-
PDF
- Split View
-
Views
-
Cite
Cite
Hongyan Wang, Xie Tang, Mohan K Balasubramanian, Rho3p Regulates Cell Separation by Modulating Exocyst Function in Schizosaccharomyces pombe, Genetics, Volume 164, Issue 4, 1 August 2003, Pages 1323–1331, https://doi.org/10.1093/genetics/164.4.1323
Close - Share Icon Share
Abstract
Cytokinesis is the final stage of the cell division cycle in which the mother cell is physically divided into two daughters. In recent years the fission yeast Schizosaccharomyces pombe has emerged as an attractive model organism for the study of cytokinesis, since it divides using an actomyosin ring whose constriction is coordinated with the centripetal deposition of new membranes and a division septum. The final step of cytokinesis in S. pombe requires the digestion of the primary septum to liberate two daughters. We have previously shown that the multiprotein exocyst complex is essential for this process. Here we report the isolation of rho3+, encoding a Rho family GTPase, as a high-copy suppressor of an exocyst mutant, sec8-1. Overproduction of Rho3p also suppressed the temperature-sensitive growth phenotype observed in cells lacking Exo70p, another conserved component of the S. pombe exocyst complex. Cells deleted for rho3 arrest at higher growth temperatures with two or more nuclei and uncleaved division septa between pairs of nuclei. rho3Δ cells accumulate ∼100-nm vesicle-like structures. These phenotypes are all similar to those observed in exocyst component mutants, consistent with a role for Rho3p in modulation of exocyst function. Taken together, our results suggest the possibility that S. pombe Rho3p regulates cell separation by modulation of exocyst function.
CYTOKINESIS is the stage of cell cycle that physically divides the mother cell into two daughters following DNA synthesis and chromosome segregation (for review, Guertin et al. 2002). Cytokinesis in Schizosaccharomyces pombe is achieved through the use of a constricting actomyosin ring followed by septum deposition. The division septum consists of one layer of primary septum and two adjacent layers of secondary septa. Finally, cell separation liberates two daughter cells upon dissolution of the primary septum. In comparison to studies of the mechanisms of actomyosin ring and division septum assembly, the process of cell separation remains poorly understood.
Our previous studies indicated that the fission yeast exocyst, a multiprotein complex, is involved in cell separation possibly by mediating docking and fusion vesicles containing cell-wall-degrading enzymes at the division site (Wang et al. 2002). The exocyst complex is conserved from yeasts to mammals and has been extensively characterized in budding yeast (Hsu et al. 1999). The exocyst in Saccharomyces cerevisiae consists of eight components, Sec3p, Sec5p, Sec6p, Sec8p, Sec10p, Sec15p, Exo70p, and Exo84p. A similar complex, termed the Sec6/8 complex, has also been identified in mammals (Grindstaff et al. 1998). It has been established that the exocyst is involved in late stages of exocytosis, targeting and tethering post-Golgi vesicles to the plasma membrane (TerBush et al. 1996; Grindstaff et al. 1998). Unlike target-soluble NSF attachment protein receptor proteins (t-SNAREs) that are evenly distributed along the plasma membrane, the exocyst is specifically localized at sites of active secretion.
In S. pombe cells, only five exocyst proteins, Sec6p, Sec8p, Sec10p, Sec15p, and Exo70p, have been identified to date (Wang et al. 2002). These proteins form a complex in vivo as in many other eukaryotes. They are concentrated at the cell tip as well as at the division site. They first localize to the division site as a ring structure at early mitosis and split into two rings adjacent to the constricting actomyosin ring, indicative of participation in cytokinesis. A few exocyst deletion mutants have been analyzed and shown to have defects in cell separation. Surprisingly, cell growth, elongation, and septum assembly are not affected in these mutants, suggesting that the primary function of the exocyst in S. pombe is cell separation. The exocyst is also implicated in exocytosis in S.pombe. In exocyst mutants, the post-Golgi secretory vesicles accumulate in the cytoplasm presumably due to a defect in tethering these vesicles to the plasma membrane.
Recently, Rho GTPase proteins have attracted increasing attention for their role in exocytosis (Novick and Guo 2002). In S. cerevisiae, Rho3p regulates exocytosis by interacting with Exo70p (Robinson et al. 1999). Although S. pombe Rho3p was recently shown to be involved in polarized cell growth (Nakano et al. 2002), its role in exocytosis was not known. Here we report the isolation of the rho3 gene as a multicopy suppressor of sec8-1, a temperature-sensitive exocyst mutant. We also show that both loss of Rho3p function and expression of a dominant-negative mutant lead to a cell separation defect similar to that observed in exocyst mutants.
