. The genotype of the strain carrying the integration event is unc-119(ed3) dpy-31(e2770); eIs101.
IF staining of N2, sqt-3, and dpy-31 worms using a SQT-3-specific polyclonal antiserum:
To investigate the effects of the sqt-3 and dpy-17 lesions as well as of the loss of dpy-31 on SQT-3 assembly, we made use of the R584 anti-SQT-3 rabbit polyclonal serum. In initial experiments aimed at determining its specificity, the antiserum was tested on wild type and sqt-3(e2924, e2117) worms.
Consistent with the ubiquitous expression of sqt-3 throughout the life cycle (Kramer et al. 1985), the R584 antiserum intensely stains the hypodermal cells and cuticles of all stages. For the purposes of this investigation, we focused on embryonic staining pattern.
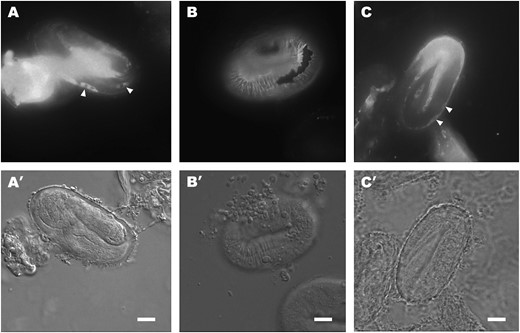
Reactive antigens are first detected in wild-type comma-stage embryos within the hypodermal cells (Figure 5A). The signal localizes perinuclearly, presumably in association with the secretory pathway (Figure 5A). At the threefold stage, coincident with cuticle secretion, the signal becomes progressively extracellular: by the late pretzel stage, all antigen is detected in alignment with the annular ridges of the embryonic cuticle (Figure 5B).
IF staining experiments of N2 worms and sqt-3(e2924, e2117) mutants with the R584 SQT-3-specific serum. (A and B) IF Staining of N2 embryos with the R584 serum. A′ and B′ are the differential interference contrast (DIC) micrographs of A and B. In comma-stage embryos (A), the reactive antigen is detected intracellularly in a perinuclear distribution. By the late threefold stage (B), all antigen is assembled into the cuticle. (C) e2924 embryos labeled with the R584 serum. No specific signal is detected. The puncta represent background fluorescence. (D and E) Staining of e2117 embryos with the R584 serum. D′ and E′ are the DIC micrographs of D and E. The animals in D and E were raised at 15° and at 25°, respectively. In both cases, SQT-3 failed to be secreted. The animal in D is a threefold-stage embryo, while the one in E is an arrested L1 larva. Note the persistence of the retained collagens in the hypodermis of the L1 specimen (E, arrowheads). Bars, 10 μm.
This labeling pattern is consistent with the antigen being a secreted cuticle component. In similar experiments using monoclonal antiserum specific for the collagen DPY-7, McMahon et al. (2003) obtained comparable results, with the difference that the DPY-7 antigen localized extracellularly to the annular furrows, not ridges.
Staining of sqt-3(0) embryos consistently failed to reveal any specific signal (Figure 5C). This finding supports the specificity of the antiserum for SQT-3 and confirms e2924 as a sqt-3 molecular null. Several other cuticle collagens (such as COL-43, COL-97, and COL-109) might be expected to react with the antiserum, because of sequence similarity to SQT-3, but these may be expressed only at low level or at later stages and consequently are not detected by IF staining of embryos with R584.
We also tested SQT-3 immunolocalization in sqt-3(e2117ts) mutants. As mentioned above, the e2117 lesion was characterized previously and causes substitution of an obligate glycine in the repeat region (van der Keyl et al. 1994). Phenotypically, e2117 mutants are wild-type-like at 15° and embryonically lethal at 25°. e2117 embryos were reared at the two growth extremes before staining with the R584 serum. In both cases, SQT-3 was retained within the hypodermal cells (Figure 5, D and E). While no secretion was detected, retained collagen forms did not appear to be degraded efficiently and could be seen to persist in the hypodermis of L1 larvae (Figure 5E). The temperature-independent intracellular accumulation of SQT-3 observed in e2117 animals reinforces the hypothesis that lack of SQT-3 in the cuticle leads to temperature-dependent lethality. Alternatively, small amounts of SQT-3 may be secreted at 15° and suffice to rescue the lethal phenotype; such temperature-dependent effects on secretion have been reported to occur for type IV collagen (Gupta et al. 1997).
