-
PDF
- Split View
-
Views
-
Cite
Cite
Cristel C Carles, Kvin Lertpiriyapong, Keira Reville, Jennifer C Fletcher, The ULTRAPETALA1 Gene Functions Early in Arabidopsis Development to Restrict Shoot Apical Meristem Activity and Acts Through WUSCHEL to Regulate Floral Meristem Determinacy, Genetics, Volume 167, Issue 4, 1 August 2004, Pages 1893–1903, https://doi.org/10.1534/genetics.104.028787
Close - Share Icon Share
Abstract
Shoot and floral meristem activity in higher plants is controlled by complex signaling networks consisting of positive and negative regulators. The Arabidopsis ULTRAPETALA1 (ULT1) gene has been shown to act as a negative regulator of meristem cell accumulation in inflorescence and floral meristems, as loss-of-function ult1 mutations cause inflorescence meristem enlargement, the production of extra flowers and floral organs, and a decrease in floral meristem determinacy. To investigate whether ULT1 functions in known meristem regulatory pathways, we generated double mutants between ult1 alleles and null alleles of the meristem-promoting genes SHOOTMERISTEMLESS (STM) and WUSCHEL (WUS). We found that, although the ult1 alleles have no detectable embryonic or vegetative phenotypes, ult1 mutations restored extensive organ-forming capability to stm null mutants after germination and increased leaf and floral organ production in stm partial loss-of-function mutants. Mutations in ULT1 also partially suppressed the wus shoot and floral meristem phenotypes. However, wus was epistatic to ult1 in the center of the flower, and WUS transcriptional repression was delayed in ult1 floral meristems. Our results show that during the majority of the Arabidopsis life cycle, ULT1 acts oppositely to STM and WUS in maintaining meristem activity and functions in a separate genetic pathway. However, ULT1 negatively regulates WUS to establish floral meristem determinacy, acting through the WUS-AG temporal feedback loop.
HIGHER plants continuously produce organs such as leaves and flowers from small groups of cells at their growing tips, called apical meristems. During the development of Arabidopsis thaliana, the shoot apical meristem (SAM) provides all of the cells for above-ground organ formation while simultaneously maintaining a reservoir of pluripotent stem cells (Steeves and Sussex 1989). The stem cell population resides at the very apex of the meristem and replenishes those cells that are lost during organogenesis on the meristem flanks. The SAM forms during embryonic development, but generates the vast majority of the lateral organs after germination. The SAM generates leaves during the vegetative phase of development, followed by stem tissue, axillary meristems, and an indeterminate number of flowers during the reproductive phase. Flowers arise from floral meristems, which sequentially produce sepals, petals, stamens, and carpels in a whorled pattern from the outside to the inside of the flower. Unlike SAMs, floral meristems are determinate structures that terminate stem cell activity at the time of carpel formation.
Overlapping networks of meristem-promoting and meristem-restricting factors regulate shoot apical and floral meristem activity during Arabidopsis development. One key meristem-promoting factor is SHOOTMERISTEMLESS (STM). Plants carrying strong stm alleles fail to maintain a functional SAM during embryogenesis (Barton and Poethig 1993), while plants carrying weaker stm alleles have reduced shoot and floral meristem function (Clark et al. 1996). STM encodes a Knotted1-like homeobox (KNOX) gene that is expressed only in shoot and floral meristem cells (Long et al. 1996). STM activity prevents SAM cells from undergoing differentiation by restricting the expression of the ASYMMETRIC LEAVES1 (AS1) and AS2 genes to organ primordia, thereby preventing the inappropriate development of leaves across the shoot apex (Byrne et al. 2000, 2002). Thus, STM provides an environment in which stem cell derivatives can become amplified to the appropriate extent prior to their incorporation into organ primordia.
The homeodomain transcription factor WUSCHEL (WUS; Mayer et al. 1998) maintains stem cell identity as part of a spatial negative feedback loop that functions in both shoot and floral meristems. WUS is expressed in a small group of cells in the interior of these meristems (Mayer et al. 1998), called the organizing center, where it is required to specify the overlying cells as stem cells (Schoof et al. 2000). In floral meristems, WUS is also required to induce the expression of its own repressor, AGAMOUS (AG; Lenhard et al. 2001; Lohmann et al. 2001). Repression of WUS by AG is necessary to terminate stem cell activity at the appropriate time during flower development to permit the cells in the center of the flower to differentiate into carpels.
The stem-cell-promoting activity of WUS is modulated by the CLAVATA (CLV) signaling pathway. The function of the CLV pathway is to restrict excess stem cell accumulation by limiting the size of the WUS expression domain (Brand et al. 2000; Schoof et al. 2000). The CLV3 gene is expressed in the stem cell population of shoot and floral meristems and encodes a small, secreted signaling molecule (Fletcher et al. 1999; Rojo et al. 2002). CLV3 protein spreads through the extracellular space to the interior regions of the meristems, where it is proposed to interact with a receptor complex (Trotochaud et al. 1999) consisting of the leucine-rich-repeat (LRR) receptor kinase CLV1 (Clark et al. 1997) and the LRR receptor-like protein CLV2 (Jeong et al. 1999). CLV3 represses WUS transcription in the stem cells and their lateral neighbors; however, the movement of CLV3 protein into the meristem interior is limited by CLV1, allowing WUS to be transcribed in the organizing center (Lenhard and Laux 2003).
An additional negative regulator of meristem cell accumulation is the ULTRAPETALA1 (ULT1) gene. ULT1 encodes a novel cysteine-rich protein with a B box-like domain and is expressed throughout shoot and floral meristems and in developing stamens and carpels (C. Carles, D. Choffnes-Inada, K. Reville, K. Lertpiriyapong and J. Fletcher, unpublished results). The loss-of-function ult1-1 and ult1-2 mutations cause the reproductive (inflorescence) meristems to produce more floral meristems than normal and the floral meristems to produce extra organs (Fletcher 2001). These phenotypes correlate with an increase in the size of ult1 inflorescence and floral meristems and an enlargement of the CLV1 expression domain in the interior of the ult1 inflorescence meristem. ult1 mutations also lead to the partial loss of floral meristem determinacy, such that supernumerary whorls of carpels, stamens, and/or undifferentiated tissue are observed in the center of ult1 gynoecia.