S. pombe strains analyzed in this study
| Name . | Relevant genotype . | Source . |
|---|---|---|
| MBY 919 | exo70Δ::ura4, h- | This study |
| MBY1303 | sec8-1, pREP3-1-leu1+ | This study |
| MBY1304 | sec8-1, pREP3-1-pot1-leu1+ | This study |
| MBY1305 | sec8-1, pREP3-1-rho3-leu1+ | This study |
| MBY1325 | rho3Δ::ura4+, h+ | This study |
| MBY1370 | rho4Δ::ura4+, h- | This study |
| MBY1393 | rho3Δ::ura4+, Sec8-GFP::ura4+ | This study |
| MBY1396 | sec8Δ::ura4+, pREP3-1-rho3-leu1+ | This study |
| MBY1443 | rho3Δ::ura4+, Sec6-GFP::ura4+ | This study |
| MBY1452 | pREP1-GFP-rho3-leu1+, ura4-D18 | This study |
| MBY1453 | GFP-Rho3::ura4+, leu1-32, | This study |
| MBY1459 | pREP1-rho3-G25V-leu1+, ura4-D18, h- | This study |
| MBY1460 | pREP1-rho3-T30N-leu1+, ura4-D18, h- | This study |
| MBY1672 | sec8-1, pREP1-GFP-rho3 | This study |
| MBY1676 | rho3Δ::ura4+, exo70Δ::ura4 | This study |
| Name . | Relevant genotype . | Source . |
|---|---|---|
| MBY 919 | exo70Δ::ura4, h- | This study |
| MBY1303 | sec8-1, pREP3-1-leu1+ | This study |
| MBY1304 | sec8-1, pREP3-1-pot1-leu1+ | This study |
| MBY1305 | sec8-1, pREP3-1-rho3-leu1+ | This study |
| MBY1325 | rho3Δ::ura4+, h+ | This study |
| MBY1370 | rho4Δ::ura4+, h- | This study |
| MBY1393 | rho3Δ::ura4+, Sec8-GFP::ura4+ | This study |
| MBY1396 | sec8Δ::ura4+, pREP3-1-rho3-leu1+ | This study |
| MBY1443 | rho3Δ::ura4+, Sec6-GFP::ura4+ | This study |
| MBY1452 | pREP1-GFP-rho3-leu1+, ura4-D18 | This study |
| MBY1453 | GFP-Rho3::ura4+, leu1-32, | This study |
| MBY1459 | pREP1-rho3-G25V-leu1+, ura4-D18, h- | This study |
| MBY1460 | pREP1-rho3-T30N-leu1+, ura4-D18, h- | This study |
| MBY1672 | sec8-1, pREP1-GFP-rho3 | This study |
| MBY1676 | rho3Δ::ura4+, exo70Δ::ura4 | This study |
S. pombe strains analyzed in this study
| Name . | Relevant genotype . | Source . |
|---|---|---|
| MBY 919 | exo70Δ::ura4, h- | This study |
| MBY1303 | sec8-1, pREP3-1-leu1+ | This study |
| MBY1304 | sec8-1, pREP3-1-pot1-leu1+ | This study |
| MBY1305 | sec8-1, pREP3-1-rho3-leu1+ | This study |
| MBY1325 | rho3Δ::ura4+, h+ | This study |
| MBY1370 | rho4Δ::ura4+, h- | This study |
| MBY1393 | rho3Δ::ura4+, Sec8-GFP::ura4+ | This study |
| MBY1396 | sec8Δ::ura4+, pREP3-1-rho3-leu1+ | This study |
| MBY1443 | rho3Δ::ura4+, Sec6-GFP::ura4+ | This study |
| MBY1452 | pREP1-GFP-rho3-leu1+, ura4-D18 | This study |
| MBY1453 | GFP-Rho3::ura4+, leu1-32, | This study |
| MBY1459 | pREP1-rho3-G25V-leu1+, ura4-D18, h- | This study |
| MBY1460 | pREP1-rho3-T30N-leu1+, ura4-D18, h- | This study |
| MBY1672 | sec8-1, pREP1-GFP-rho3 | This study |
| MBY1676 | rho3Δ::ura4+, exo70Δ::ura4 | This study |
| Name . | Relevant genotype . | Source . |
|---|---|---|
| MBY 919 | exo70Δ::ura4, h- | This study |
| MBY1303 | sec8-1, pREP3-1-leu1+ | This study |
| MBY1304 | sec8-1, pREP3-1-pot1-leu1+ | This study |
| MBY1305 | sec8-1, pREP3-1-rho3-leu1+ | This study |
| MBY1325 | rho3Δ::ura4+, h+ | This study |
| MBY1370 | rho4Δ::ura4+, h- | This study |
| MBY1393 | rho3Δ::ura4+, Sec8-GFP::ura4+ | This study |
| MBY1396 | sec8Δ::ura4+, pREP3-1-rho3-leu1+ | This study |
| MBY1443 | rho3Δ::ura4+, Sec6-GFP::ura4+ | This study |
| MBY1452 | pREP1-GFP-rho3-leu1+, ura4-D18 | This study |
| MBY1453 | GFP-Rho3::ura4+, leu1-32, | This study |
| MBY1459 | pREP1-rho3-G25V-leu1+, ura4-D18, h- | This study |
| MBY1460 | pREP1-rho3-T30N-leu1+, ura4-D18, h- | This study |
| MBY1672 | sec8-1, pREP1-GFP-rho3 | This study |
| MBY1676 | rho3Δ::ura4+, exo70Δ::ura4 | This study |
MATERIALS AND METHODS
Media, reagents, and genetics: S. pombe strains used in this study are listed in Table 1. Yeast cells were grown on yeast extract with supplements (YES) medium or minimal media with appropriate supplements (Moreno et al. 1991). Crosses were performed by mixing appropriate strains directly on YPD plates. Recombinant strains were obtained by tetrad analysis. Yeast transformations were performed by the lithium acetate method (Okazaki et al. 1990).