We next examined dpy-31 probable null mutants by IF. Since dpy-31 mutants also display ts lethality (Novelli et al. 2004), staining was performed on worms raised at semipermissive temperature (20°) as well as at restrictive temperature (25°).
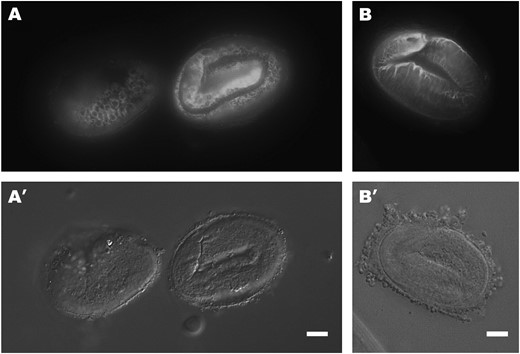
At any temperature, the intracellular distribution of SQT-3 in developing dpy-31 embryos is indistinguishable from that of wild type (Figure 6A). However, after secretion, the extracellular assembly of SQT-3 is aberrant in the mutant. In dpy-31 embryos reared at 20° (Figure 6B), localization of SQT-3 to the annuli can still be detected, but in comparison to wild type (Figure 5B) the signal is diffuse and the annular staining appears intermittent. At 25°, concurrently with the disruption of body morphology occurring at the threefold stage, the distribution of SQT-3 is severely affected (Figure 6C). These results show that in dpy-31 mutants SQT-3 is secreted normally both at semipermissive and at restrictive temperature but its extracellular assembly is affected in a temperature-dependent fashion.
IF staining of unsuppressed and suppressed dpy-31 mutants with the R584 serum. (A) e2770 comma-stage embryo raised at 20°, showing an apparently normal pattern of intracellular staining. (B) e2770 threefold-stage embryo raised at 20°. In comparison to wild type (Figure 5B), annular staining is aberrant. Gaps lacking SQT-3 are visible (arrowhead); moreover, the signal appears generally blurred, indicating that the antigen could diffuse within the cuticle. (C) e2770 threefold-stage embryo raised at 25°. Disruption of SQT-3 localization is enhanced. Areas of intense fluorescence are visualized (arrowhead), which may represent aggregates of misassembled SQT-3 molecules. (D) e2770; e2902 threefold-stage embryo raised at 25°. SQT-3 assembly is restored to wild type. Bars, 10 μm.
To determine how suppression of dpy-31 lethality by the sqt-3(sup) alleles occurs, we examined by IF staining dpy-31(e2770); sqt-3(e2902) homozygous animals raised at 25°, finding that assembly of SQT-3 in the embryonic cuticle is indistinguishable from that of wild type (Figure 6D). Identical results were obtained by staining other dpy-31; sqt-3(sup) double mutants (data not shown).
To examine how the cs-lethal lesions affect viability, sqt-3(e2909) worms raised at the two growth extremes were stained with the R584 antiserum. This showed that SQT-3 is retained intracellularly in e2909 worms that complete embryogenesis at 15° (Figure 7A), while at 25° SQT-3 is secreted and assembles into the cuticle as in wild type (Figure 7B). Likewise, intracellular retention at 15° and secretion at 25° was detected in the other two strongest cs-lethal alleles, e2908 (Figure 7C) and e2810 (data not shown). The pattern of retention at 15° differed from that detected in e2117 embryos in an important aspect: in cs-lethal worms, retained collagen forms appear to be rapidly degraded intracellularly, as shown by the progressive disappearance of the reactive antigen without concurrent secretion from the cells (Figure 7A). Paradoxically, cs lethality is seen in strains in which retained collagens are rapidly eliminated, whereas the persistently retained collagen forms in e2117 Gly substitution mutants do not cause significant lethality at 15°.