The relationship between ULT1 and the CLV loci, all of which negatively regulate shoot and floral meristem cell accumulation, was investigated using genetic analysis (Fletcher 2001). Double mutants generated between ult1 alleles and strong clv1 or clv3 alleles showed synergistic effects on inflorescence meristem size, indicating that these genes have overlapping functions in controlling SAM growth but act in separate genetic pathways. In addition, the central tissue of some ult1 clv1 and ult1 clv3 double-mutant flowers developed into entirely new inflorescence meristems, revealing that ULT1 and the CLV genes also interact to regulate floral meristem determinacy. One point at which the ULT1 pathway and the CLV pathway might intersect is at the level of WUS regulation, as limiting WUS activity is essential for both proper SAM maintenance and floral meristem determinacy.
ULT1 acts to restrict shoot and floral meristem cell accumulation, while STM and WUS both function to promote meristem activity. To further investigate the role of ULT1 in meristem growth control, we have analyzed the interactions between ULT1 and the STM and WUS genes at the genetic and molecular levels. We find that ULT1 functions in a genetic pathway separate from STM and WUS in restricting shoot and floral meristem size, but that ULT1 and WUS act antagonistically in the same pathway to control floral meristem determinacy.
MATERIALS AND METHODS
Plant materials and growth conditions:
Plants were grown in a 1:1:1 mixture of perlite:vermiculite:topsoil under 140 mmol/m2 sec of continuous constant illumination at 22° and watered daily with a dilute (1:1500) solution of Miracle-Gro 20-20-20 fertilizer. Putative double-mutant plants were identified in the F2 generation and confirmed through segregation analysis in the F3 generation. All plants were in the Landsberg erecta (Ler) ecotype. The stm-11 allele was provided by Jeff Long and Kathy Barton, and the wus-1 allele by Thomas Laux.
Microscopy:
Confocal laser scanning microscopy was performed as described previously (Running et al. 1995) using a Zeiss 510 confocal microscope. Scanning electron microscopy was performed as described previously (Bowman et al. 1989) using a Hitachi 4700 electron microscope with digital imaging capability.
Histology:
Tissues were fixed for histological analysis by formaldehyde acetic acid vacuum infiltration, dehydrated, processed through paraffin wax, sectioned at 8 μm thickness, stained with toluidine blue, and visualized using a Zeiss Axiophot microscope. Images were acquired with a 12-bit MicroMax cooled CCD camera operated by IPLab software.
In situ hybridization:
A WUS antisense probe for in situ hybridization was generated as described previously (Mayer et al. 1998) using a digoxigenin labeling mix (Roche). Tissue fixation and in situ hybridization were performed as described previously (Jackson 1991). Slides were prehybridized at 55° for 5 hr, and 200 μg of probe per slide was then added for an overnight incubation at 55°. After two washes in 0.2× SSC 0.1% SDS of 10 min each at 55°, the slides were treated with 10 μg/ml RNAse A for 30 min at 37°. Two more washes (0.2× SSC 0.1% SDS, 10 min each at 55°) were then performed. For signal detection the NBT/BCPIP reagents (Roche) were applied for 42 hr.
RESULTS
ult1 embryonic and vegetative development:
Prior analysis revealed that ult1 mutants accumulate excess SAM cells during the reproductive phase of development—both inflorescence and floral meristems are larger in ult1-1 and ult1-2 mutant plants than in wild-type plants, and the flowers produce more whorls and floral organs than normal (Fletcher 2001). The ult1-1 mutation has more severe effects on meristem size and on sepal and petal number than the ult1-2 mutation. The phenotypes of plants carrying the ult1-3 mutation, which is caused by a T-DNA insertion that abolishes ULT1 transcription (C. Carles, D. Choffnes-Inada, K. Reville, K. Lertpiriyapong and J. Fletcher, unpublished results), are statistically indistinguishable from those of ult1-2 plants. Thus, ult1-2 is a phenotypic null allele for the ULT1 locus, while the ult1-1 allele has slightly more severe effects on meristem size and sepal/petal number than the ult1-3 knockout allele and is weakly semidominant (C. Carles, D. Choffnes-Inada, K. Reville, K. Lertpiriyapong and J. Fletcher, unpublished results).
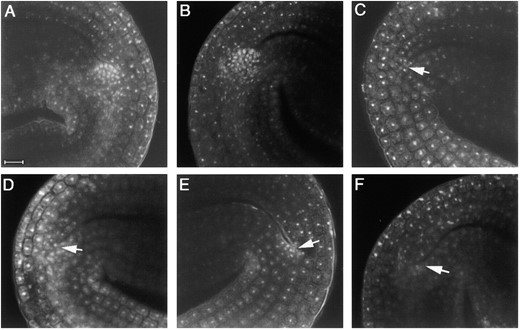
To determine at what stage plant development is first affected by mutations in ULT1, we examined wild-type Ler, ult1-1, and ult1-2 embryos and seedlings. Shoot apical meristem cell layering, organogenesis, and organ morphology appear normal in ult1-1 and ult1-2 embryos and seedlings (Figure 1B
Embryonic shoot apical meristem formation in wild-type, ult1, stm, and wus mutant plants. (A) Wild-type Ler embryonic SAM appears as a dome-shaped structure at the base of the two cotyledons. (B) ult1-2 embryonic SAM resembles that of the wild type. (C) stm-11 embryos lack a dome-shaped SAM structure at the base of the cotyledons (arrow). (D) ult1-2 stm-11 embryos resemble stm-11 single-mutant embryos. (E) wus-1 embryos have only a few densely staining cells at the base of the cotyledons (arrow) and lack a dome-shaped SAM structure. (F) ult1-2 wus-1 embryos resemble wus-1 single-mutant embryos.
