-
PDF
- Split View
-
Views
-
Cite
Cite
Fabio Santagati, Kuniya Abe, Volker Schmidt, Thomas Schmitt-John, Misao Suzuki, Ken-ichi Yamamura, Kenji Imai, Identification of Cis-regulatory Elements in the Mouse Pax9/Nkx2-9 Genomic Region: Implication for Evolutionary Conserved Synteny, Genetics, Volume 165, Issue 1, 1 September 2003, Pages 235–242, https://doi.org/10.1093/genetics/165.1.235
Close - Share Icon Share
Abstract
We previously reported close physical linkage between Pax9 and Nkx2-9 in the human, mouse, and pufferfish (Fugu rubripes) genomes. In this study, we analyzed cis-regulatory elements of the two genes by comparative sequencing in the three species and by transgenesis in the mouse. We identified two regions including conserved noncoding sequences that possessed specific enhancer activities for expression of Pax9 in the medial nasal process and of Nkx2-9 in the ventral neural tube. Remarkably, the latter contained the consensus Gli-binding motif. Interestingly, the identified Pax9 cis-regulatory sequences were located in an intron of the neighboring gene Slc25a21. Close examination of an extended genomic interval around Pax9 revealed the presence of strong synteny conservation in the human, mouse, and Fugu genomes. We propose such an intersecting organization of cis-regulatory sequences in multigenic regions as a possible mechanism that maintains evolutionary conserved synteny.
THE sequencing of genomes has opened new research perspectives for the full interpretation of genomic codes, not only by allowing the comprehensive recognition of protein coding and noncoding transcribed sequences, but also by facilitating the identification of regulatory sequences responsible for the control of correct gene expression in time and space. The identification of evolutionarily conserved noncoding sequences (CNSs) among orthologous genomic regions of different species has been proven to be a powerful guide for the localization of cis-acting transcriptional regulatory elements (Koop and Nadeau 1996; Hardison 2000; Wasserman et al. 2000). The elucidation of cis-regulatory elements in a genome is an important starting point for the identification of the specific binding factors and therefore for the subsequent discovery of the molecular pathways that control the mechanisms of cellular differentiation and physiology.
Pax9 belongs to the paired-box (Pax) transcription factor gene family. The Pax family in vertebrates includes nine members that are further subdivided into four paralogous subgroups. The products of the Pax genes have major roles during embryonic development in the processes of tissue patterning and organogenesis (Dahl et al. 1997). One subgroup consists of Pax9 and its paralog Pax1. The two genes share a high sequence homology and show a very widely overlapping expression pattern. Pax9 is expressed mainly in the sclerotome of the somites, in the pharyngeal pouch endoderm and its derivatives, in the developing limb buds, and in the facial mesenchyme of neural-crest cell origin, including nasal and jaw processes and tooth buds (Neubüser et al. 1995). A targeted mutagenesis approach has shown that Pax9 exerts a fundamental function for the normal development of several facial structures, such as the secondary palate, teeth, and laryngeal cartilage, of the third and fourth pharyngeal pouch derivatives like parathyroid glands, ultimobranchial bodies, and the thymus, and of the limbs (Peters et al. 1998). A role of Pax9 in vertebral column development was observed only in Pax1;Pax9 double-mutant mice, which show a much more severe phenotype than that of single Pax1 mutants, while single Pax9 null mutants do not exhibit vertebral column defects (Peters et al. 1999).
No cis-regulatory mechanism that controls specific expression of Pax9 in time and space has been described. In the framework of the search for cis-regulatory elements of Pax9, we previously described the exonintron structure of Pax9 in Homo sapiens, Mus musculus, and Fugu rubripes (Santagati et al. 2001). The analysis of the Pax9 genomic region in the three species demonstrated a conserved physical association with another transcription factor gene, Nkx2-9. Nkx2-9 codes for a member of the Nk2 transcription factor family, characterized by the Nk2 homeobox. Nkx2-9 is initially expressed in the ventral half of the developing neural tube of the mouse embryo in the entire axis and, as development progresses, its expression becomes restricted to the brain region (Pabst et al. 1998). Pax9 and Nkx2-9 lie ∼80 kb apart from each other in a head-to-head orientation on human chromosome (HSA) 14q13 and on mouse chromosome (MMU) 12, while they are only 10 kb apart in Fugu.