IF staining of sqt-3(sup) cs-lethal mutants with the R584 serum. (A) e2909 threefold-stage embryo reared at 15°. Note the presence of retained collagen forms in some hypodermal cells (arrowheads). (B) e2909 threefold-stage embryo reared at 25°. SQT-3 is assembled normally into the cuticle. (C) e2908 threefold-stage embryo reared at 15°. The arrowheads indicate intracellularly retained SQT-3. Bars, 10 μm.
To examine the relationship between SQT-3 and DPY-17 assembly, we tested SQT-3 immunolocalization in dpy-17(e164) putative null mutants raised at 25°. In comma-stage embryos, the intracellular pattern of staining appears grossly normal (Figure 8A, left embryo). Unexpectedly, however, SQT-3 fails almost entirely to be secreted in threefold-stage e164 embryos (Figure 8A, right embryo). The pattern of intracellular accumulation of SQT-3 observed in dpy-17 mutants closely resembles that seen in sqt-3(e2117) embryos (compare Figures 8A and 5D), as retained collagens could be seen to persist within the hypodermal cells throughout the L1 stage. In contrast to e2117 embryos, however, e164 animals secrete low levels of SQT-3 molecules, which apparently assemble normally into the cuticle, resulting in a faint and fragmented pattern of annular staining (data not shown). Likewise, limited secretion of SQT-3 was detected in dpy-17(e164) animals raised at 15° (data not shown). To confirm that intracellular retention of SQT-3 is specific to dpy-17(0) mutants, we tested SQT-3 immunolocalization in dpy-7(e88), dpy-10(e128), and dpy-13(e184) cuticle collagen mutants (von Mende et al. 1988; Johnstone et al. 1992; Levy et al. 1993). In all cases, SQT-3 is secreted and assembles in the cuticle normally at 20° (Figure 9). Taken together, these results suggest that loss of dpy-17 specifically affects intracellular assembly of SQT-3, impairing its secretion, which in turn leads to reduced extracellular levels of the collagen.
IF staining of dpy-17(−) and dpy-17(sup) dpy-31(−) mutants with the R584 serum. (A) e164 comma-stage embryo (left) and threefold-stage embryo (right) raised at 25°. While the comma-stage pattern appears normal, SQT-3 is retained intracellularly in thethreefold-stage embryo. (B) dpy-17(e2899) dpy-31(e2770) threefold-stage embryo raised at 25°. SQT-3 extracellular assembly is restored to wild type. Bars, 10 μm.
SQT-3 immunolocalization in some cuticle collagen mutants. (A) dpy-7(e88) L1 larva stained with R584. SQT-3 is secreted and its assembly appears grossly normal. Likewise, dpy-10(e128) (B) and dpy-13(e184) (C) threefold-stage embryos show normal patterns of SQT-3 secretion and extracellular assembly. Animals were raised at 20°. Bars, 10 μm.
To examine the effect of the dpy-17(sup) mutations on SQT-3 assembly in a dpy-31(−) background, we tested SQT-3 immunolocalization in dpy-17(sup) dpy-31-suppressed animals. Similar to what is seen in dpy-31; sqt-3(sup) worms, SQT-3 extracellular assembly is restored to wild type in both dpy-17(e2899) dpy-31(e2770) (Figure 8B) and dpy-17(e2910) dpy-31(e2770) double mutants raised at 25° (data not shown). We also confirmed that SQT-3 assembly occurs normally in dpy-17(e2898) animals at all temperatures (data not shown).