and data not shown). Confocal laser scanning microscopy reveals that the ult1-2 mature embryonic meristem size and cell number is not significantly different from that of wild-type meristems (Figure 1, A and B). ult1-1 mutant embryonic meristems on average are 33.0 ± 1.2 μm wide and 8.5 ± 0.5 μm tall (n = 7), while Ler embryonic meristems on average are 31.2 ± 1.2 μm wide and 9.3 ± 0.4 μm tall (n = 12). Seven-day-old ult1-1 seedlings (52.4 ± 2.0 μm wide, 22.4 ± 1.1 μm tall, n = 18) likewise have approximately the same size shoot apical meristem as Ler seedlings (48.9 ± 2.3 μm wide, 23.9 ± 1.8 μm tall, n = 8). ult1-3 null mutant plants are also indistinguishable from wild-type plants during the embryonic and vegetative periods. Thus ult1 mutant phenotypes are not detectable until the reproductive phase of development.
ult1 interactions with stm:
Since ult1 mutants accumulate excess meristem cells during reproductive development, we hypothesized that the ult1 alleles might restore shoot and/or floral meristem activity to stm mutants, which fail to maintain meristem cells in an undifferentiated state. This has been shown to be the case for the clv1 and clv3 mutants, which partially suppress stm mutant phenotypes in a dose-dependent manner (Clark et al. 1996). To test this hypothesis, we generated double mutants between the ult1 alleles and strong and weak stm alleles and followed their embryonic and postembryonic development.
Plants homozygous for the stm-11 null allele (Long and Barton 1998) form a pair of normal embryonic leaves (cotyledons) during embryogenesis, but fail to develop a densely staining dome of meristematic cells at the base between them (Figure 1C). Among the progeny of ult1-2 F2 plants that segregated stm-11, we found that ∼25% of embryos lacked a dome-shaped SAM between the cotyledons (Figure 1D). In addition, progeny of ult1-1 F2 plants that segregated stm-11 also yielded ∼25% embryos lacking a discernible SAM. Thus neither the ult1-1 mutation nor the ult1-2 mutation rescues the stm-11 embryo shoot-meristemless phenotype.
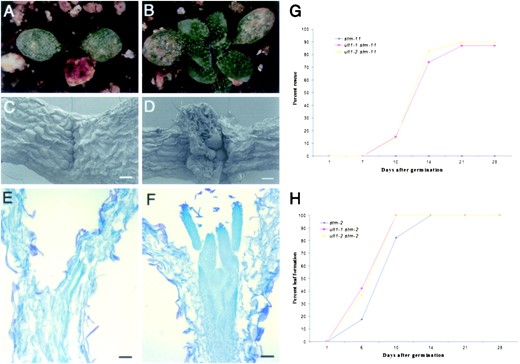
Following germination, stm-11 mutant seedlings do not produce postembryonic organs between the cotyledons (Figure 2, A and C)
Shoot apical meristem activity in ult1 stm-11 seedlings. (A) stm-11 seedling 14 days after germination. No organs have formed between the cotyledons. (B) ult1-2 stm-11 seedling 14 days after germination. Leaves are developing from between the cotyledons. (C) SEM of an stm-11 seedling 10 days after germination. (D) SEM of an ult1-1 stm-11 seedling 10 days after germination. Leaves are forming between the cotyledons at the position where they are normally produced by the shoot apical meristem in wild-type plants. A pair of developing leaves arch over the SAM. (E) Section through an stm-11 seedling 10 days after germination. There is no evidence of a dome-shaped SAM. (F) Section through an ult1-2 stm-11 seedling 10 days after germination. A correctly positioned SAM is producing leaves from its flanks, and the organization of the meristem cell layers is intact. (G) Percentage of ult1-1 stm-11 (red, n = 46) and ult1-2 stm-11 (yellow, n = 29) mutant seedlings that formed a shoot apical meristem and produce true leaves following germination and growth under constant light. No stm-11 plants (blue, n = 41) grown at the same time under the same conditions produced any leaves or any sign of a shoot apical meristem. (H) Percentage of stm-2 (blue, n = 26), ult1-1 stm-2 (red, n = 35), and ult1-2 stm-2 (yellow, n = 27) mutant seedlings that formed a shoot apical meristem and produced true leaves following germination and growth under constant light. Nearly all of the single and double-mutant plants produced organs from the shoot apex, but the ult1-1 and ult1-2 mutations increased the rate at which stm-2 mutants developed postembryonic leaves. Bars: 100 μm, C and D; 30 μm, E and F.
and completely lack a dome-shaped shoot apical meristem (Figure 2E). Double mutants generated between stm-11 and either ult1-1 or ult1-2 initially resembled stm-11 single-mutant plants. After 7 days of growth, the ult1-1 stm-11 and ult1-2 stm-11 double-mutant seedlings showed no evidence of organ formation between the cotyledons (Figure 2G). However, after 10 days ∼15% of the ult1-1 stm-11 and ult1-2 stm-11 double-mutant plants began to develop leaves (Figure 2, B, D, and G). Scanning electron microscopy and sectioning revealed that the leaves produced by the double-mutant plants originated at the seedling shoot apex from the flanks of a dome of meristematic cells between the cotyledons (Figure 2, D and F). In contrast, leaves that are occasionally produced by single-mutant plants carrying the weaker stm-1 allele arise from the hypocotyl region (Clark et al. 1996). After 21 days nearly 90% of ult1-1 stm-11 and ult1-2 stm-11 plants displayed this “restored” phenotype, while none of the stm-11 single mutants showed signs of postembryonic organ formation (Figure 2G). Thus, a shoot apical meristem structure and organogenic capability is eventually restored to stm-11 mutant plants when ULT1 activity is absent. This experiment also reveals that ULT1 is active during the vegetative phase of development, despite the fact that the ult1 mutants lack a detectable vegetative phenotype.