In this work, we investigated Nkx2-9 and Pax9 tissue-specific enhancers by interspecies sequence comparison and by transgenesis. Through an extended genomic analysis and the identification of more associated genes that enlarged the region of the conserved synteny, we propose a model for the regulatory sequence organization of this region and suggest a possible interpretation for the strong synteny conservation with evolutionary considerations.
MATERIALS AND METHODS
Genomic sequence comparison: Alignment and search for homologous regions between large genomic sequences of different species were carried out with the PipMaker program (Schwartz et al. 2000) available on the web site at Pennsylvania State University (http://bio.cse.psu.edu/pipmaker). The reference human sequence was masked against repetitive DNA with RepeatMasker (http://ftp.genome.washington.edu/RM/RepeatMasker.html).
Construction of transgenic constructs with CNSs: A 1-kb fragment containing the CNS-6 segment was amplified from bacterial artificial chromosome (BAC) DNA by PCR with primers 5′-CATTTTGCCAGAGGCAGAGG-3′ and 5′-AAGGGAC AGTGAGCGGTCTG-3′ and was cloned in SmaI-linearized pASShsp68lacZpA (provided by H. Sasaki). Its original orientation with respect to the mouse Nkx2-9 promoter was maintained in the construct. A 2.5-kb fragment containing the CNS+2 segment was amplified with primers 5′-GGACCAGG CCTTTGTATAAGGC-3′ and 5′-TGATTGTGACCCCTGGTT TAGC-3′ and cloned as above. The integrity of all constructs was verified by sequencing, and their inserts were excised from the vector sequence by SalI digestion.
Generation of transgenic mice: Transgenic mice were generated by pronucleus injection of linearized transgene constructs into fertilized eggs that were subsequently transferred into the oviducts of pseudopregnant foster females. X-gal staining was performed as described (Peters et al. 1998) on founder transgenic embryos. To obtain sectioned views, X-galstained embryos were cut by scalpel at levels as indicated (Figure 3, A–D). Yolk sacs were used for DNA preparation for PCR genotyping (primer pairs 5′-AGGTGACACTATAGAAG GATCCG-3′, 5′-GGTGCTAGCTCAACTGGTGG-3′ and 5′-TCT CATGCTGGAGTTCTTCG-3′, 5′-AGCATTTGTATTTCTGAT GCCAAC-3′).
Whole-mount RNA in situ hybridization: A mouse Nkx2-9 probe was amplified by RT-PCR from total RNA of embryonic day 11.5 mouse embryos and cloned in pCRII-TOPO (Invitrogen, San Diego). An antisense probe was transcribed with SP6 RNA polymerase on XhoI-linearized template. A sense probe was transcribed with T7 RNA polymerase on BamHIlinearized template. Whole-mount in situ hybridization in mouse embryos was performed according to the protocol described by Spörle and Schughart (1998). After rehydration of paraformaldehide-fixed embryos in methanol/PBS, all incubation and washing steps were automatically processed by InsituPro (INTAVIS Bioanalytical Instruments AG, Köln, Germany).