DISCUSSION
In this study, we have analyzed the interaction between dpy-31, coding for an essential C. elegans BMP-1 ortholog, and sqt-3 and dpy-17, two cuticle collagen genes that we isolated as extragenic suppressors of dpy-31 lethality. The development of a SQT-3-specific polyclonal serum has enabled us to investigate the cellular mechanisms underlying suppression of dpy-31 by sqt-3 and dpy-17. Our results suggest that DPY-31 is responsible for extracellular processing of SQT-3 and implicate the SQT-3 C-terminal region in intracellular and extracellular events during processing. Furthermore, we infer that assembly of DPY-17 and SQT-3, which belong to distinct collagen subfamilies, are coupled at multiple points before and after secretion.
BMP-1-mediated C-terminal processing of vertebrate fibrillar procollagens is believed to occur in the extracellular milieu prior to fibril formation, as indicated by the presence of the functional protease in the culture media of collagen-secreting cells (Hojima et al. 1985). BMP-1 is synthesized as a zymogen that is activated by furin-mediated proteolytic maturation. The activation takes place late in the secretory pathway, presumably immediately before secretion (Leighton and Kadler 2003). Like most BMP-1 homologs (Bond and Beynon 1995), DPY-31 also contains a furin recognition motif located at the junction between the N-terminal prodomain and the catalytic domain. This suggests that the mechanisms of activation of BMP-1 and DPY-31 as well as their sites of action are conserved. Consequently, assuming that DPY-31 mediates cleavage of the SQT-3 C terminus, loss of dpy-31 would be expected to exclusively affect extracellular assembly of the collagen. The results of the IF experiments show that this is indeed the case and are therefore consistent with the hypothesis that SQT-3 is a DPY-31 target. Our results also suggest that in dpy-31 mutants SQT-3 is capable of residual assembly at low temperatures.
We have clarified the role of sqt-3 by isolating a null allele of this gene. Previously, it had been assumed that the conditionally lethal phenotype of some existing sqt-3 mutants (such as e2117 animals) derived from reduced thermal stability of the cuticle as a result of the incorporation of abnormal collagen molecules. Our results show that simple loss of SQT-3 is sufficient to cause temperature-dependent lethality. Hence, lack of assembled SQT-3 may cause a structural derangement, which renders the whole cuticle or its assembly thermolabile. Significantly, the dpy-31 null phenotype is similarly ts lethal. The similarity of the amorphic phenotypes of dpy-31 and sqt-3 strongly supports the interrelatedness of their functions. Furthermore, this finding indicates that the cs-lethal effect seen in some sqt-3(sup) alleles does not reflect the null state of the gene, which is compatible with viability at 15°. Consequently, in the cs-lethal strains the aberrant collagen chains produced at 15° must have a toxic effect and the Gly substitutions somehow counteract or eliminate the toxicity.
The finding that missense mutations affecting the SQT-3 C terminus are sufficient to rescue extracellular assembly of the collagen in the absence of dpy-31 function provides further support to the hypothesis that DPY-31 is required for C-terminal proteolytic processing of SQT-3 and suggests that alterations in the C terminus of the collagen can bypass the requirement for cleavage by DPY-31. In turn, successful assembly of SQT-3 must rescue the process of embryonic cuticle formation. The actual molecular mechanisms underlying suppression of dpy-31 lethality by the sqt-3(sup) alleles remain unclear. The structural modifications resulting from the sqt-3(sup) lesions may render cleavage of collagen trimers unnecessary; more probably, they may allow action of an alternative protease substituting for DPY-31. The present data do not permit discrimination between these two possibilities, because the R584 antiserum was found to be unsuitable for Western blot analysis (data not shown) and therefore cannot be employed to examine cleavage of SQT-3 by DPY-31.
