-
PDF
- Split View
-
Views
-
Cite
Cite
Shu-hei Yoshida, Hiba Al-Amodi, Taro Nakamura, Christopher J McInerny, Chikashi Shimoda, The Schizosaccharomyces pombe cdt2+ Gene, a Target of G1-S Phase-Specific Transcription Factor Complex DSC1, Is Required for Mitotic and Premeiotic DNA Replication, Genetics, Volume 164, Issue 3, 1 July 2003, Pages 881–893, https://doi.org/10.1093/genetics/164.3.881
Close - Share Icon Share
Abstract
We have defined five sev genes by genetic analysis of Schizosaccharomyces pombe mutants, which are defective in both proliferation and sporulation. sev1+/cdt2+ was transcribed during the G1-S phase of the mitotic cell cycle, as well as during the premeiotic S phase. The mitotic expression of cdt2+ was regulated by the MCB-DSC1 system. A mutant of a component of DSC1 affected cdt2+ expression in vivo, and a cdt2+ promoter fragment containing MCB motifs bound DSC1 in vitro. Cdt2 protein also accumulated in S phase and localized to the nucleus. cdt2 null mutants grew slowly at 30° and were unable to grow at 19°. These cdt2 mutants were also medially sensitive to hydroxyurea, camptothecin, and 4-nitroquinoline-1-oxide and were synthetically lethal in combination with DNA replication checkpoint mutations. Flow cytometry analysis and pulsed-field gel electrophoresis revealed that S-phase progression was severely retarded in cdt2 mutants, especially at low temperatures. Under sporulation conditions, premeiotic DNA replication was impaired with meiosis I blocked. Furthermore, overexpression of suc22+, a ribonucleotide reductase gene, fully complemented the sporulation defect of cdt2 mutants and alleviated their growth defect at 19°. These observations suggest that cdt2+ plays an important role in DNA replication in both the mitotic and the meiotic life cycles of fission yeast.
SPORULATION in the fission yeast Schizosaccharomyces pombe is an excellent model system for the study of cell differentiation (Egel 1971, 1989; Yamamoto et al. 1997). Ascospore formation is preceded by meiosis that itself is induced by starvation. To date, many S. pombe mutants defective solely in sporulation have been isolated. Genetic analysis of these mutants has defined sporulation-specific genes, such as spo1-spo20 (Bresch et al. 1968; Kishida and Shimoda 1986). However, gene disruption studies of these spo genes revealed that some of them are also essential for vegetative growth. It would appear that spore formation relies not only on specific spo genes but also on genes required for proliferation.
Here we report the isolation and analysis of S. pombe genes, necessary for normal growth and also required for sporulation. These genes are termed sev (sporulation genes essential or important for vegetative growth). Among five sev genes defined, sev1+ is identical to the previously reported cdt2+ (Hofmann and Beach 1994), whose function is unknown. In this article we focus on the functional analysis of sev1+/cdt2+.
The Cdc10-dependent transcript 2 (cdt2+) gene is tran- a peak of expression at the G1-S interval (Hofmann and Beach 1994). In eukaryotic cells, a set of genes required for DNA replication are periodically transcribed in the cell cycle, peaking at G1-S phase under control of specific transcription factors, such as the Mbp1/Swi6 complex in Saccharomyces cerevisiae (Verma et al. 1992; Koch et al. 1993). In fission yeast, the equivalent transcription factor complex involved in G1-S specific transcription is named DSC1 (DNA synthesis control; also MBF). This complex is composed of the products of the cdc10+, res1+, res2+, rep1+, and rep2+ genes (Lowndes et al. 1992; Caligiuri and Beach 1993; Miyamoto et al. 1994; Sugiyama et al. 1994; Zhu et al. 1994; Nakashima et al. 1995). DSC1 binds to a cis-acting promoter element, called an MCB (MluI cell-cycle box; ACGCGT; Lowndes et al. 1992). cdc22+, cdc18+, cdt1+, cdt2+, and cig2+ have been reported as target genes of DSC1 (Lowndes et al. 1992; Kelly et al. 1993; Hofmann and Beach 1994; Obara-Ishihara and Okayama 1994), all of which contain MCB elements in their promoters. Most of these genes are implicated in DNA replication: Cdt1 and Cdc18 are licensing factors controlling DNA replication in the cell cycle (Nishitani et al. 2000), and Cdc22 is a regulatory subunit of ribonucleotide reductase, which catalyzes the synthesis of deoxyribonucleoside triphosphates (dNTP; Fernandez-Sarabia et al. 1993). Here we describe evidence to show that Cdt2 plays an important role in the regulation of mitotic and premeiotic S-phase progression.