RESULTS
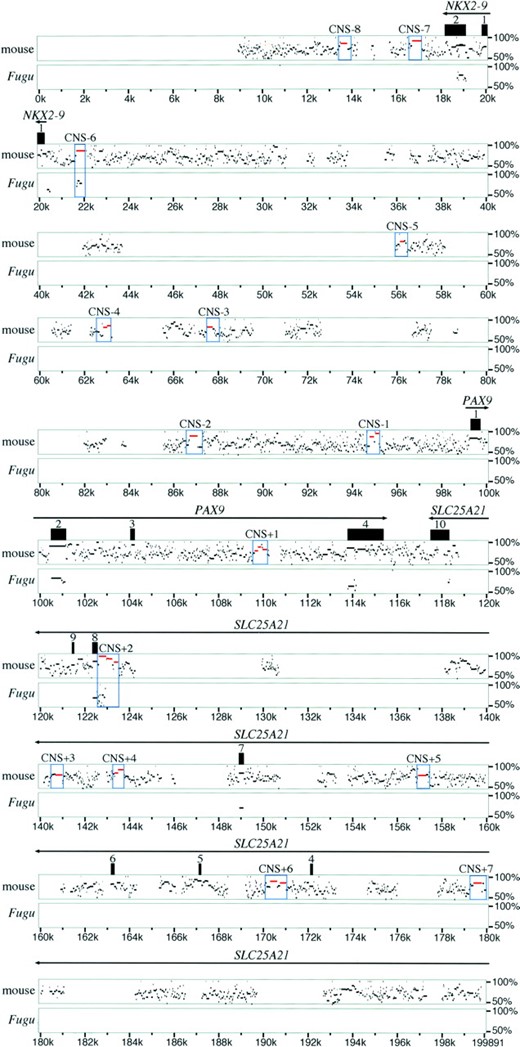
Identification of candidates for cis-regulatory elements by comparative sequencing: The human sequence (GenBank accession no. AL079303) consisted of a nearly 200-kb genomic region from 18 kb downstream of NKX2-9 to 84 kb downstream of PAX9 (note their head-to-head organization as shown in Figure 1). The mouse sequence was derived from the combination of two overlapping Pax9-positive BAC clones, 136M3 (GenBank accession no. AC040982) and 327I21 (GenBank accession no. AC079959) from the RPCI-23 mouse BAC library. The 24.4-kb Fugu sequence (GenBank accession no. AF267536) was determined by sequencing a cosmid clone including Nkx2-9 and Pax9. This Fugu sequence ranges from 1.9 kb downstream of Nkx2-9 to 6.5 kb downstream of Pax9. Consistent with the fact that the Fugu genome is eight times more compact than the human genome (Elgar et al. 1996; Koop and Nadeau 1996), this Fugu genomic interval largely overlaps with the 200-kb human reference interval. A comparison between the human and mouse sequences by PipMaker analysis revealed that the entire genomic interval displayed a significant degree of homology, indicating a remarkable conservation even outside of the coding sequences (Figure 1). The BLASTZ alignment of PipMaker analysis found numerous (∼2000) ungapped sequence segments that were similar between humans and mice (depicted as dots or bars in Figure 1). Of these conserved sequence segments, we focused on segments that were at least 100 bp long and at least 70% identical, according to the criteria suggested by Loots et al. (2000). In this way, we found 100 of the conserved segments as putative CNSs. Of these, 4 and 8 segments were found to correspond to exons of Nkx2-9 and Pax9, respectively, and were excluded from further analyses. A BLAST search was performed for each of the remaining 88 putative CNSs to verify whether they really represented noncoding sequences. Surprisingly, 7 of them lying downstream of PAX9 matched the cDNA sequence of SLC25A21. SLC25A21 codes for a mitochondrial oxodicarboxylate carrier protein, which is ubiquitously present in the inner mitochondrial membrane and plays a central role in amino acid metabolism (Fiermonte et al. 2001). The mouse counterpart of SLC25A21 (hereafter referred to as Slc25a21) lies ∼2 kb from the 3′ end of Pax9 in a tail-to-tail orientation. SLC25A21/ Slc25a21 consists of 10 exons that are distributed over a large genomic region of ∼500 kb. These 7 conserved segments that corresponded to SLC25A21 exon sequences were excluded from further investigations. Thus, the rest of 81 sequence segments were regarded as true CNSs. These CNSs often appeared in clusters, and we found 15 ∼1-kb genome segments that contained multiple CNSs, including very long ones, which were distributed over the whole region examined (CNS-8–CNS+7; Figure 1).
—PipMaker analysis of the human/mouse/Fugu sequences. The graph shows the percent identity plot output of ∼200 kb of the human genomic sequence including Nkx2-9, Pax9, and part of Slc25a21. The human sequence was used as reference. Arrows above the gene designations indicate the transcriptional direction of the three genes. Homology matches with the mouse and Fugu sequences are represented as dots and dashes, where the length corresponds to that of the matching sequence and the height in the plot to the homology degree (scaling between 50 and 100% on the right side of each row). Boxed segments (CNS-8–CNS+7, outlined in blue) contain one or more of major CNSs (highlighted in red). The CNS segments upstream and down-stream of Pax9 have negative and positive numbers, respectively.