Ultimately, 100% of the restored ult1-1 stm-11 plants and 86% of the restored ult1-2 stm-11 plants produced one or more abnormal inflorescence meristems bearing one to a few flowers (Figure 3)
Inflorescence meristem and flower phenotypes of ult1 stm and ult1 wus double mutants. (A) A wild-type Ler inflorescence meristem. (B) An ult1-1 mutant inflorescence meristem, which generates flowers containing additional organs of all types, predominantly sepals and petals. (C) ult1-1 stm-11 plants produce inflorescence meristems that generate a limited number of abnormal flowers. (D) Flowers produced by an ult1-1 stm-11 inflorescence meristem contain fewer petals, stamens, and carpels than wild-type flowers do and resemble flowers generated by plants carrying weak stm alleles. Infrequently, flowers form carpeloid structures in the center of the flower (arrow). (E) Plants carrying the weak stm-2 allele produce inflorescence meristems that generate a limited number of abnormal flowers. (F) Flowers produced by an stm-2 inflorescence meristem lack the full complement of internal organs and fail to generate carpels. (G) ult1-1 stm-2 plants produce inflorescence meristems that generate more flowers than stm-2 inflorescence meristems do. (H) Flowers produced by an ult1-1 stm-2 inflorescence meristem contain internal organs and can form unfused carpels or a normal, fused gynoecium (arrows). (I) A wus-1 inflorescence meristem, which generates a small number of abnormal flowers in a disorganized phyllotactic pattern. (J) ult1-1 wus-1 inflorescence meristems form many more flowers than do wus-1 single-mutant meristems in a normal spiral phyllotaxy. (K) Flowers produced by wus-1 plants lack the full complement of organs and generally terminate in a solitary stamen (arrow). (L) Flowers produced by ult1-1 wus-1 plants can form more sepals and petals than either wild-type or wus-1 flowers, but fail to form carpels and generally terminate in a solitary stamen (arrow).
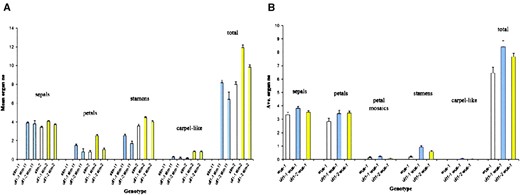
. Compared to wild-type (Figure 3A) and ult1-1 (Figure 3B) inflorescences, which produced flowers in a stereotypical spiral phyllotaxy, ult1-1 stm-11 inflorescences consisted of disorganized aerial structures with abnormal phyllotaxy, consisting of leaves and reduced numbers of flowers (Figure 3C). The flowers produced by the double-mutant inflorescences were also abnormal. Wild-type flowers normally form four sepals, four petals, five or six stamens, and two carpels that fuse to form the central gynoecium (Smyth et al. 1990). In contrast, ult1-1 stm-11 and ult1-2 stm-11 flowers often contained fused and/or mosaic organs, such as fused stamens and sepal/petal, petal/stamen, and stamen/carpel mosaics (see supplemental table at http://www.genetics.org/supplemental/). Moreover, while sepal number was similar to that observed in the wild type, the petals, stamens, and carpels were either reduced in number or absent (Figure 4A)
Organ number in ult1, stm, and wus single- and double-mutant flowers. (A) ult1 mutations restore organogenesis to stm floral meristems. The mean number of organs in the first 10 flowers of stm single-mutant and ult1 stm double-mutant plants is shown. If <10 flowers were produced by an individual plant, then all flowers on the primary inflorescence meristem were counted. At least 15 flowers were counted for each mean, and the standard error is indicated. (B) ult1 mutations restore organogenesis to wus-1 floral meristems, except in the carpel whorl in the center of the flower. The mean number of organs in the first 10 flowers of 10 wus-1 single-mutant and ult1 wus-1 double-mutant plants is shown. If <10 flowers were produced by an individual plant, then all flowers on the primary inflorescence meristem were counted. At least 38 flowers were counted for each mean, and the standard error is indicated.
. Although the center of the flower was the most severely affected, rare ult1-1 stm-11 flowers with central structures bearing ovules and/or stigmatic tissue were observed (Figure 3D), indicating that stm-11 plants are capable of forming all organ types when ULT1 activity is lost. ult1-1 stm-11 and ult1-2 stm-11 plants displaying these inflorescence and floral phenotypes resembled plants homozygous for the weak stm-2 allele (Clark et al. 1996). Thus, significant inflorescence and floral meristem activity is restored to stm null mutant plants in the absence of ULT1 function.
stm-2 mutant plants retain some meristematic activity, as evidenced by their ability to form abnormal rosettes of leaves followed by inflorescence stems that produce reduced numbers of flowers (Clark et al. 1996). To determine whether the ult1 mutations affected the production of these postembryonic structures, we generated ult1 stm-2 double mutants and compared their growth with that of stm-2 single-mutant plants. Because nearly all stm-2 plants eventually form leaves, we compared the rate of postembryonic leaf production between the different genotypes. After 6 days of growth, 43% of ult1-1 stm-2 and 37% of ult1-2 stm-2 seedlings had produced one or more leaves, compared to 17% of stm-2 seedlings (Figure 2H). After 10 days of growth, 100% of ult1-1 stm-2 and ult1-2 stm-2 seedlings had produced leaves, compared to 82% of stm-2 seedlings (Figure 2H). Therefore, stm-2 seedlings generate leaves at a slightly accelerated rate when ULT1 activity is reduced or absent.
The extent of inflorescence and floral meristem development was also greater in ult1 stm-2 plants compared to stm-2 mutants. Approximately one-third of stm-2 plants formed a solitary, terminal flower. The other two-thirds produced more than one flower, frequently four or five. Rarely, an stm-2 plant formed >10 flowers before terminating (Figure 3E). In contrast, 100% of ult1-1 stm-2 and ult1-2 stm-2 plants generated inflorescence meristems bearing more than one flower, and 30–40% of the double-mutant plants produced >10 flowers in a normal spiral phyllotaxy (Figure 3G). The number of floral organs generated per whorl, and per flower, was likewise increased in ult1 stm-2 plants compared to stm-2 plants (Figure 4A). Flowers produced by stm-2 plants contained reduced numbers of sepals, petals, and stamens and rarely formed carpels (Figures 3F and 4A). We observed partial restoration of organogenesis in each whorl of ult1 stm-2 flowers, including the formation of fused or unfused carpel structures in the center of the flower (Figures 3H and 4A). However, we did not detect dose dependence between ult1 alleles and stm alleles in any combination, indicating that while these two genes appear to have opposite activities they do not function in a directly competitive manner.