Strains used in this study
| Strain . | Genotype . | Reference/Source . |
|---|---|---|
| C982-16C | h90 ura4-D18 | C. Shimoda |
| C996-11D | h90 leu1-32 | C. Shimoda |
| GG1 | h- | C. J. McInerny |
| GG51 | h- cdc25-22 | C. J. McInerny |
| GG147 | h- res2::ura4+ ura4-D18 | C. J. McInerny |
| GG149 | h- rep2::ura4+ ura4-D18 | C. J. McInerny |
| GG154 | h- res1::ura4+ ura4-D18 | C. J. McInerny |
| GG214 | h- leu1-32 | C. J. McInerny |
| GG219 | h- cdc10-129 | C. J. McInerny |
| GG250 | h- cdc10-C4 | C. J. McInerny |
| SY2 | h90 cdt2-M1 ura4-D18 | This study |
| SY3 | h90 cdt2-M1 leu1-32 | This study |
| SY6 | h90 cdt2::ura4+ ura4-D18 leu1-32 | This study |
| SY7 | h90 cdt2::HA-cdt2+ ura4-D18 leu1-32 | This study |
| SY8 | h- cdc25-22 | This study |
| SY9 | h- cdt2-M1 cdc25-22 | This study |
| SY11 | h- cdt2::HA-cdt2+ cdc25-22 | This study |
| SY12 | h90 rad3::ura4+ ura4-D18 leu1-32 | This study |
| TN29 | h90 ura4-D18 leu1-32 Ikemoto et al. (2000) | |
| MKD3 | h90 spo3::ura4+ ura4-D18 leu1-32 | Nakamura et al. (2001) |
| SI51 | h90 spo15::ura4+ ura4-D18 leu1-32 | Ikemoto et al. (2000) |
| JZ670 | h-/h- pat1-114/pat1-114 ade6-M210/ade6-M216 leu1-32/leu1-32 | M. Yamamoto |
| Strain . | Genotype . | Reference/Source . |
|---|---|---|
| C982-16C | h90 ura4-D18 | C. Shimoda |
| C996-11D | h90 leu1-32 | C. Shimoda |
| GG1 | h- | C. J. McInerny |
| GG51 | h- cdc25-22 | C. J. McInerny |
| GG147 | h- res2::ura4+ ura4-D18 | C. J. McInerny |
| GG149 | h- rep2::ura4+ ura4-D18 | C. J. McInerny |
| GG154 | h- res1::ura4+ ura4-D18 | C. J. McInerny |
| GG214 | h- leu1-32 | C. J. McInerny |
| GG219 | h- cdc10-129 | C. J. McInerny |
| GG250 | h- cdc10-C4 | C. J. McInerny |
| SY2 | h90 cdt2-M1 ura4-D18 | This study |
| SY3 | h90 cdt2-M1 leu1-32 | This study |
| SY6 | h90 cdt2::ura4+ ura4-D18 leu1-32 | This study |
| SY7 | h90 cdt2::HA-cdt2+ ura4-D18 leu1-32 | This study |
| SY8 | h- cdc25-22 | This study |
| SY9 | h- cdt2-M1 cdc25-22 | This study |
| SY11 | h- cdt2::HA-cdt2+ cdc25-22 | This study |
| SY12 | h90 rad3::ura4+ ura4-D18 leu1-32 | This study |
| TN29 | h90 ura4-D18 leu1-32 Ikemoto et al. (2000) | |
| MKD3 | h90 spo3::ura4+ ura4-D18 leu1-32 | Nakamura et al. (2001) |
| SI51 | h90 spo15::ura4+ ura4-D18 leu1-32 | Ikemoto et al. (2000) |
| JZ670 | h-/h- pat1-114/pat1-114 ade6-M210/ade6-M216 leu1-32/leu1-32 | M. Yamamoto |
Strains used in this study
| Strain . | Genotype . | Reference/Source . |
|---|---|---|
| C982-16C | h90 ura4-D18 | C. Shimoda |
| C996-11D | h90 leu1-32 | C. Shimoda |
| GG1 | h- | C. J. McInerny |
| GG51 | h- cdc25-22 | C. J. McInerny |
| GG147 | h- res2::ura4+ ura4-D18 | C. J. McInerny |
| GG149 | h- rep2::ura4+ ura4-D18 | C. J. McInerny |
| GG154 | h- res1::ura4+ ura4-D18 | C. J. McInerny |