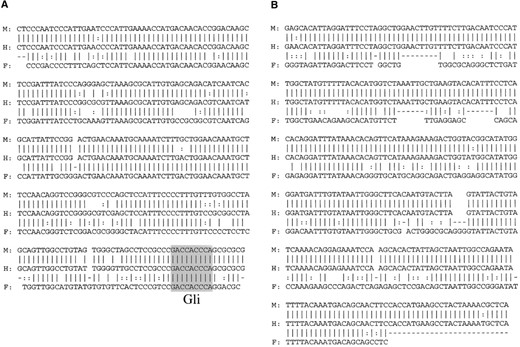
—Sequence alignment of CNS-6 (A) and CNS+2 (B). Within the 200-kb interval analyzed by PipMaker, these regions are the only two segments of noncoding sequences that show a significant degree of homology among humans, mice, and Fugu. Vertical bars, double dots, and dashes indicate perfect matches, transitions, and gaps, respectively. In CNS-6, the potential Glibinding sites with the complete consensus motif are shaded. M, mouse; H, human; and F, Fugu.
In contrast, in the alignment of the Fugu and human sequences, only a small number of homologous segments were detected by PipMaker analysis (Figure 1). In the intergenic region between Pax9 and Nkx2-9, sequences within the CNS-6 region also were found conserved in Fugu (Figures 1 and 2A). Downstream of Pax9, four distinct hits were identified by PipMaker analysis. Interestingly, three of these conserved segments fell into three exons of SLC25A21 (exons 7, 8, and 10) in the corresponding human sequence. Although Slc25a21 in the mouse and in Fugu has not been described, this result provides evidence for the presence of the orthologous gene of SLC25A21 in the two species. This finding was very intriguing, because it extended the region of conserved synteny among the three vertebrate species, already described for Pax9 and Nkx2-9 (Santagati et al. 2001), to include an additional gene, Slc25a21. The fourth hit was detected inside Slc25a21 intron 7 in the close vicinity of exon 8 (Figure 2B). This region coincided with the mouse-human CNS+2.
Transient transgenesis with CNS constructs: To test the potential regulatory activity of the selected CNS segments, we performed a transient transgenic assay in founder embryos. The general design of the experiment was to place a test genomic fragment upstream of a lacZ reporter cassette with a minimal promoter from mouse Hsp68.(Kothary et al. 1989; Sasaki and Hogan 1996).
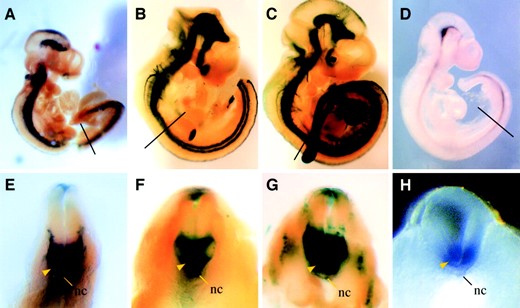
CNS-6 segment drives Nkx2-9 expression in the ventral neural tube: CNS-6 and CNS+2, which are conserved in all three species, were tested by this enhancer assay. CNS-6 is only ∼1.5 kb from the 5′ end of Nkx2-9 and consists of a 422-bp sequence with an overall 85% identity between humans and mice. Within this sequence a 244-bp segment had 80% conservation with the Fugu DNA (Figure 2A). Five independent mouse embryos at E10.5 carried the CNS-6 transgene, and all of them showed lacZ expression upon X-gal staining. As compared to endogenous Nkx2-9 expression (Figure 3, D and H), expression of the CNS-6 transgene in the transgenic embryos varied in both levels and tissue-specific patterns, including irreproducible ectopic staining in diverse structures (Figure 3, A–C and E–G). Nevertheless, all of the samples shared a common positive domain in the ventral neural tube (Figure 3, E–G). This lacZ expression in the ventral neural tube corresponds well to that of endogenous Nkx2-9 (Figure 3, D and H). Thus, our data strongly suggest that the CNS-6 segment includes cis-acting sequences that regulate Nkx2-9 expression in the ventral neural tube. This Nkx2-9 expression pattern has been shown to be dependent on Sonic hedgehog (Shh) signaling from the floor plate (Pabst et al. 2000), which is mediated by Gli transcription factors as the final effectors. Consistent with this model, a fully conserved consensus sequence of nine bases for the Gli-binding site (GACCACCCA), originally found in Foxa2 (Sasaki et al. 1997), was recognized within the CNS-6 sequence in all three species (Figure 2A).