We used scanning electron microscopy to determine the earliest stage at which ult1-1 stm-2 flower development deviated from stm-2 flower development. At stage 2, when floral meristems first become distinguishable as bulges on the flanks of the shoot apical meristem (stages according to Smyth et al. 1990), we observed no difference between stm-2 and ult1-1 stm-2 floral meristems (see supplemental figure at http://www.genetics.org/supplemental/). However, a distinction was clearly detected when stage 3 floral meristems were compared. At this stage, wild-type floral meristems assume a dome-shaped structure, surrounded by four sepal primordia at the periphery (Smyth et al. 1990). The stage 3 floral meristems of stm-2 plants formed a very reduced apex between the developing sepal primordia (see supplemental figure at http://www.genetics.org/supplemental/). In contrast, the stage 3 floral meristems of ult1-1 stm-2 plants formed a dome between the developing sepal primordia and more closely resembled the wild type (see supplemental figure at http://www.genetics.org/supplemental/). Thus, the effect of the ult1-1 mutation on stm-2 flower development could be detected at the time of sepal initiation, after the floral meristems had formed, consistent with the idea that ULT1 acts competitively with STM during meristem maintenance but not meristem initiation.
ult1 interactions with wus:
The WUSCHEL (WUS) gene is required for proper meristem function, as wus mutant plants are defective in shoot and floral meristem maintenance (Laux et al. 1996). Plants homozygous for the wus-1 null allele form a normal pair of cotyledons during embryogenesis, but produce only a few disorganized meristematic cells at their base (Figure 1E). Because wus-1 plants are sterile, we crossed ult1-1 and ult1-2 plants to wus-1/+ heterozygous plants and identified homozygous ult1 plants in the F2. Among the progeny of ult1-1 and ult1-2 F2 plants that segregated wus-1, we found that ∼25% of embryos lacked a dome-shaped SAM between the cotyledons (Figure 1F). Thus neither the ult1-1 nor the ult1-2 mutation rescues the wus-1 embryo shoot apical meristem defect. After germination the wus-1 plants pause in their development and then produce multiple abbreviated rosettes of leaves from the axils of the cotyledons and across the flat shoot apex (Laux et al. 1996). We compared postembryonic development between wus-1 plants and ult1 wus-1 plants and found that the rate of leaf production in the double mutants was indistinguishable from that of the single mutants. Thus the ult1 mutations do not accelerate vegetative organ formation in wus-1 mutant plants, as they do in stm mutant plants.
After the transition to flowering, wus mutant plants produce abnormal inflorescences and flowers due to reduced meristem activity (Laux et al. 1996; Schoof et al. 2000). wus-1 inflorescence meristems generate far fewer flowers than wild-type meristems do, and the flowers that do form arise in aerial rosettes with a disorganized phyllotaxic pattern (Figure 3I). Under our growth conditions, 45% of wus-1 plants terminated development without flowering, and an additional 37% generated a solitary flower (Table 1)
Terminal structures produced by wus-1 plants and ult1 wus-1 double-mutant plants
Structure . | wus-1 (%) . | ult1-1 wus-1 (%) . | ult1-2 wus-1 (%) . |
|---|---|---|---|
| Basal rosette | 15 | 0 | 0 |
| Aerial rosette | 30 | 0 | 18 |
| Solitary flower | 37 | 28 | 18 |
| Multiple flowers | 18 | 72 | 64 |
Structure . | wus-1 (%) . | ult1-1 wus-1 (%) . | ult1-2 wus-1 (%) . |
|---|---|---|---|
| Basal rosette | 15 | 0 | 0 |
| Aerial rosette | 30 | 0 | 18 |
| Solitary flower | 37 | 28 | 18 |
| Multiple flowers | 18 | 72 | 64 |
Numbers represent the percentage of plants with the indicated structure as a terminal phenotype. Plants terminating in either a basal or an aerial rosette did not produce flowers. wus-1, n = 33; ult1-1 wus-1, n = 36; and ult1-2 wus-1, n = 27 plants scored.
Terminal structures produced by wus-1 plants and ult1 wus-1 double-mutant plants
Structure . | wus-1 (%) . | ult1-1 wus-1 (%) . | ult1-2 wus-1 (%) . |
|---|---|---|---|
| Basal rosette | 15 | 0 | 0 |
| Aerial rosette | 30 | 0 | 18 |
| Solitary flower | 37 | 28 | 18 |
| Multiple flowers | 18 | 72 | 64 |
Structure . | wus-1 (%) . | ult1-1 wus-1 (%) . | ult1-2 wus-1 (%) . |
|---|---|---|---|
| Basal rosette | 15 | 0 | 0 |
| Aerial rosette | 30 | 0 | 18 |
| Solitary flower | 37 | 28 | 18 |
| Multiple flowers | 18 | 72 | 64 |
Numbers represent the percentage of plants with the indicated structure as a terminal phenotype. Plants terminating in either a basal or an aerial rosette did not produce flowers. wus-1, n = 33; ult1-1 wus-1, n = 36; and ult1-2 wus-1, n = 27 plants scored.
. Only 18% of wus-1 plants generated multiple flowers from their adventitious inflorescence meristems (Table 1). In contrast, 100% of ult1-1 wus-1 plants and 82% of ult1-2 wus-1 plants produced one or more flowers in a normal spiral phyllotaxy (Figure 3J, Table 1), indicating that mutations in ULT1 restore some function to wus-1 inflorescence meristems. wus-1 floral meristems generate reduced numbers of floral organs, producing on average three to four sepals, three to four petals, and zero to one stamen per flower (Figures 3K and 4B). wus-1 flowers do not contain more than four sepals and petals and never form carpels (Figure 4B). ult1-1 wus-1 and ult1-2 wus-1 flowers have slightly more sepals and petals on average than wus-1 flowers (Figure 4B). However, unlike wus-1 flowers, ult1-1 wus-1 flowers and ult1-2 wus-1 flowers can contain up to six or seven sepals and petals (Figure 3L). The sepal and petal number increase observed in ult1 flowers is therefore at least partially independent of WUS.