| GG214 | h- leu1-32 | C. J. McInerny |
| GG219 | h- cdc10-129 | C. J. McInerny |
| GG250 | h- cdc10-C4 | C. J. McInerny |
| SY2 | h90 cdt2-M1 ura4-D18 | This study |
| SY3 | h90 cdt2-M1 leu1-32 | This study |
| SY6 | h90 cdt2::ura4+ ura4-D18 leu1-32 | This study |
| SY7 | h90 cdt2::HA-cdt2+ ura4-D18 leu1-32 | This study |
| SY8 | h- cdc25-22 | This study |
| SY9 | h- cdt2-M1 cdc25-22 | This study |
| SY11 | h- cdt2::HA-cdt2+ cdc25-22 | This study |
| SY12 | h90 rad3::ura4+ ura4-D18 leu1-32 | This study |
| TN29 | h90 ura4-D18 leu1-32 Ikemoto et al. (2000) | |
| MKD3 | h90 spo3::ura4+ ura4-D18 leu1-32 | Nakamura et al. (2001) |
| SI51 | h90 spo15::ura4+ ura4-D18 leu1-32 | Ikemoto et al. (2000) |
| JZ670 | h-/h- pat1-114/pat1-114 ade6-M210/ade6-M216 leu1-32/leu1-32 | M. Yamamoto |
| Strain . | Genotype . | Reference/Source . |
|---|---|---|
| C982-16C | h90 ura4-D18 | C. Shimoda |
| C996-11D | h90 leu1-32 | C. Shimoda |
| GG1 | h- | C. J. McInerny |
| GG51 | h- cdc25-22 | C. J. McInerny |
| GG147 | h- res2::ura4+ ura4-D18 | C. J. McInerny |
| GG149 | h- rep2::ura4+ ura4-D18 | C. J. McInerny |
| GG154 | h- res1::ura4+ ura4-D18 | C. J. McInerny |
| GG214 | h- leu1-32 | C. J. McInerny |
| GG219 | h- cdc10-129 | C. J. McInerny |
| GG250 | h- cdc10-C4 | C. J. McInerny |
| SY2 | h90 cdt2-M1 ura4-D18 | This study |
| SY3 | h90 cdt2-M1 leu1-32 | This study |
| SY6 | h90 cdt2::ura4+ ura4-D18 leu1-32 | This study |
| SY7 | h90 cdt2::HA-cdt2+ ura4-D18 leu1-32 | This study |
| SY8 | h- cdc25-22 | This study |
| SY9 | h- cdt2-M1 cdc25-22 | This study |
| SY11 | h- cdt2::HA-cdt2+ cdc25-22 | This study |
| SY12 | h90 rad3::ura4+ ura4-D18 leu1-32 | This study |
| TN29 | h90 ura4-D18 leu1-32 Ikemoto et al. (2000) | |
| MKD3 | h90 spo3::ura4+ ura4-D18 leu1-32 | Nakamura et al. (2001) |
| SI51 | h90 spo15::ura4+ ura4-D18 leu1-32 | Ikemoto et al. (2000) |
| JZ670 | h-/h- pat1-114/pat1-114 ade6-M210/ade6-M216 leu1-32/leu1-32 | M. Yamamoto |
MATERIALS AND METHODS
Yeast strains, media, and genetic techniques: S. pombe strains used in this study are listed in Table 1. Complete medium (YEA) was used for routine growth. Sporulation media used were MEA and SSA for plate culture and Edinburgh minimal medium - N (EMM-N) for liquid culture. Minimal media used were MM, EMM, and SD. These standard fission yeast media have been described (Egel and Egel-Mitani 1974; Gutz et al. 1974; Moreno et al. 1991). Synchronous cultures were prepared by transient temperature shifts using a cdc25-22 temperature-sensitive mutant. Cells were first cultured at 36° for 3.5 hr in EMM liquid medium, and G2 block was then released by shifting the temperature to the permissive temperature of 25°. In meiotic experiments, a synchronous sporulation protocol using a pat1-114ts mutant was adopted (Iino and Yamamoto 1985; Nurse 1985; Bahler et al. 1991). Transformation was performed by the lithium acetate method (Okazaki et al. 1990). Conventional procedures for S. pombe genetic manipulation were followed (Gutz et al. 1974; Alfa et al. 1993). Flow cytometry was performed as previously described (Tanaka et al. 1992; McInerny et al. 1995).