—X-gal staining of CNS-6 transgenic embryos. (A–C) Wholemount overview of transgenic founder embryos at E10.5-E11. lacZ expression is clearly visible along the whole neural tube. (D) RNA in situ hybridization of Nkx2-9 at E10.5 as control. E–H show transversal dissections of the whole embryos depicted in A–D, respectively. The section level is indicated in A–D with a line. Note that X-gal staining is consistently seen in the ventral part of the neural tube (yellow arrowheads in E–G). The location of the notochord is indicated (nc in E–H).
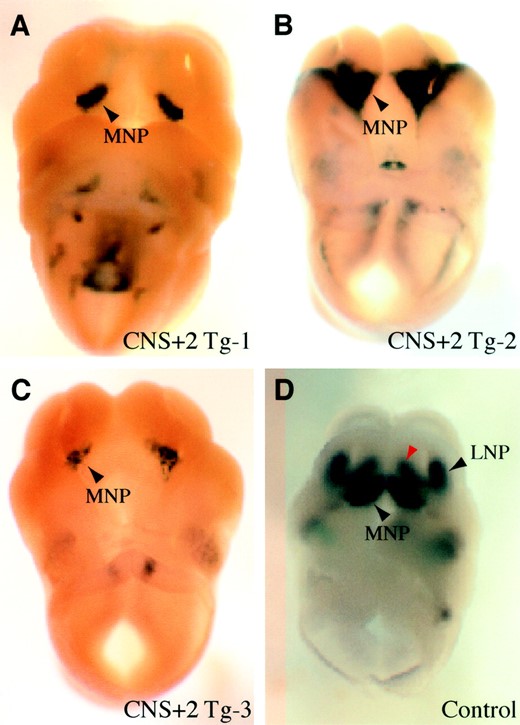
CNS+2 segment possessed the enhancer activity of Pax9 expression in the medial nasal process: The second fragment tested was CNS+2. This segment was located very close to exon 8 of Slc25a21 inside the preceding intron. The human/mouse homologous region was 594 bp long with an overall identity of 95%, 300 bp of which were homologous to the Fugu sequence with 64% identity (Figure 2B). A 2.2-kb segment of the mouse sequence, including CNS+2 and part of Slc25a21 exon 8, was used for the transgenic construct. Three transgenic founder embryos at E11.5 were produced with this construct. Besides inconsistent X-gal staining in several domains that did not match with the endogenous Pax9 expression pattern, a very specific expression was observed in all of the embryos in the ventromedial region of the medial nasal processes (Figure 4, A–C). This domain overlapped with the two stripes of endogenous Pax9 expression in the same structures (Figure 4D), suggesting that CNS+2 includes cis-regulatory elements that drive Pax9 expression in this domain. However, endogenous Pax9 expression can also be detected in the more internal medial nasal processes as well as in the lateral nasal process (Figure 4D). Thus the CNS+2 segment does not appear to contain all the elements required for the complete expression of Pax9 in the nasal processes.
DISCUSSION
Identification of enhancers for expression of Nkx2-9 and Pax9: This work provides the first evidence for the elucidation of cis-regulatory elements that control expression of the physically linked but distinctly regulated genes Pax9 and Nkx2-9. The CNS-6 segment directed the transcription of the reporter gene in the ventral part of the neural tube. The analysis of the CNS-6 sequence revealed not only a high degree of sequence conservation among the three species, but also a putative binding site for Gli proteins with a complete match to the consensus sequence identified in Foxa2 (Sasaki et al. 1997). In the 200-kb Nkx2-9/Pax9 genome interval of the mouse, we found three of the nine-base Gli-binding motif, but the one included in CNS-6 is the only one that is conserved in humans, mice, and Fugu. Whether the identified sequence in CNS-6 functions as a Glibinding site remains to be confirmed in future studies.