In contrast to the other wus-1 reproductive meristem phenotypes, the premature floral meristem termination phenotype is not rescued by the ult1-1 or ult1-2 mutations. wus-1 flowers contain an average of less than one stamen per flower and completely lack carpels (Figures 3K and 4B). Similarly, ult1-1 wus-1 flowers contain an average of less than one stamen per flower and lack carpels (Figures 3L and 4B; of 88 counted, a single carpel-like structure was formed in 1 ult1-1 wus-1 flower). Examination of floral meristem development using scanning electron microscopy revealed that the floral primordia of single- and double-mutant plants are indistinguishable at both stage 2 and stage 3 (see supplemental figure at http://www.genetics.org/supplemental/). Like wus1 stage 3 floral meristems, ult1-1 wus-1 stage 3 floral meristems lack a detectable dome of meristematic cells interior to the developing sepal primordia (see supplemental figure at http://www.genetics.org/supplemental/). Thus, wus is epistatic to ult1 in the center of the floral meristem, revealing that the WUS and ULT1 genes play antagonistic roles in the same genetic pathway that controls floral meristem determinacy.
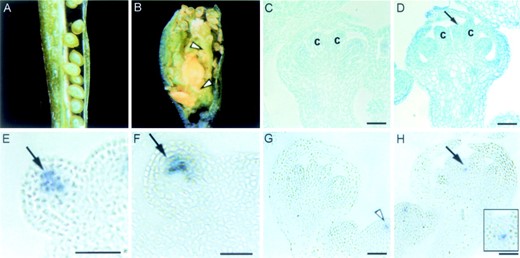
wus and ult1 mutant plants have opposite phenotypes in the center of the flower. wus-1 floral meristems are smaller than those of the wild type, generate reduced numbers of stamens, and terminate prematurely prior to carpel formation (Laux et al. 1996; Schoof et al. 2000). ult1-1 and ult1-2 floral meristems, in contrast, are larger than those of the wild type and can generate extra whorls of floral organs within the normal fourth whorl of carpels (Fletcher 2001; Figure 5B
WUS expression in wild-type and ult1-1 floral meristems. (A) A wild-type silique dissected lengthwise to reveal the ovules within one valve. (B) An ult1-1 silique dissected lengthwise to reveal the presence of supernumerary whorls of stamens and carpels (arrowheads). (C) Wild-type stage 7 flower with two carpel primordia (c) developing in the center of the flower. (D) ult1-1 stage 7 flower with a dome of tissue (arrow) between the two developing carpel primordia (c). (E–H) Wild-type and ult1-1 floral meristems hybridized with an antisense WUS riboprobe. (E) A wild-type stage 2 floral meristem. WUS expression is detected in a small group of cells beneath the outermost two cell layers (arrow). (F) An ult1-1 stage 2 floral meristem. The pattern and level of WUS expression (arrow) are similar to those observed in wild-type stage 2 floral meristems. (G) A wild-type stage 7 flower. After the carpel primordia have emerged, WUS transcription is not detected in the center of the flower. The arrowhead points to the signal detected in the inflorescence meristem, testifying to the integrity of the tissue section. (H) An ult1-1 stage 7 flower. WUS expression is observed in the cells within the dome of tissue between the two carpel primordia (arrow). The inset shows the region depicted by the arrow at higher magnification. Bars, 30 μm.
: compare with wild type in Figure 5A). Since no fourth whorl carpels or internal fifth whorl organs are formed by ult1-1 wus-1 double mutants, the partial loss of determinacy observed in ult1-1 flowers depends upon WUS activity. To confirm this result, we used in situ hybridization to visualize the WUS expression pattern in developing wild-type and ult1-1 mutant flowers.
In wild-type floral meristems, WUS transcripts are first detected in stage 2 primordia budding from the flanks of the inflorescence meristem. In wild-type stage 2 floral meristems, WUS expression is restricted to the interior cells in the central zone (Figure 5E). This area of the meristem has been proposed to act as an organizing center, specifying the overlying neighbor cells to maintain their pluripotent state (Mayer et al. 1998). WUS expression in ult1-1 floral meristems at this stage of development (Figure 5F) is indistinguishable from that in the wild type. WUS transcription is repressed in wild-type floral meristems after stage 6 (Mayer et al. 1998), when two carpel primordia are initiated in the center of the flower and meristematic activity ceases (Figure 5G). The formation of additional whorls of floral organs in ult1-1 and ult1-2 flowers is correlated with the presence of a dome of cells that separate the two developing carpel primordia detectable in stage 6 and stage 7 flowers (Figure 5D). In contrast, the carpel primordia produced by wild-type flowers abut each other (Figure 5C). In ult1-1 stage 7 flowers displaying such a dome of tissue between the carpel primordia, WUS transcription is still detectable in cells underlying this dome (Figure 5H). This WUS expression domain corresponds to cells for which floral organ identity specification has been delayed, on the basis of our observation that AG induction in the center of the floral bud occurs later in ult1-1 than in wild-type plants (Fletcher 2001). These data show that ULT1 negatively regulates WUS to establish floral meristem determinacy and that the partial loss of determinacy observed in ult1 flowers depends on WUS activity.
DISCUSSION
Shoot and floral meristem maintenance in Arabidopsis depends upon the activity of networks of meristem-restricting and meristem-promoting factors. Our previous experiments have shown that ULT is an important meristem-restricting factor that limits the accumulation of cells in both inflorescence and floral meristems. STM and WUS represent two meristem-promoting factors that act in separate genetic pathways, with the STM pathway maintaining meristem cells in an uncommitted, proliferative state and the WUS/CLV pathway maintaining stem cell fate at the meristem apex. To determine the genetic interaction between the meristem-restricting ULT1 factor and the STM and WUS pathways, we generated double mutants between strong and weak ult1 alleles and stm and wus alleles. We determined that while the ult1 alleles cause no detectable phenotypes during embryonic or vegetative development, they can partially suppress the vegetative and inflorescence meristem defects that result from reduced meristem activity in both stm and wus mutant plants. ult1 mutations also restored organogenic potential to stm floral meristems, leading to increased organ production in all whorls. However, we found that wus mutations are epistatic to ult1 mutations in the center of the flower and that WUS transcripts persist longer than normal in developing ult1 flowers. Thus, ULT1 controls floral determinacy by negatively regulating WUS expression during floral meristem development.