Mutagenesis: Two S. pombe strains, C982-16C and C996-11D, were used as parents for mutagenesis. Cells (3 × 108) from a log-phase culture were treated with ethyl methanesulfonate (EMS) at a final concentration of 2% (v/v) for 10 min in 5 ml MM liquid medium. At the end of the treatment, 20 ml of 5% sodium thiosulfate was added, and cells were washed twice with 10 ml 5% sodium thiosulfate and then three times with 1 ml MM liquid culture. Finally, aliquots were spread on YE agar plates. Temperature-dependent growth was determined by incubation of replica plates grown at 19°, 30°, and 37°. Sporulation ability was tested on MEA plates by iodine vapor method and direct observation of asci under a phase-contrast microscope.
Cloning of the sev genes: Molecular cloning of wild-type genes was conducted by phenotypic complementation of sev mutant strains transformed with the S. pombe genomic library, pTN-L1 (Nakamura et al. 2001), which contained Sau3AI fragments constructed on a multicopy plasmid, pAL-KS (Tanaka et al. 2000). Transformants on SSA sporulation plates for 3 days were treated with iodine vapor. Positive colonies were taken and their sporulation efficiency was examined by phase-contrast microscopy. A multicopy suppressor of the cdt2-M1 mutant (SY2) was isolated by selecting for cDNA clones that complemented its sporulation deficiency. An S. pombe meiotic cDNA library, pTN-RC5 (Nakamura et al. 2002), constructed on an expression vector, pREP42 (Maundrell 1993), was used.
To show that the cloned genes were identical to the genetically defined sev genes, we constructed integration plasmids by inserting the cloned fragments into the pIL(II) integration vector (Nakamura et al. 2001). The integration plasmids were cut with appropriate restriction enzymes and then transformed into the wild-type strain TN29. The integrant strains were crossed with the respective sev mutants, and spores derived from the resultant diploids were analyzed by tetrad dissection.
Nucleotide sequence analysis of the cdt2-M1 allele: The entire cdt2-M1 open reading frame (ORF) was amplified by PCR, using genomic DNA of cdt2-M1 as a template. The amplified DNA fragment was cloned into pGEM easy vector (Promega, Madison, WI), and its nucleotide sequence determined using an ABI dye terminator cycle sequence kit (PE Applied Biosystems, Foster City, CA).