The results of IF on e2810, e2908, and e2909 mutants reveal that the cs-lethal lesions, which at 25° can rescue the extracellular assembly of SQT-3 in a dpy-31(−) background, interfere at 15° with intracellular processing of the collagen. However, we find that intracellular retention of SQT-3 per se does not cause cs lethality because sqt-3(e2117) Gly substitution mutants are viable at 15° despite retention of SQT-3. The IF staining experiments also showed that retained collagen forms are degraded at different rates in e2117 and e2810-e2908-e2909 mutants. Furthermore, sqt-3 Gly substitution alleles were recovered as intragenic suppressors of the cs lethality associated with e2810, e2908, and e2909. Taken together, the biochemical and genetic data indicate that the Gly substitution and cs-lethal lesions must necessarily affect SQT-3 assembly in distinct ways, most likely by causing collagen biosynthesis to stall at different intracellular steps.
Intriguingly, cs-lethal lesions are not distributed at random across the SQT-3 C-terminal region: sqt-3(sup) alleles altering aa 288–291 are generally not associated with cold sensitivity, while 5/6 of the remaining suppressors show this phenotypic trait (Figure 1A). Indirect evidence supports the hypothesis that the YCALD conserved motif between aa 286 and 290 represents the DPY-31 cleavage site in SQT-3: this stretch of residues matches the Y(xx)xD consensus sequence identified in several BMP-1 substrates (Steiglitz et al. 2004). The observation that cs lethality is characteristic of lesions located outside the putative cleavage site suggests a role for these residues in events taking place in the intracellular milieu before secretion and proteolytic maturation.
Because of the paucity of structural and biochemical data, our knowledge of intracellular processing of nematode collagens remains sketchy and is mostly based on indirect genetic inferences. It is, however, clear that at least three intracellular steps must necessarily be shared between vertebrate and nematode collagens, namely collagen chain oligomerization, trimerization, and intracellular transport of folded procollagen chains.
In vertebrates, oligomerization of fibrillar procollagens occurs post-translationally in the endoplasmic reticulum (ER), as reviewed by McLaughlin and Bulleid (1998). Briefly, the three collagen chains first associate via a portion of their nontrimeric C-terminal extension. A variable stretch of residues in the C terminus, termed the chain recognition sequence, ensures selective oligomerization of structurally compatible monomers. After chain association, nucleation of the triple helix begins from the C-terminal-most Gly–X–Y repeats and propagates toward the N terminus in a zipper-like fashion. Later, folded collagen chains are translocated through the Golgi to the extracellular milieu via cisternal transport (Bonfanti et al. 1998; Mironov et al. 2003).
It has not been hitherto determined whether nematode collagen trimerization proceeds from the C terminus toward the N terminus, or vice versa. No clear equivalent of the variable chain recognition sequence can be identified in nematode cuticle collagens, and selective oligomerization may be primarily achieved through temporal regulation of collagen gene transcription, which ensures that cuticle collagens are expressed in discrete waves of abundance (Johnstone and Barry 1996; McMahon et al. 2003). Genetic evidence indicates that nematode collagens are transported from the ER to the Golgi via COPII-coated vesicles (Roberts et al. 2003), but the mechanisms regulating collagen vesicular transport remain uncharacterized.
Studies of vertebrate collagens have shown that Gly substitutions cause prolonged ER retention of misfolded chains (reviewed in Engel and Prockop 1991). Assuming that the sequence of intracellular processing steps is conserved in nematodes, ER retention should also occur in nematode collagen Gly substitution mutants, such as sqt-3(e2117) animals. Consequently, recovery of Gly substitutions as intragenic suppressors of cold sensitivity indicates that at 15° the SQT-3 chains must become toxic downstream of oligomerization and trimerization. Possibly, the cs-lethal lesions conditionally affect collagen processing taking place in the Golgi. In an alternative scenario, it could be hypothesized that part of the C-terminal sequence is required for targeting to the Golgi and/or to the extracellular environment and that the SQT-3 toxic forms block transport at 15°, thereby impeding secretion of other collagen chains as well. These models hold that ER retention of SQT-3 toxic forms due to the Gly substitutions could permit Golgi processing/secretion of other collagen chains during cuticle formation, thereby relieving the embryonic lethality. The cs-lethal effect can be further investigated by characterizing additional suppressors of cold sensitivity, some of which appear to be extragenic. This approach may uncover novel intracellular components of the nematode collagen assembly pathway.