Our finding that CNS+2 is able to direct transgene expression selectively in the oral edge of the medial nasal processes but not in the remaining mesenchymal tissues suggests that each of the Pax9-expressing structures is independently regulated. Thus, the regulatory mechanisms that control the whole cranio-facial development may be very complex and diverse, consistent with the composite distribution of signaling molecules and transcription factors involved in facial development (Francis-West et al. 1998). CNS+2 is located only 8 kb downstream of the last exon of Pax9, but remarkably this region is in the seventh intron of the neighboring gene Slc25a21. The entire genomic interval that contains all cis-regulatory elements of Pax9 has not been determined. By BAC transgenesis we tested whether a 195-kb BAC clone, including 130 kb upstream and 50 kb downstream of Pax9, contained all cis-regulatory elements of Pax9. Our preliminary result showed that the Pax9-BAC transgene could only partially reproduce endogenous expression of Pax9 (see the supplemental figures available online at http://www.genetics.org/supplemental). This observation suggests that additional cis-regulatory elements of Pax9 are located outside this BAC interval. It should be noted that this BAC clone contained not only Pax9 but also its flanking genes, Nkx2-9 and part of Slc25a21, on either side. Taken together, our results suggest that cis-regulatory elements of Pax9 are interspersed in a wide genomic interval, even in the regions of neighboring genes. The correct expression of Pax9 in a particular domain may be determined by the concomitant function of separate control elements, even at a long genomic distance from each other, as exemplified in the case of mouse Myf5 and Mrf4 (Carvajal et al. 2001). The regulatory elements of both Myf5 and Mrf4 trespass the limits of gene boundary and, within the introns of a neighboring gene, localize the protein tyrosine phosphatase-RQ gene. Recently, another remarkable example of a complex organization of genes in mammals has been reported for Shh (Lettice et al. 2002). The genomic region containing the limb-specific control elements of Shh was identified to be ∼800 kb away from the gene itself inside the introns of the Lmbr1 gene in humans and mice. Moreover, at least one more gene has been located between Shh and Lmbr1, suggesting that these elements can exert their specific function on the target gene despite large genomic distances and the presence of other intervening genes.
—X-gal staining of CNS+2 transgenic embryos. A–D show reporter (lacZ) expression in the ventral part of the facial structures from three independent transgenic founder embryos at E11.5 (A–C) in comparison to endogenous Pax9 expression detected by RNA in situ hybridization at E11.0 (D). (A–C) The reporter expression is consistently observed in the oral edge of the medial nasal processes (MNP) as indicated by black arrowheads. (D) Pax9 is very strongly expressed in the corresponding part of the MNP. Note that Pax9 is also expressed in the dorsal part of the MNP (red arrowhead) and in the lateral nasal processes (LNP).
Conserved synteny around Pax9: Our finding that Slc25a21 physically links to Pax9 in the human, mouse, and Fugu genomes has extended the region of conserved synteny in the three species that we described earlier (Santagati et al. 2001). Moreover, close examination of the mouse and human genome sequences from GenBank (mouse, NW_000053; human, NT_025892) has revealed that this region of conserved synteny extends even further with at least four additional genes included. In the mouse and human genomes, Nkx2-1 (or Titf1) and Mbip (Fukuyama et al. 2000) are found on the centromeric side of Nkx2-9, and Mipol1 (Kondoh et al. 2002) and Foxa1 are located on the telomeric side of Slc25a21. Thus, the order of the genes in the synteny group is: Mbip—Nkx2-1—Nkx2-9—Pax9—Slc25a21—Mipol1—Foxa1. In the Fugu genome (http://www.ensembl.org), five of the seven genes also physically link together: Mbip, Nkx2-1, Nkx2-9, Pax9, and Slc25a21. Whether Fugu counterparts of Mipol1 and Foxa1 are also associated with these linked genes cannot be determined on the basis of the currently available Fugu sequence data.
Interestingly, a similar physical linkage is encountered for mouse and human paralogs of many of these genes. The paralogous genes of Pax9, Nkx2-9, and Nkx2-1 are Pax1, Nkx2-2, and Nkx2-4, respectively, and they are known to physically link in MMU2 and in HSA20p11 (Wang et al. 2000; Santagati et al. 2001). By close examination of genome sequence data (mouse, NW_000178; human, NT_011387), we found that Foxa2, the paralog of Foxa1, also fell into this synteny group. Furthermore, within the human genome sequence between PAX1 and FOXA2, a computer-predicted gene with homology to SLC 25A21 (named LOC200259 or SLC21A5L) was present. Thus, the locus order in this synteny group is: Nkx2-4— Nkx2-2—Pax1—Slc25a5l—Foxa2. Similarly, in Fugu, we could confirm the physical association of Nkx2-4, Nkx2-2, and Pax1 in the genome annotation data (http://www.ensembl.org). Together, gene order in the two paralogous genome segments including Pax9 or Pax1 is highly conserved in the human, mouse, and Fugu genomes.