Gene disruption: To disrupt the cdt2+ gene, the whole ORF was replaced by S. pombe ura4+. A 5.3-kb BamHI-XhoI fragment containing the cdt2+ ORF was inserted into the pBluescript II-KS(-) vector (Stratagene, La Jolla, CA) to yield pSY38. The plasmid pSY38 was then subjected to PCR amplification using a pair of oligonucleotide primers, GGGAGATCT(BglII)GGAT TTAGTGAAAAATGA and CCCAGATCT(BglII)TCCAATGT CCATATCAT. The amplified DNA lacking the cdt2+ ORF was digested with BglII and ligated with a BamHI fragment containing ura4+ to yield pSY38(ura4+). A 5.6-kb BamHI/XhoI fragment containing the disrupted Δcdt2::ura4+ allele was introduced into TN29 and Ura+ transformants were selected. Correct chromosomal integration at the cdt2+ locus was verified by genomic Southern blot analysis.
Southern and Northern blot analysis: Genomic DNA was digested, separated in a 1% agarose gel, and then transferred onto nylon membranes (Biodyne B; Pall Bio-Support, New York). Total RNA was prepared as described in McInerny et al. (1995) using a Ribolyser (Hybaid), and Northern blotting was carried out using GeneScreen membrane (NEN, Life Science Products), following the manufacturer’s protocol. DNA probes (∼1 kb) were labeled with [α-32P]dCTP using the random hexanucleotide labeling procedure of Feinberg and Volgelstein (1983). Equal loading of RNA in each lane was confirmed by hybridization with an adh1+ probe. Transcripts were quantified using National Institutes of Health software.
HA-cdt2+ fusion construct: A triple hemagglutinin (HA) epitope-tagged cdt2+ was constructed as follows. First, the cdt2+ coding region was amplified by PCR using primers CCC GTCGAC(SalI)CATGAATATAGGACATT and GGGGCGGC CGC(NotI)ATTTTTCACTAAATCCC. The amplified DNA fragment was digested with SalI and NotI and then inserted into pSLF273 (Forsburg and Sherman 1997) containing the nmt41 promoter and HA(3×) to yield a plasmid, pHA-cdt2C. Next the cdt2 promoter region was amplified by PCR with primers M13 and CCCCTCGAG(XhoI)GGTATAATCATGA ATCTT. After digestion with SphI and XhoI, it was inserted into pHA-cdt2C instead of the nmt41 promoter. This plasmid, pHA-cdt2PC, was then digested with SacI and replaced by the SacI restriction fragment carrying the C-terminal and 3′ terminator region of cdt2. The resultant plasmid, pHA-cdt 2PCT, was then restricted by SphI and NheI. The 3.6-kb SphI/ NheI fragment bearing the HA(3×)-cdt2+ was introduced into the disruptant strain SY6 harboring cdt2::ura4+. A few Ura- colonies were selected as candidates of integrants. Correct chromosomal integration of HA-cdt2+ at the cdt2 locus was verified by genomic Southern blot analysis (data not shown). The HA-cdt2+ integrant was efficiently sporulated on MEA plates.
Western blotting: Crude cell extract was prepared from strain SY11 expressing HA-Cdt2 as described by Masai et al. (1995). Polypeptides were fractionated by SDS-PAGE on a 10% gel and then blotted onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Filters were probed with the rat anti-HA antibody 3F10 (Boehringer Mannheim, Mannheim, Germany) at a 1:1000 dilution. Blots were also probed with the anti-α-tubulin antibody TAT-1 (Woods et al. 1989) to compare the amount of loaded proteins.
Gel retardation assay: Analysis was performed as previously described (Ng et al. 2001) using MCB promoter fragments from cdt2+ and cdc22+. These fragments were amplified by PCR using oligonucleotides as follows: cdc22+, GTAGTTCA ATCTCATAGA and CTCTGT TTACGCTGAATG; cdt2+, TAT TCACTCCGCGAGTTGAGA and ATGATATAATGAGCGCT CGAA.