A similarly complex picture emerges from analysis of the dpy-17(sup) alleles. Genetic evidence shows that the dpy-17(sup) mutations are gain-of-function alleles capable of suppressing loss of dpy-31 but not of sqt-3, suggesting that they do not act by bypassing the requirement for SQT-3 within the cuticle (for instance, by endowing DPY-17 with a SQT-3-like role), but rather rescue assembly of SQT-3 in the absence of DPY-31. This is confirmed by the IF staining experiments, which revealed that in dpy-17(sup) dpy-31 double mutants embryonic assembly of SQT-3 is approximately normal at 25°. What might be the effect of the DPY-17 gain-of-function chains on SQT-3 assembly? As discussed above, the dpy-17(sup) alleles have unusual missense mutations causing replacement of two X residues in two Gly–X–Y repeats located in proximity to the C-terminal end of DPY-17. That the dpy-17(sup) alleles are not loss-of-function alleles is consistent with what was observed in the case of vertebrate collagens, where some X and Y position substitutions occur as nonpathogenic polymorphisms (reviewed in Myllyharju and Kivirikko 2001). In folded collagen trimers, the glycine residues in the Gly–X–Y repeats of each chain face into the center of the triple helix, while X and Y residues have their side chains pointing outward from the helix (Ramachandran 1967). The side chains of residues X and Y are therefore available for lateral interactions, which are known to play an important role in formation of homotypic and heterotypic collagen fibrils in vertebrates (reviewed in van der Rest and Garrone 1991). Given that both e2898 and e2899 lead to charge/hydrophilicity changes (Figure 4C), it is conceivable that they affect packing of collagen trimers into fibrils. McMahon et al. (2003) demonstrated that C. elegans collagens form discrete matrix substructures composed of genetically distinct collagen trimers. A tempting speculation is that the dpy-17(sup) alleles could modify the conformation of a matrix substructure composed of SQT-3 and DPY-17 trimers, thereby allowing SQT-3 to be incorporated into the DPY-17–SQT-3 polymeric matrix in the absence of C-terminal processing by DPY-31.
In addition to the interdependence of DPY-17 and SQT-3 extracellular assemblies, the IF staining experiments also show that, unexpectedly, loss of dpy-17 specifically causes SQT-3 intracellular retention. A simple interpretation for this finding is that SQT-3 and DPY-17 might form heterotrimers, like C. elegans basement membrane collagens LET-2 and EMB-9 do, which are obligate heterotrimeric partners (Gupta et al. 1997). Heterotrimer formation is also well documented in several instances for vertebrate collagens (reviewed in van der Rest and Garrone 1991). However, we think this explanation unlikely for the following reasons: direct proof that nematode cuticle collagens assemble into heterotrimers is lacking and structural considerations suggest that if heterotrimerization does occur, it is limited to chains belonging to the same collagen subgroup (Johnstone 1994). SQT-3 and DPY-17 are members of different collagen subfamilies and share only 30.3% of overall primary sequence identity. It is difficult to conceive how stable SQT-3–DPY-17 heterotrimers could be formed. Instead, the intracellular accumulation of SQT-3 observed in a dpy-17(0) background suggests that assembly of the matrix substructure formed by inclusion of DPY-17 and SQT-3 trimers could commence intracellularly prior to secretion. This hypothesis can be reconciled with recent observations revealing that assembly of vertebrate collagen fibrils is initiated in the late secretory pathway, rather than in the extracellular environment as previously thought (Kadler et al. 2003). It can be imagined that, after folding, DPY-17 and SQT-3 trimers begin immediately forming higher-order aggregates, which are then secreted and stabilized by crosslinks, thus yielding the final SQT-3–DPY-17 heteropolymer. Under this model, the partial retention of SQT-3 observed in dpy-17 mutants would result from failure of intracellular formation of higher-order SQT-3 multimers due to the absence of DPY-17 trimers. This model is consistent with the observation that the Dpy-17 embryonic phenotype resembles the Sqt-3 phenotype, but is milder and is not associated with ts lethality: the partial secretion of SQT-3 trimers occurring in dpy-17(0) mutants may be sufficient to ensure viability at 25°. It seems possible that SQT-3 is a DPY-17 obligate partner, but is capable of residual assembly on its own. This hypothesis can be further tested by analyzing dpy-17 and sqt-3 mutants with a DPY-17-specific antiserum.