Isolation of the rho3 gene: A genomic DNA library (Balasundaram et al. 1999) was introduced into sec8-1 cells to isolate colonies that were able to reverse the temperature sensitivity of sec8-1. Two colonies grew at 36°, one of which turned out to carry a plasmid containing the rho3 gene that is located on cosmid SPAC23C4.08.
Construction of rho3 and the exo70 null mutant: A PCR-based deletion strategy was utilized to construct the rho3Δ. Primers MOH730 (TGGTTCGCATCGCCTTAATTATTCTAAATTTGA TTGTTACCTGAAATGTAGAAGCCAATCTACAGCGCAC AAGGACATGTAATCCCACTGGCTATATGTA) and MOH731 (ACCCACACTAACGTCATATACAATAATAAACTTCGAACA TTAGAAATAAGACTTTTAGGCGCTTTCAAAAGAAAGTGC TTTAGGTAAAGATAAACCGTAC) were used to amplify a product containing the uar4 gene as well as two flanking sequences of rho3. The PCR product was purified and transformed into wild-type diploid cells to select on medium lacking uracil. Correct integration was confirmed by colony PCR analysis.
The exo70Δ was generated using the standard cloning-based homologous recombination method. Primer pairs MOH655 (GGCGGGATCCTGCGCTGTGCGCAATTACTGATCT) and MOH656 (GGCGGAATTCTTTGCATATAATTTTTACGAA GGA) and MOH657 (GGCGCTCGAGTTCAATATGATGAA TTTGGGTCC) and MOH658 (GGCGGGTACCCAAACATC AAATAATATGGTAATTT) were used to amplify ∼500 bp of exo70 5′ untranslated region (UTR) and the 3′ UTR region, respectively. They were subsequently cloned into pSK-ura4 in BamHI-EcoRI and XhoI-KpnI fragments. This resulting plasmid was linearized with BamHI and KpnI and introduced into a wild-type diploid strain. Colonies were selected on medium lacking uracil and correct integration was confirmed by PCR.
Generation of mutations in rho3: The mutant alleles, rho3Val-25 and rho3Asn-30, were constructed by an oligonucleotide-directed PCR. For construction of the rho3Val-25 mutation, primers with 18-mer overlapping sequences containing the mutations MOH-738 (CTTGGTGATGTTGCTGCTGGTAAAACCAGTTTG) and MOH739 (CCAGCAGCAACATCACCAAGAATTACGATTTTC) were designed to amplify the rho3 gene as two fragments with both carrying the mutation. These two fragments were mixed and used as templates for another round of PCR to generate a complete open reading frame (ORF) of rho3 with the rho3Val-25 (Rho3 G25V) mutation, which was cloned into pREP1. The rho3Asn-30 (Rho3T30N) mutation was constructed by employing a similar strategy using primers MOH740 (GCTGGTAAAAA CAGTTTGTTAAATGTATTTACT) and MOH741 (TAACAAA CTGTTTTTACCAGCAGCACCATCACC).
Microscopy: Fluorescence microscopy was performed essentially as described (Balasubramanian et al. 1997). Cells were viewed using a Leica DMLB microscope with appropriate filters. To visualize DNA, F-actin, and septum material, cells were fixed in 3.7% formaldehyde for 1 min and stained with 4′,6-diamidino-2-phenylindole (DAPI), rhodamine-conjugated phalloidin, and calcofluor (Sigma, St. Louis), respectively, as described (Balasubramanian et al. 1997).
Electron microscopy: Electron microscopy was performed essentially as described (Wang et al. 2002). Cells were fixed in 2% potassium permanganate, dehydrated, and treated with propylene oxide prior to infiltration with Spurr’s medium, followed by another change of medium, and incubation at 65° for 1 hr. Finally, cells were embedded in Spurr’s resin and the resin was allowed to polymerize at 60° overnight. Ultrathin sections were cut on a Jung Reichert microtome (Leica Mikroskopie and Systeme GmbH, Wetzlar, Germany) and examined using a JEM1010 transmission electron microscope (Jeol, Tokyo) at 100 kV.
Measurement of acid phosphatase secretion: Levels of acid phosphatase secretion were assayed as described (Wang et al. 2002). Briefly, cell culture supernatant was assayed by using p-nitrophenyl phosphate as the substrate for acid phosphatase. The amount of released nitrophenyl was measured with a spectrophotometer at 405 nm.
RESULTS
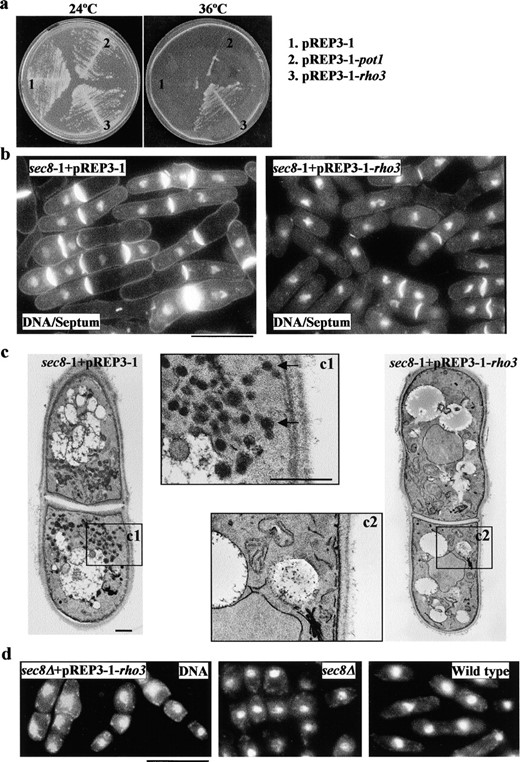
Isolation of rho3+ as a multicopy suppressor of sec8-1: A temperature-sensitive mutant, sec8-1, was previously found to be defective in cell separation and vesicle fusion in S. pombe (Wang et al. 2002). In the process of cloning the sec8 gene by complementation, we isolated a plasmid from a S. pombe genomic library that was able to suppress the growth defect of sec8-1 at 36°. Sequencing of the plasmid insert revealed that it contained the rho3 gene, encoding a rho GTPase protein. As shown in Figure 1a, sec8-1 cells transformed with rho3+ grew well and formed healthy colonies at 36°, whereas cells transformed with control plasmids were unable to do so. Thus, the rho3+ gene is a multicopy suppressor of the sec8-1 mutation.