Pulsed-field gel electrophoresis (PFGE) analysis: Preparation of chromosomal DNA in agarose plugs was as described (Tanaka et al. 1999) with slight modifications. Cells (5 × 108) from a log-phase culture were washed with CSE [20 mm citrate-phosphate (pH 5.6), 1.2 m sorbitol, 40 mm EDTA (pH 8.0), and 150 mm β-mercaptoethanol] and resuspended in 10 ml of CSE containing 30 mm β-mercaptoethanol and 1 mg/ml of Zymolyase 100T (Seikagaku Kogyo, Tokyo). After incubation at 37° for 1.5 hr, spheroplasts were pelleted and resuspended in TSE [10 mm Tris-HCl (pH 7.5), 0.9 m sorbitol, 45 mm EDTA (pH 8.0)] at a cell density of 8 × 108 cells/ml. The suspension was incubated at 37° for 1 min, then added to an equal volume of 1% GTG low-melting-temperature agarose (SeaPlaque, BMA, Rockland, ME) in TSE, and finally dispensed in plug molds. Spheroplasts in agarose plugs were lysed in 0.25 m EDTA (pH 8.0), 50 mm Tris-HCl (pH 7.5), 1% sodium dodecyl sulfate (SDS) at 55° for 90 min, in NDS [0.5 m EDTA (pH 9.5), 1% lauryl sarcosine sulfate] containing 0.5 mg/ml of proteinase K (Takara Shuzo, Shiga, Japan) at 55° for 24 hr. The proteinase K treatment was repeated once more. After cell lysis, plugs were equilibrated with TE [10 mm Tris-HCl (pH 7.5), 1 mm EDTA (pH 8.0)] three times for 5 min each and stored in 0.5 m EDTA (pH 8.0) at 4°. Before electrophoresis, plugs were equilibrated with TE again. PFGE was carried out in a 0.8% agarose gel for 46 hr in 0.5× TBE buffer [44.5 mm Tris, 44.5 mm boric acid, 1 mm EDTA (pH 8.0)] at 50 V with a switching time of 30 min.
Fluorescence microscopy: HA-Cdt2 cells were fixed by cold methanol. HA-Cdt2 was visualized by indirect immunofluorescence microscopy with the use of rat anti-HA antibody 3F10 (Boehringer Mannheim) and Alexa 488-conjugated goat anti-rat IgG (Molecular Probes, Eugene, OR). For immunostaining of microtubules and spindle pole bodies (SPB), cells were fixed as described (Hagan and Hyams 1988). Microtubules and SPB were stained by mouse anti-α-tubulin antibody, TAT-1 (a generous gift from K. Gull, University of Manchester), and rabbit anti-Sad1 antibody (a generous gift from O. Niwa, Kazusa DNA Institute), respectively. Alexa 488-conjugated goat anti-mouse IgG (Molecular Probes) and Alexa 546-conjugated goat anti-rabbit IgG (Molecular Probes) were used as secondary antibodies. Nuclear chromatin region was stained with 4′,6-diamidino-2-phenylindole (DAPI).
RESULTS
Isolation and characterization of sev mutants: To obtain mutants defective in both vegetative growth and sporulation, two homothallic haploid strains (C982-16C and C996-11D) were mutagenized by EMS (materials and methods). Conditional lethal mutants unable to grow at 19° (cold sensitive) or at 37° (temperature sensitive) on YE medium were then examined for their ability to sporulate at the permissive temperature of 30°. Mutants that produced only very few asci were then subjected to tetrad analysis to examine whether the mutant phenotypes of defective sporulation and growth cosegregated. The isolated nonsporulating mutants defined several recessive mutations, named sev. Complementation tests of these mutants defined five genetic loci, sev1-sev5. Although sev2 and sev4 mutants normally complete meiosis, sev1, sev3, and sev5 mutants are defective in meiosis (data not shown).