Footnotes
Communicating editor: B. J. Meyer
References
Bond, J. S., and R. J. Beynon,
Bonfanti, L., A. A. Mironov, Jr., J. A. Martinez-Menarguez, O. Martella, A. Fusella et al.,
Burglin, T. R., E. Lobos and M. L. Blaxter,
Cox, G. N., J. S. Laufer, M. Kusch and R. S. Edgar,
Cox, G. N., L. M. Shamansky and R. J. Boisvenue,
Davis, M. W., A. J. Birnie, A. C. Chan, A. P. Page and E. M. Jorgensen,
Engel, J., and D. J. Prockop,
Frand, A. R., S. Russel and G. Ruvkun,
Gupta, M. C., P. L. Graham and J. M. Kramer,
Hishida, R., T. Ishihara, K. Kondo and I. Katsura,
Hodgkin, J.,
Hojima, Y., M. van der Rest and D. J. Prockop,
Hutter, H., B. E. Vogel, J. D. Plenefisch, C. R. Norris, R. B. Proenca et al.,
Johnstone, I. L.,
Johnstone, I. L.,
Johnstone, I. L., and J. D. Barry,
Johnstone, I. L., Y. Shafi and J. D. Barry,
Kadler, K. E., E. G. Canty and L. Yinhui,
Kenning, C., I. Kipping and R. J. Sommer,
Kessler, E., K. Takahara, L. Biniaminov, M. Brusel and D. S. Greenspan,
Kramer, J. M.,
Kramer, J. M., G. N. Cox and D. Hirsh,
Leighton, M., and K. E. Kadler,
Levy, A. D., J. Yang and J. M. Kramer,
Li, S. W., A. L. Sieron, A. Fertala, Y. Hojima, W. V. Arnold et al.,
Maduro, M., and D. Pilgrim,
McLaughlin, S. H., and N. J. Bulleid,
McMahon, L., J. M. Muriel, B. Roberts, M. Quinn and I. L. Johnstone,
Miller, D. M., and D. C. Shakes,
Mironov, A. A., A. A. Mironov, Jr., G. V. Beznoussenko, A. Trucco, P. Lupetti et al.,
Mohrlen, F., H. Hutter and R. Zwilling,
Myllyharju, J., and K. I. Kivirikko,
Myllyharju, J., and K. I. Kivirikko,
Novelli, J., S. Ahmed and J. Hodgkin,
Page, A. P., and A. D. Winter,
Park, E. C., and H. R. Horvitz,
Praitis, V., E. Casey, D. Collar and J. Austin,
Priess, J. R., and D. I. Hirsh,
Ramachandran, G. N.,
Roberts, B., C. Clucas and I. L. Johnstone,
Sambrook, J., E. F. Fritsch and T. Maniatis,
Steiglitz, B. M., M. Ayala, K. Narayanan, A. George and D. S. Greenspan,
Sulston, J., and J. Hodgkin,
Suzuki, M., N. Sagoh, H. Iwasaki, H. Inoue and K. Takahashi,
Thacker, C., K. Peters, M. Srayko and A. M. Rose,
van der Keyl, H., H. Kim, R. Espey, C. V. Oke and M. K. Edwards,
van der Rest, M., and R. Garrone,
von Mende, N., D. M. Bird, P. S. Albert and D. L. Riddle,
Wicks, S. R., R. T. Yeh, W. R. Gish, R. H. Waterston and R. H. Plasterk,
Yang, J., and J. M. Kramer,