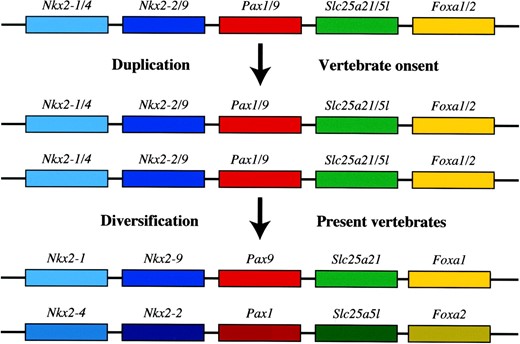
In lower chordates, only one member for each paralogous gene pair is present, strongly suggesting that the genomic regions evolved through duplication of a common ancestral genome (Figure 5). This genomic region surrounding the Pax1/9 gene may include at least four other linked genes that have maintained their physical association from the primitive situation predating the locus duplication up to the present time. Following this duplication event, these paralogous gene pairs diversified their roles, contributing to the variety of functionalities in vertebrates (Figure 5). To our best knowledge, such a synteny conservation between mammals and fish involving five to seven genes maintaining the same gene order has never been described in detail before. Moreover, the comparison between two paralogous genomic segments including Pax9 or Pax1 allowed us to speculate on the organization of a putative ancestral genome. This physical association might reflect a fixed multigenic transcriptional domain whose members (at least the five genes in Figure 5) cannot be separated without compromising their normal function. The nature of this functional bond has still to be investigated, but the strong indication from our study that at least some of the Pax9 regulatory elements are interspersed or interdigitated over a widespread multigenic region can already account for a decisive factor to maintain their physical linkage. As mentioned above, the tight physical linkage between Myf5 and Mrf4 is likewise conserved in all the vertebrate species so far analyzed (Carvajal et al. 2001). It is logical to deduce that this kind of genomic organization of genes and their regulatory elements represents an irresolvable constraint for neighboring genes.
—A model of the origin and conservation of the syntenic regions including Nkx2-2/9 and Pax1/9 paralogous gene pairs. From the conserved synteny of the Pax1/Pax9 genomic regions and the genetic information about lower chordates such as amphioxus, it can be assumed that in the ancestral genome of chordate progenitors a set of at least five genes (ancestors of Nkx2-1/4, Nkx2-2/9, Pax1/9, Slc25a21/5l, and Foxa1/2) were tightly associated. A series of genome duplication events brought about the evolutionary burst that caused the origin of vertebrates. The Pax1/9 syntenic region also underwent duplication, originating two sets of paralogous genes that afterward independently diversified, acquiring distinct functions up to the present time. The presence of interspersed regulatory cis-elements throughout this genomic region may have been working as the driving force that has kept these genes tightly associated through evolution.
In conclusion, interpretation of our results in light of similar examples in the literature leads to some new general considerations. The presence of intersecting regulatory sequences in multigenic genomic regions conceivably represents a key point in genome evolution. The onset and fixation of regulatory elements inside the territory of neighboring genes constitutes a functional bond resulting in the physical association between the genes. These associations in time might have extended, involving entire blocks of genes. Thus, it logically follows that the plasticity of the genome in the events of shuffling and reorganization during evolution has been inevitably limited to some extent. The rearrangement units may not have been single genes but the blocks of physically linked genes. Hence, in the future, the search for cis-regulatory elements not only will lead to a better understanding of the molecular mechanisms that control gene expression, but also will provide insights into genome evolution.
Footnotes
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession no. AF267536.
Communicating editor: C. Kozak
Acknowledgement
We acknowledge H. Sasaki, P. A. Iannou, K. Araki, and F. Stewart for providing plasmid constructs as indicated in the text. We thank B. Rey, A. Krause, and G. Michel for their contribution to the generation of transgenic mice; M. Wahl for providing informatics tools for genomic sequence analyses; and R. Balling, L. Bally-Cuif, and J. Favor for critical reading of the manuscript and helpful comments. This work was supported in part by the Deutsche Forschungsgemeinschaft and the GSF-Forschungszentrum and in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan, with Special Coordinating Funds for Promoting Science and Technology (K.A.).
LITERATURE CITED
Author notes
Present address: Institut de Génétique et de Biologie Moléculaire et Cellulaire, CNRS/INSERM/ULP, Collège de France, 67404 Illkirch, Strasbourg, France.