We isolated the sev1+ and sev4+ genes by phenotypic complementation. Integration mapping demonstrated that these were genetically defined sev genes, but not multicopy suppressors. Subcloning of the plasmids, pAL(sev1)1 and pAL(sev4)1, and partial sequencing of their genomic inserts indicated that SPAC17H9.19c and SPACUNK4.07c (The S. pombe Genome Sequencing project, The Wellcome Trust Sanger Institute, Hixton, UK) are responsible for complementation of sev1-M1 and sev4-L5, respectively. The sev4+ gene has the highest homology with S. cerevisiae SPF1, which encodes a P-type ATPase (Suzuki and Shimma 1999). The detailed analysis of sev4+ will be reported elsewhere.
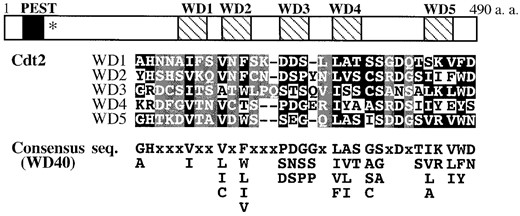
—Characteristic domains of Cdt2. Cdt2 contains a potential PEST sequence (amino acid residues 21-38) and five WD40 repeats (178-208, 226-257, 285-317, 339-369, and 435-464), which are shown as solid and hatched boxes, respectively. The nonsense mutation site in the cdt2-M1 allele is indicated by an asterisk. Amino acid alignment of the five WD40 repeats with the consensus sequence is presented. Conserved and nonconserved (“x” in the consensus) amino acid residues are indicated in white letters against solid or shaded backgrounds, respectively.
The sev1+ is identical to the known gene cdt2+ whose promoter is recognized by the G1-S phase-specific transcription factor, Cdc10 (Hofmann and Beach 1994). Hereafter sev1+ is designated as cdt2+. As the biological function and biochemical identity of Cdt2 is totally unknown, we further analyzed the cdt2+ gene. The cdt2+ gene encodes a 54-kD protein composed of 490 amino acid residues. Cdt2 contains a putative PEST motif in the N-terminal region and five conserved WD40 repeats in the rest of the protein (Figure 1). Otherwise, Cdt2 has no significant similarity with known proteins.
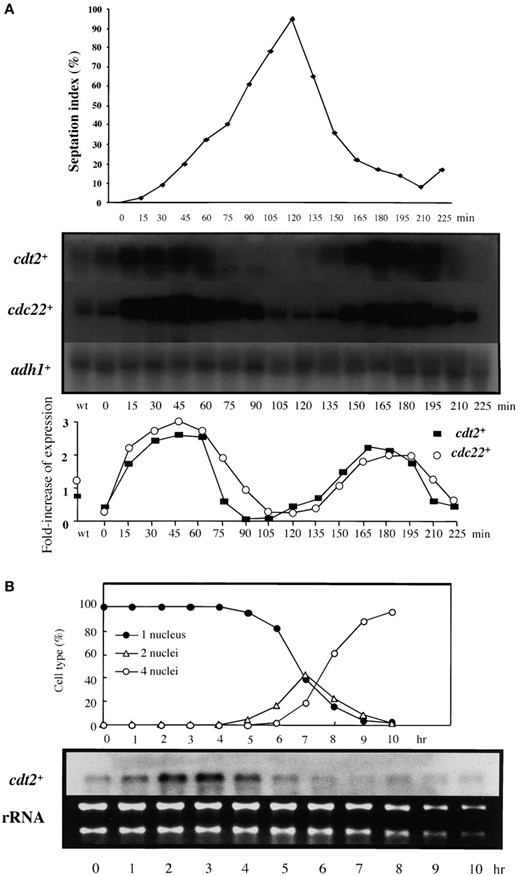
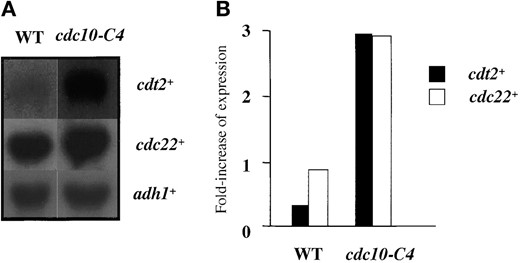
cdt2+ expression is affected by cdc10-C4 in vivo, and a cdt2+ promoter fragment binds DSC1 in vitro: cdt2+ is a possible target of Cdc10 (Hofmann and Beach 1994), which is a major component of the G1-S phase-specific transcription factor DSC1 (Lowndes et al. 1992). As previously reported, transcription of cdt2+ fluctuates through the mitotic cell cycle with a peak at G1-S phase, similar to the expression pattern of cdc22+, another target of DSC1 (Figure 2A). To test whether cdt2+ is under direct DSC1 control, we examined its transcript levels in a mutant of cdc10+, cdc10-C4. This allele contains a