ULT1 function early in Arabidopsis development:
Plants carrying loss-of-function ult1 alleles are indistinguishable from wild-type plants during embryonic and vegetative development. This may be because either ULT1 does not function prior to the inflorescence phase or its activity earlier in development is masked by the activity of another gene or genes. We find that loss of ULT1 activity restores postembryonic SAM structure and organogenesis function to stm mutant plants and also partially suppresses the wus vegetative terminal meristem phenotype. Thus our analysis of ult1 stm and ult1 wus double mutants reveals that ULT1 is functional during vegetative development, prior to the stage at which an ult1 single-mutant phenotype is detectable. However, lack of ULT1 activity does not restore embryonic SAM structure in stm-11 and wus-1 mutants.
ULT1 regulation of shoot and floral meristem activity:
Genetic and molecular studies have defined the homeobox genes STM and WUS as essential regulators of shoot and floral meristem formation and maintenance. WUS and STM are induced independently of one another in embryonic SAMs (Long and Barton 1998; Mayer et al. 1998), and the evidence to date indicates that these two genes promote meristem activity in independent but complementary ways and function in distinct regulatory pathways (Lenhard et al. 2002). The STM pathway suppresses cell differentiation throughout the meristem, while the WUS pathway specifies a subset of cells at the meristem apex as stem cells. These pathways ultimately converge to maintain meristem cells in an undifferentiated state (Gallois et al. 2002; Lenhard et al. 2002).
The CLV loci act to restrict meristem activity, having the opposite effect to STM and WUS on shoot and floral meristems. Genetic analysis showed that wus mutations were epistatic to clv mutations in both shoot and floral meristems, placing WUS and the CLV genes in the same genetic pathway (Schoof et al. 2000). In contrast, clv mutations partially suppressed the stm mutant phenotypes and vice versa, and the suppression occurred in a dominant fashion (Clark et al. 1996). The occurrence of dominant interactions between clv and stm mutations was interpreted to mean that these genes play opposing and possibly competitive roles in shoot and floral meristem regulation.
Like the CLV loci, ULT1 acts opposite to STM and WUS in that it functions to restrict the excess accumulation of cells in shoot and floral meristems. We have shown that ult1 mutations restore organized vegetative shoot apical meristems to stm mutant plants, allowing postembryonic organ formation to proceed, albeit in an abbreviated manner. In addition, we observe restoration of floral meristem and floral organ formation in both ult1 stm-11 and ult1 stm-2 plants, including the development of carpel tissue in the latter genotype. In sum, the ult1 alleles reverse many of the effects of weak and strong stm alleles, but do not suppress them completely. Similarly, the stm mutations partially suppress the ult1 phenotypes, showing that a wild-type level of STM activity is necessary for excess meristem cell accumulation in ult1 plants. The ult1 stm double-mutant phenotypes can therefore be considered additive, from which we conclude that STM and ULT1 act oppositely through separate genetic pathways to regulate shoot and floral meristem activity. However, the lack of dose dependency between stm and ult1 alleles suggests that the two genes do not function competitively to regulate the same process.
The interaction between ULT1 and WUS is more complex. Similar to stm-2 plants, wus-1 plants produced some lateral organs from disorganized meristems that initiate randomly across the entire differentiated shoot apex (Laux et al. 1996). Yet unlike stm-2 plants, the rate at which wus-1 plants produced leaves was not accelerated in the absence of ULT1. However, ult1 wus-1 double mutants bolted at a higher frequency and formed many more floral meristems than did wus-1 single mutants. These results indicate that ult1 mutations partially suppress wus, restoring a greater amount of shoot and floral meristem activity. However, ult1 wus-1 double-mutant inflorescences still terminated prematurely and produced flowers with fewer organs than ult1 single mutants did, indicating that the wus-1 mutation also partially suppresses the ult1 mutations. We also observe significant sepal and petal restoration in ult1-1 wus-1 and ult1-2 wus-1 flowers, and, in fact, supernumerary sepals and petals could be produced by ult1 floral meristems even in the absence of WUS. Thus ULT1 acts in a separate pathway from WUS to control shoot apical meristem activity, and the sepal and petal number increase in ult1 flowers is largely WUS independent. However, the wus-1 mutation is epistatic to the ult1 mutations in the inner two whorls of the floral meristem, indicating that WUS is absolutely required for the formation of supernumerary whorls of organs by ult1 floral meristems.
ULT1 regulation of floral meristem determinacy:
Normal Arabidopsis flower development requires that floral stem cell activity terminate upon formation of the central carpel primordia, which consumes the floral meristem. Floral meristem termination occurs via a temporal autoregulatory loop involving WUS and AG (Lenhard et al. 2001; Lohmann et al. 2001). AG encodes a MADS domain transcription factor that is required to terminate floral meristem activity and also to specify stamen and carpel identity (Yanofsky et al. 1990). Early in flower development, WUS and the floral meristem identity factor LEAFY (LFY) activate AG transcription by binding to adjacent sites in the second intron (Lohmann et al. 2001). AG expression is restricted to the interior two whorls of the flower bud, where the stamen and carpel primordia form (Drews et al. 1991). At stage 6 of flower development, AG switches off the organizing center activity by repressing WUS expression, resulting in the differentiation of the remaining stem cells into carpel tissues. However, genetic evidence indicates that AG alone is not sufficient to repress WUS in the center of the flower, because ectopic activation of AG in the inflorescence meristem does not cause meristem arrest (Mizukami and Ma 1997). Therefore AG requires an additional factor or factors to achieve downregulation of WUS transcription (Lenhard et al. 2001).
ULT1 also plays a role in specifying floral meristem determinacy. Mature ult1 flowers can contain more than four whorls of organs, such as fifth and sixth whorls of carpels or a fifth whorl of stamens and a sixth whorl of carpels (Fletcher 2001; Figure 5B). In this way the ult1 flowers are reminiscent of ag flowers, which produce an indeterminate number of floral whorls as a result of active maintenance of a stem cell reservoir and organizing center at the apex of the floral meristem. When the stamen and carpel specification functions of AG are separated from the floral meristem determinacy function via site-directed mutagenesis, the resemblance is even more striking: a synthetic partial loss-of-function ag mutation, AG-Met205, causes production of extra whorls of stamens and carpels in the ag-3 background (Sieburth et al. 1995), closely resembling the ult1 mutant phenotype. Transgenic plants carrying an antisense AG construct in which AG expression is reduced to approximately half the normal level also display the nested stamen and carpel phenotype (Mizukami and Ma 1995). However, ult1 mutant flowers, unlike ag null mutant flowers, are never completely converted to an indeterminate fate, and, as expected, ag mutations are epistatic to ult1 mutations with respect to floral meristem determinacy (data not shown). Since floral stem cell termination eventually occurs in ult1 mutants, it appears that AG can partially compensate for the absence of ULT1, but ULT1 cannot compensate for the absence of AG.