We next tested whether the cell separation defect of the sec8-1 mutant could be suppressed by overproduction of Rho3p. sec8-1 cells overproducing Rho3p were stained to visualize DNA and septum. Whereas sec8-1 cells carrying the vector alone (Figure 1b, left) were unable to separate following cytokinesis, sec8-1 cells overexpressing Rho3p were capable of separation following cytokinesis (Figure 1b, right). Given that the cell separation phenotype of sec8-1 was suppressed upon overproduction of Rho3p, we performed electron microscopy to examine whether the secretion defect associated with sec8-1 was also suppressed in these cells. As previously described (Wang et al. 2002), sec8-1 cells accumulate presumed secretory vesicles (∼100 nm in diameter, intensely stained after permanganate fixation) in the cytoplasm due to a defect in post-Golgi vesicle trafficking. Interestingly, very few secretory vesicles were detected in sec8-1 cells overexpressing Rho3p (Figure 1c, right, and bottom box c2), whereas control cells accumulated a large number of secretory vesicles as in sec8-1 alone (Figure 1c, left, and top box c1). Thus, overproduction of Rho3p rescues all deleterious phenotypes associated with sec8-1.
—(a) Isolation of rho3 as a multicopy suppressor of sec8-1 in S. pombe. A sec8-1 strain transformed with a blank control plasmid pREP3-1 (1), a random insert control plasmid pREP3-1-pot1 (2), and a rho3-containing plasmid pREP3-1-rho3 (3) were grown on plates at 24° (left) for 4 days and at 36° (right) for 2 days, respectively. (b) Cell separation phenotype of sec8-1 is suppressed upon overproduction of Rho3p. sec8-1 cells carrying pREP3-1 (left) or pREP3-1-rho3 (right) were grown at 36° and stained with DAPI/aniline blue to visualize DNA and septum. Bar, 10 μm. (c) sec8-1 cells carrying pREP3-1 (left) or pREP3-1-rho3 (right) were grown at 36°, fixed, and processed for electron microscopy. c1 and c2 are magnified regions. Arrows, putative secretory vesicles. Bar, 500 μm. (d) Overexpression of Rho3p is unable to suppress a sec8Δ strain. A diploid strain carrying the sec8Δ mutation and a pREP3-1-rho3 plasmid was sporulated and germinated in minimal medium lacking uracil and leucine to select for the sec8Δ and pREP3-1-rho3 plasmid at 30° for 24 hr. Cells were then stained with DAPI to visualize DNA. DAPI-stained sec8Δ (middle) and wild-type cells (right) are shown for comparison. Bar, 10 μm.
rho3 is unable to suppress the sec8 null mutant: The suppression of the sec8-1 mutant by ectopic expression of Rho3p could be explained by two possibilities. One possibility is that increased levels of Rho3p might bypass the requirement for Sec8p function. In this case, high dosage expression of Rho3p would rescue a sec8-null mutation. Alternatively, high levels of Rho3p might stimulate residual activity of the mutant Sec8-1p. If so, high dosage expression of Rho3p would not rescue the sec8Δ. To distinguish between these possibilities, we investigated whether the high-copy-number rho3 gene was able to suppress a sec8Δ mutant. A heterozygous diploid carrying a sec8 null mutation marked with ura4+ was transformed with plasmid carrying rho3 marked with leu1+ and sporulated, and spores were germinated in minimal medium selecting for the sec8Δ and the plasmid carrying rho3. These germinated spores (Figure 1d, left) were found to exhibit a phenotype similar to that of the sec8Δ alone (Figure 1d, middle), but were dramatically different from wild type (Figure 1d, right). Thus, overexpression of Rho3p was unable to rescue the loss of Sec8p, indicating that Rho3p suppresses the sec8-1 mutant by stimulating residual function of the mutant Sec8-1p, rather than bypassing the requirement of Sec8p.
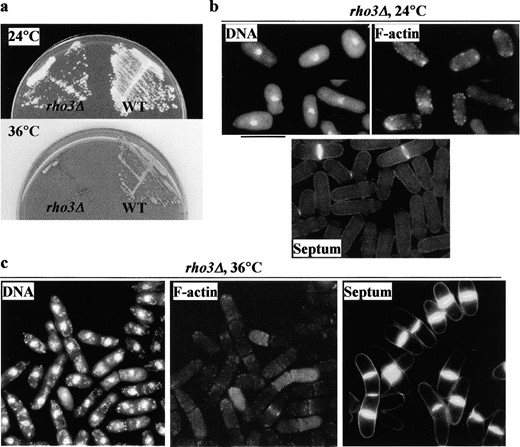
—(a-c) A rho3Δ mutant is defective in cell separation at restrictive temperature. (a) To compare relative growth rates, rho3Δ (left) and wild-type (right) cells were streaked to single colonies on plates and incubated at 24° (top) and at 36° (bottom). (b and c) Phenotype of rho3Δ cells. rho3Δ cells were either grown at 24° (b) or shifted to 36° for 4 hr (c) and fixed and stained with DAPI, rhodamine-conjugated phalloidin and calcofluor to visualize DNA, F-actin, and the cell wall, respectively. Bar, 10 μm.
rho3Δ mutant is defective in cell separation and shows genetic interaction with sec8-1 and exo70Δ: To examine whether Rho3p plays a role in cell separation, we constructed the rho3 deletion mutant (rho3Δ) by replacing the entire rho3 ORF with the ura4 gene. rho3Δ formed colonies at 24°, showing that the rho3 gene was not essential. Interestingly, rho3Δ cells exhibited a temperature-sensitive phenotype (Figure 2a). At the permissive temperature (24°), rho3Δ cells resembled wild-type cells (Figure 2b). Following the shift to the restrictive temperature (36°), rho3Δ cells were defective in cell separation (Figure 2c). These cells were able to grow and to assemble the actomyosin ring and division septa, but were defective in proper cleavage of the division septum. A majority of the mutant cells failed to separate and showed thickened septa despite normal mitosis and F-actin distribution. This phenotype mimics that of exocyst mutants (Wang et al. 2002), indicating that Rho3p is also required for cell separation.
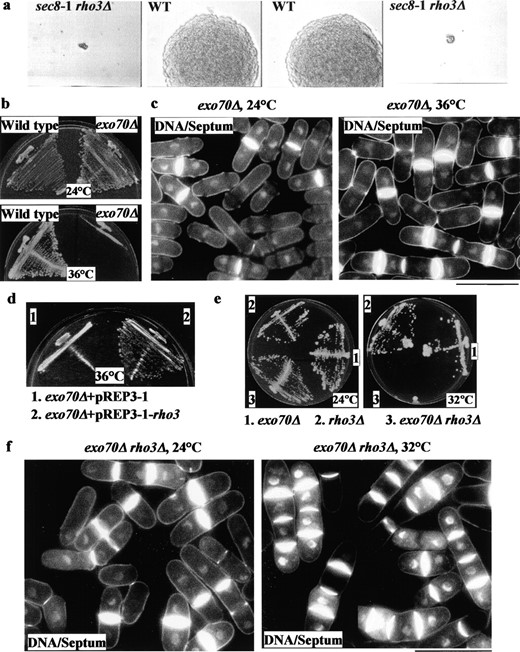
To test for genetic interactions between rho3 and sec8, we crossed a rho3Δ strain to a sec8-1 strain. Interestingly, the double mutant was inviable whereas both single mutants survived at 24°, indicating that rho3Δ was synthetically lethal with sec8-1 (Figure 3a). From this cross, there were six parental ditypes, 12 tetrad types, and three NPDs (nonparental ditypes) in which the double mutant(s) died in each tetrad, suggesting a genetic interaction between Rho3p and Sec8p.
To test whether rho3 interacted with other exocyst components, we tested the genetic interaction between rho3 and another exocyst mutant, exo70Δ, which exhibited a temperature-sensitive lethal phenotype (Figure 3b) and had a cell separation defect at 36° (Figure 3c). Overproduction of Rho3p was able to suppress growth (Figure 3d) and partially cell separation phenotypes (data not shown) of exo70Δ, confirming a genetic interaction between these gene products. In addition, the double mutant rho3Δ exo70Δ failed to grow at 32°, which was permissive for either of the single mutants (Figure 3e), further confirming this genetic interaction. While rho3Δ exo70Δ cells showed a mild cell separation defect at 24° (Figure 3f, left), this phenotype was exacerbated at 32° (Figure 3f, right) with most cells arresting with multiple septa, similar to the sec8-1 mutant phenotype. Thus, S. pombe rho3 shows strong genetic interactions with all exocyst components for which conditionally lethal mutants are currently available.
—(a) sec8-1 is synthetically lethal with rho3Δ. Asci from a cross of a sec8-1 mutant strain with a rho3Δ strain were dissected on YES and grown at 24°. One NPD is shown. Double mutants were unable to form colonies at 24°. (b) exo70Δ is temperature sensitive. To compare relative growth rates, wild-type (left side) and exo70Δ (right side) cells were streaked onto single colonies on plates and incubated at 24° (top) and 36° (bottom). (c) exo70Δ cells were grown at 24° (left) or shifted to 36° for 4 hr (right), fixed, and stained with DAPI/aniline blue to visualize DNA and septum. (d) Overproduction of Rho3p suppresses growth defects of exo70Δ. exo70Δ cells transformed with pREP3-1 (left side) or pREP3-1-rho3 (right side) were grown on plates and were incubated at 24° or 36°. (e) exo70 shows a negative genetic interaction with rho3. Strains exo70Δ (1), rho3Δ (2), and exo70Δ rho3Δ (3) were grown at either 24° (left) or 32° (right) for 3 days. (f) The exo70Δ rho3Δ double mutant shows more severe defects in cell separation than each single null mutant does. exo70Δ rho3Δ cells were grown at 24° (left) or shifted to 32° for 4 hr (right), fixed, and stained with DAPI/aniline blue to visualize DNA and septum.