Our results demonstrate that ULT1 is a new component of the AG-WUS temporal feedback loop that controls floral meristem termination. We have shown that ult1 floral meristems contain a dome of cells between the developing carpel primordia, which normally abut one another. Proliferating cells that separate the two carpel primordia are also observed in pAG::WUS transgenic plants in which WUS activity is prolonged in the center of the flower (Lenhard et al. 2001). Similarly, ag mutants maintain a dome of proliferating cells in the center of the flower, even after multiple whorls of organs have formed, which sustains WUS expression beneath the two outermost cell layers (Lenhard et al. 2001; Lohmann et al. 2001). In addition, carpel number is not restored in ult1 wus double mutants, revealing that the ULT1 indeterminacy phenotype is dependent on the activity of WUS. wus mutations are in fact epistatic to ult1 mutations with respect to floral meristem determinacy, indicating that ULT1 acts in the same pathway as WUS to control floral stem cell termination. Finally, we have previously observed that AG expression is delayed in the center of ult1 mutant floral meristems (Fletcher 2001), which correlates with delayed floral meristem termination. This result suggests that ULT1 repression of stem cell activity may work through AG.
We can invoke two possible models for ULT1 function in floral meristem termination that are consistent with our data. In the first model, ULT1 is necessary to induce AG at the correct time during flower development to ensure that WUS repression occurs at the time of carpel initiation. Therefore we propose that in wild-type floral meristems, ULT1 acts (directly or indirectly) with LFY and WUS to activate AG at the proper time and place in the most central region of the flower. AG activation leads to WUS repression and consequent stem cell termination. In ult1 floral meristems, AG activation in the central region is delayed, causing a delay in WUS repression and thus permitting amplification of additional stem cells that become incorporated into extra whorls of organs. This model is attractive in that it accounts for the delayed activation of AG in the center of the flower, although we note that, because AG is properly induced in the third whorl even when ULT1 is absent, some other factor(s) is likely to activate AG in this region. An alternative scenario is that ULT1 may be required in addition to and independently of AG to repress WUS in the center of the flower. In this case, we predict that, in the absence of ULT1, AG is eventually able to repress WUS on its own, but only after a delay, during which the level of AG itself or a secondary activator may rise sufficiently to terminate WUS transcription. While we favor the first model because of its simplicity (it invokes only a single regulatory pathway rather than two separate pathways), its confirmation or rejection will await further experiments to determine whether AG is directly or indirectly activated by ULT1.
Footnotes
Communicating editor: D. Weigel
Acknowledgement
We thank Jeff Long, Kathy Barton, and Thomas Laux for providing stm and wus alleles, and Michael Lenhard for the WUS antisense construct. We are grateful to Karen Osmont, Leor Williams, Dan Choffnes, Robert Blanvillain, and George Chuck for helpful comments on the manuscript. This work was supported by the National Science Foundation (IBN-0110667).
References
Barton, M. K., and R. S. Poethig,
Bowman, J. L., D. R. Smyth and E. M. Meyerowitz,
Brand, U., J. C. Fletcher, M. Hobe, E. M. Meyerowitz and R. Simon,
Byrne, M. E., R. Barley, M. Curtis, J. M. Arroyo, M. Dunham et al.,
Byrne, M. E., J. Simorowski and R. A. Martienssen,
Clark, S. E., S. E. Jacobsen, J. Z. Levin and E. M. Meyerowitz,
Clark, S. E., R. W. Williams and E. M. Meyerowitz,
Drews, G. N., J. L. Bowman and E. M. Meyerowitz,
Fletcher, J. C.,
Fletcher, J. C., U. Brand, M. P. Running, R. Simon and E. M. Meyerowitz,
Gallois, J.-L., C. Woodward, G. V. Reddy and R. Sablowski,
Jackson, D., 1991 In situ hybridization in plants, pp. 163–174 in Molecular Plant Pathology: A Practical Approach, edited by D. J. Bowles, S. J. Gurr and R. McPherson. Oxford University Press, Oxford.
Jeong, S., A. E. Trotochaud and S. E. Clark,
Laux, T., K. F. X. Mayer, J. Berger and G. Jurgens,
Lenhard, M., and T. Laux,
Lenhard, M., A. Bohnert, G. Jurgens and T. Laux,
Lenhard, M., G. Jurgens and T. Laux,
Lohmann, J. U., R. L. Hong, M. Hobe, M. A. Busch, F. Parcy et al.,
Long, J. A., and M. K. Barton,
Long, J. A., E. I. Moan, J. I. Medford and M. K. Barton,
Mayer, K. F. X., H. Schoof, A. Haecker, M. Lenhard, G. Jurgens et al.,
Mizukami, Y., and H. Ma,
Mizukami, Y., and H. Ma,
Rojo, E., V. K. Sharma, V. Kovaleva, N. V. Raikhel and J. C. Fletcher,
Running, M. P., S. E. Clark and E. M. Meyerowitz,
Schoof, H., M. Lenhard, A. Haecker, K. F. X. Mayer, G. Jurgens et al.,
Sieburth, L. E., M. P. Running and E. M. Meyerowitz,
Smyth, D. R., J. L. Bowman and E. M. Meyerowitz,
Steeves, T. A., and I. M. Sussex, 1989 Patterns in Plant Development. Cambridge University Press, New York.
Trotochaud, A. E., T. Hao, G. Wu, Z. Yang and S. E. Clark,
Yanofsky, M. F., H. Ma, J. L. Bowman, G. N. Drews, K. A. Feldmann et al.,
Author notes
Present address: Department of Medicine and Therapeutics, Conway Institute of Biomolecular and Biomedical Research, University College, Dublin 4, Ireland.