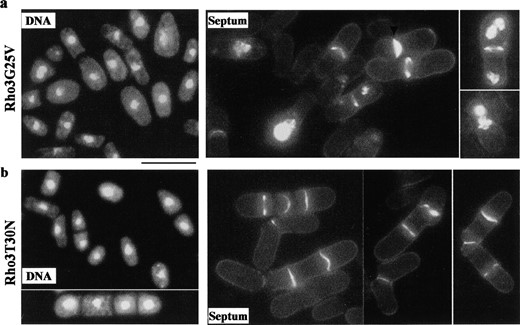
Phenotypes of dominant rho3 mutations: Rho3p is a member of a family of small GTPases that function as molecular switches. Given that activating and inactivating mutations have been extensively characterized in several ras-related GTPases, we studied the function of Rho3p by characterization of dominant-active and dominant-inactive versions of Rho3p. To analyze the dominant phenotypes of rho3 alleles, we introduced two mutations in rho3 that predict a constitutively active (Rho3G25V) form or a constitutively inactive (Rho3T30N) form by analogy to ras oncogene (Bourne et al. 1991). Cells overexpressing Rho3G25V (Figure 4a) had abnormal cell wall deposition, an effect similar to that reported by Nakano et al. (2002) using a different active Rho3Q71L mutation. In addition, the position of the division septum was altered in some cells (Figure 4a, arrowhead). Cells overproducing Rho3T30N (Figure 3b) were defective in cell separation as observed with the rho3-deletion mutant. These data suggest that Rho3p is involved in cell separation and might be required for cell polarity and cell wall integrity. The cell wall integrity of Rho3G25V and Rho3T30N mutants was measured by testing their sensitivity to zymolyase digestion. Neither mutant exhibited increased sensitivity to zymolyase (data not shown). However, given that the cells expressing Rho3G25V and RhoT30N, as with the exocyst mutants, lyse upon incubation at the restrictive temperature, it is likely that the integrity of the cell wall is compromised in these cells under restrictive growth conditions.
—Phenotypes of dominant forms of rho3 mutants. Cells expressing either Rho3-Val-25 (a) or Rho3-Asn-30 (b) under control of an nmt1 promoter were grown in the absence of thiamine at 30° for 24 hr, fixed, and stained with DAPI and calcofluor to visualize DNA and septum, respectively. Bar, 10 μm.
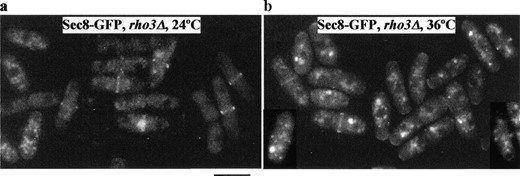
The localization of exocyst proteins is independent of Rho3p: To investigate whether Rho3p regulates the localization of the exocyst complex at the division site, we examined the localization of exocyst components Sec8-GFP and Sec6-GFP in a rho3Δ strain. At both 24° (Figure 5a) and 36° (Figure 5b), Sec8-GFP was clearly localized to the division site and growing tip(s) in rho3Δ, suggesting that the localization of Sec8p was independent of Rho3p. Similarly, the localization of Sec6p also seemed to be unaffected in rho3Δ (data not shown). On the basis of these observations, we propose that regulation of localization of the exocyst complex to the division site is not a likely function of Rho3p.
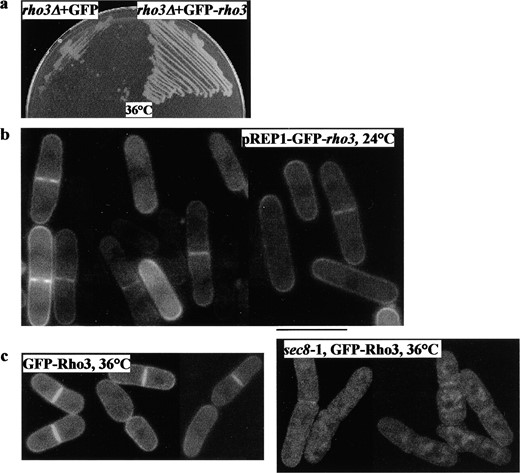
GFP-Rho3p localizes to the division site and its localization requires a functional exocyst: To investigate the intracellular localization of Rho3p, we tagged the rho3 gene with green fluorescent protein (GFP) under its native promoter at the chromosomal locus. However, no discernible GFP signal could be observed in these cells, presumably due to low expression levels (data not shown). We then fused the rho3 gene at the amino terminus with GFP, expressed it from the nmt1 promoter of the pREP1 plasmid (Basi et al. 1993), and introduced this plasmid into wild-type cells and into a rho3Δ strain. This plasmid expressing GFP-Rho3p under conditions of repression was able to rescue rho3Δ at 36° (Figure 6a), indicating that this fusion protein was functional. Under conditions of nmt1 promoter repression, we found that the fusion protein localized to the plasma membrane in interphase. During cell division, GFP-Rho3p was localized to the division site (Figure 6b), similar to exocyst proteins. This suggests that rho3 is a plasma-membrane-bound protein that localizes to the division site during cytokinesis.
—Sec8-GFP localization is rho3 independent. rho3Δ cells expressing Sec8-GFP were either grown at 24° (a) or shifted to 36° for 4 hr (b) and examined for GFP epifluorescence. Bar, 10 μm.
To investigate whether the localization of Rho3p required the function of the exocyst, the plasmid expressing GFP-Rho3p was introduced into sec8-1. Whereas GFP-Rho3p alone localized in wild-type cells (Figure 6c, left), its localization was partially compromised in most sec8-1 cells at 36° in that a significant proportion of the protein was detected in intracellular structures (Figure 6c, right). Even at 24°, Rho3p localization was compromised in a significant portion of sec8-1 cells (data not shown). These data indicate that efficient Rho3 localization to the plasma membrane depends on functional Sec8p, but that Rho3p localization is not solely dependent on exocyst function.
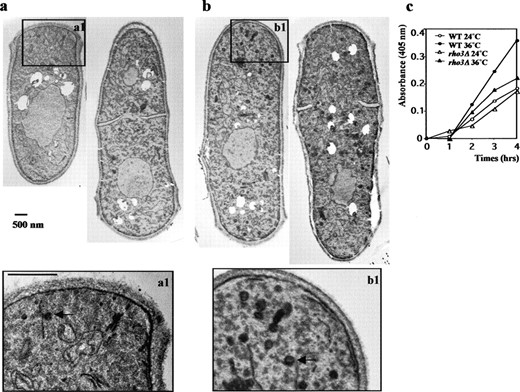
Rho3p is involved in vesicle transport: Given that we had isolated rho3 as a high-copy suppressor of a mutant defective in vesicle transport, we tested whether Rho3p was also involved in vesicle transport by performing electron microscopy on the rho3Δ cells. Secretory vesicles were rarely observed in rho3Δ cells growing at 24° (Figure 7a), similar to observation of wild-type cells (Wang et al. 2002). Interestingly, in rho3Δ cells growing at 36°, a large number of putative secretory vesicles accumulated in the cytoplasm in both uninucleate and binucleate interphase cells, suggesting that Rho3p might play a role in trafficking of secretory vesicles at all stages of the cell cycle (Figure 7b). To further test whether rho3Δ had defects in secretion, we monitored the transport of acid phosphatase, a protein that transits through the secretory pathway in rho3Δ cells. rho3Δ cells secreted much less acid phosphatase at 36° compared to that secreted by wild-type cells (Figure 7c). These results suggest that Rho3p is likely to be involved in post-Golgi vesicle trafficking, possibly by coordinating with the exocyst complex.
—Localization of GFP-Rho3p in S. pombe. (a) GFP-Rho3p rescues the growth defect of rho3Δ at 36°. rho3Δ cells were transformed with plasmids expressing GFP alone (cells on left) or GFP-rho3 (cells on right) from nmt1 promoter at the repressed condition. Cells were grown at 24° (not shown) and 36°. (b) GFP-Rho3p localizes to the plasma membrane. The plasmid containing a fusion of GFP at the amino terminus of Rho3p and under control of the nmt1 promoter was introduced into S. pombe wild-type cells. Cells were grown overnight in the presence of thiamine at 30° and visualized microscopically to examine GFP epifluorescence. (c) GFP-Rho3p localization is dependent on functional Sec8p. Wild type (left) or sec8-1 (right) expressing GFP-Rho3p were examined for GFP-Rho3p epifluorescence at 36°. Bar, 10 μm.
—Rho3p is required for a late stage of exocytosis. (a) Electron microscopic analysis of rho3Δ cells. rho3Δ cells were either grown at 24° (a) or shifted to 36° for 4 hr (b) overnight, fixed in permanganate, and processed for ultrastructural analysis. The ∼100-nm darkly stained structures are presumptive secretory vesicles (arrow in magnified boxes). Bar, 500 nm. (c) rho3Δ cells secrete lowered levels of acid phosphatase. Wild-type (○ and •) and rho3Δ (▵ and ▴) cells were assayed for secreted acid phosphatase activity at 24° (○ and ▵) and 36° (• and ▴).
DISCUSSION
Rho3p, a Ras superfamily small GTPase, shows genetic interaction with the exocyst in fission yeast: The Ras GTPase superfamily of proteins are regulators of diverse biological processes including cell polarization, morphogenesis, cell growth, and development. By alternating between two different forms, an active GTP-bound state and an inactive GDP-bound state, they act as molecular switches in response to different signals (Bourne et al. 1990). In yeasts, the Rho subfamily consists of six members, Cdc42 and Rho1-Rho5. In S. cerevisiae, CDC42, Rho3, and Rho4 play roles in polarized bud formation, while Rho1, Rho2, and Rho5 are involved in synthesis and maintenance of the cell wall (Madaule et al. 1987; Adams et al. 1990; Johnson and Pringle 1990; Matsui and Toh-e 1992; Cabib et al. 1998; Adamo et al. 1999; Schmitz et al. 2002). In S. pombe, Cdc42p, Rho1p, and Rho2p were shown to be important for polarized growth and morphogenesis (Fawell et al. 1992; Miller and Johnson 1994; Nakano and Mabuchi 1995; Nakano et al. 1997; Hirata et al. 1998). Recently, Rho3p, a novel Rho protein from S. pombe, was shown to interact with diaphanous/formin For3p to regulate polarized cell growth (Nakano et al. 2002). In this study, we report the isolation of the rho3+ gene as a multicopy suppressor of sec8-1 and identification of a role of Rho3p in cell separation and exocytosis in S. pombe.
Our previous work established that the multiprotein exocyst complex was essential for cell separation in S. pombe (Wang et al. 2002). We proposed that the exocyst possibly targets vesicles containing primary septum-degrading enzymes to the division site. In this study we show that the rho3 gene on a multicopy plasmid is able to fully rescue the growth and cell separation defects of a sec8-1 mutant at 36°. However, rho3 was unable to rescue a sec8Δ mutant, suggesting that high levels of Rho3p stimulate the residual function of the mutant Sec8-1p, rather than bypassing a requirement for Sec8p. The synthetic lethality between sec8-1 and the rho3Δ mutant might also be explained by an additive lowering of exocyst function in the double mutant. In this context it is interesting to note that the budding yeast Rho3p has also been shown to rescue conditional mutations in sec8 and exo70. We also found that rho3 was able to suppress a null mutant lacking Exo70p, an exocyst component essential only at higher temperatures, indicating that Rho3p might activate exocyst function, thus bypassing the requirement for Exo70p. In budding yeast, Rho3p has been shown to physically interact with the exocyst complex (Robinson et al. 1999). However, we have been unable to detect a physical interaction between exocyst proteins (Sec8p, Sec10p, and Exo70p) and Rho3p or Rho3G25V mutants in immunoprecipitation experiments (data not shown). We have also been unable to detect any interaction of Sec8p with Rho3p in a yeast two-hybrid system (data not shown). It is possible that this interaction is transient and therefore difficult to observe.
Unlike in S. cerevisiae, Exo70p is nonessential in fission yeast. However, the exo70 null mutant leads to a temperature-sensitive growth phenotype. We found that double-mutant exo70Δ rho3Δ had a more severe cell separation phenotype than each single mutant, indicating that Rho3p might share a redundant function with Exo70p. The molecular basis of this interaction is currently unclear. It is possible that Rho3p or one of its effectors might play a role redundant with Exo70p, leading to the observed genetic interaction. Given similarities in the interactions between Rho3p and the exocyst complex in budding and fission yeasts, it is possible that the exocyst function might be regulated similarly in other cell types as well.
Rho3p is a regulator of cell separation and exocytosis in S. pombe: Cells depleted of Rho3 protein show defects in cell separation similar to that of exocyst mutants, suggesting that Rho3p is involved in this process. Consistently, overproduction of a dominant-inactive form of Rho3p (Rho3T30N) almost phenocopies rho3Δ. The localization of Rho3p to the division site, as with the exocyst proteins, is consistent with a role for this protein in cell division/separation. As with the exocyst null mutants, we could not observe cell growth, elongation, and cell polarity defects in a rho3Δ mutant, suggesting that Rho3p is not essential for cell elongation and division septum assembly. rho3 is essential only at higher temperatures, whereas the exocyst complex is essential for cell viability at all temperatures except exo70. It is possible that Rho3p acts redundantly with other proteins that might themselves be critical for viability at lower temperatures. Rho4p is an attractive candidate, although other Rho proteins such as Cdc42p (Zhang et al. 2001) and Rho1p (Guo et al. 2001) might also contribute to exocyst function, as shown recently in budding yeast.
We also show that putative secretory vesicles accumulate in rho3Δ at the restrictive temperature, indicating that Rho3p is required for the targeting and fusion of secretory vesicles to the plasma membrane. A role for Rho3p in exocytosis was also inferred from lowered levels of acid phosphatase secretion in a rho3Δ compared to wild-type cells. Our data lead us to suggest that Rho3p may play a role in cell separation probably by targeting vesicles containing primary cell-wall-degrading enzymes to the division site via interaction with the exocyst complex. Interestingly, these vesicles are observed in both interphase and mitotic cells, suggesting that in addition to functioning during cell separation, Rho3p might also play a nonessential role in polarized cell growth/elongation. Consistent with this possibility is our finding that Rho3p is detected at the plasma membrane of interphase cells as well.
We find that the polarized localization of Sec8p and Sec6p is not affected in a rho3Δ strain, suggesting that exocyst proteins localize to the division site in a Rho3p-independent manner. Thus, Rho3p modulates the exocyst function by mechanisms independent of those involved in their proper localization. It is conceivable that maximal activation/function of exocyst is achieved via interaction with Rho3p and other proteins redundant with it. Interestingly, the localization of Rho3p seemed to be severely affected in sec8-1, indicating that the exocyst is important for Rho3p localization. Future studies should unravel the molecular nature of interactions between Rho3p and the exocyst complex.
Acknowledgement
We acknowledge all members of the TLL-IMCB yeast and fungal biology laboratories for helpful suggestions, advice, and reagents. In particular, we thank Ventris D’Souza, Naweed Naqvi, Snezhana Oliferenko, Suniti Naqvi, and Volker Wachtler for critical reading of the manuscript. This work was supported by research funds from the National Science and Technology Board/Agency for Science, Technology and Research Singapore (until July 2002) and subsequently by internal funds from the Temasek Life Sciences Laboratory.
Footnotes
Communicating editor: P. Russell
LITERATURE CITED
Author notes
Present address: Molecular and Cell Biology, 341 LSA, University of California, Berkeley, CA 94720-3200.