nonsense mutation resulting in the loss of 61 amino acids from the C terminus of the polypeptide, which causes all known fission yeast genes regulated by DSC1/MCB to be expressed throughout the cell cycle (McInerny et al. 1995; Ng et al. 2001). In asynchronous cells, cdc10-C4 manifests as overexpression relative to wild type. The cdt2+ transcript level was also overexpressed in cdc10-C4 cells, which suggests that it is regulated in vivo by DSC1 containing Cdc10 (Figure 3A). The cdc22+ gene is upregulated in cdc10-C4, although the difference between wild type and the mutant is not as strong; this is confirmed by the quantification (Figure 3B). This result reflects the fact that basal levels of cdc22+ are much higher in wild-type cells than in cdt2+.
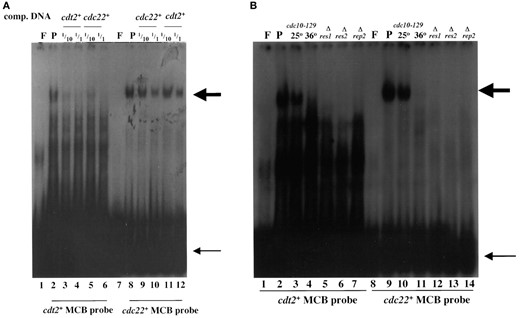
DSC1 activates G1-S transcription by binding the cis-acting promoter element MCB (Lowndes et al. 1992). The MCB DNA sequence is usually ACGCGT, although some contain only the central CGCG core thought to be essential for function (McIntosh 1993). The promoter region of cdt2+ contains seven MCB motifs in two groups. We examined whether a DNA fragment containing these potential cis elements binds DSC1. Gel retardation assays, using cell extracts from wild-type cells, showed that a cdt2+ promoter fragment, containing the three upstream MCB motifs (-314 to -453), detected a complex of low mobility (Figure 4A, lane 2). This complex was of similar size to DSC1, detected using an MCB fragment from the cdc22+ promoter (Figure 4; Lowndes et al. 1992). This complex is likely to be DSC1 for two further reasons. Both retarded complexes disappeared when the same cdt2+ and cdc22+ MCB DNAs were added as cold competitors (Figure 4A, lanes 5-6 and 11-12), suggesting that the recognized complex is the same. Finally, gel retardation assays with extracts from mutants of components of DSC1, such as cdc10ts, Δres1, Δres2, and Δrep2, revealed loss of the retarded complex, confirming that it was DSC1 (Figure 4B).
We also examined the transcription of cdt2+ during meiosis, because this gene is required for sporulation. A synchronous meiosis was induced in a diploid pat1-114 strain (JZ670), a temperature-sensitive repressor of meiosis. Northern blot analysis of RNA collected from such cells indicated that the level of cdt2+ mRNA was transiently enhanced before the first meiotic division and was weakly elevated again during meiosis II (Figure 2B). This expression pattern has been observed by DNA microarray analysis (Mata et al. 2002).
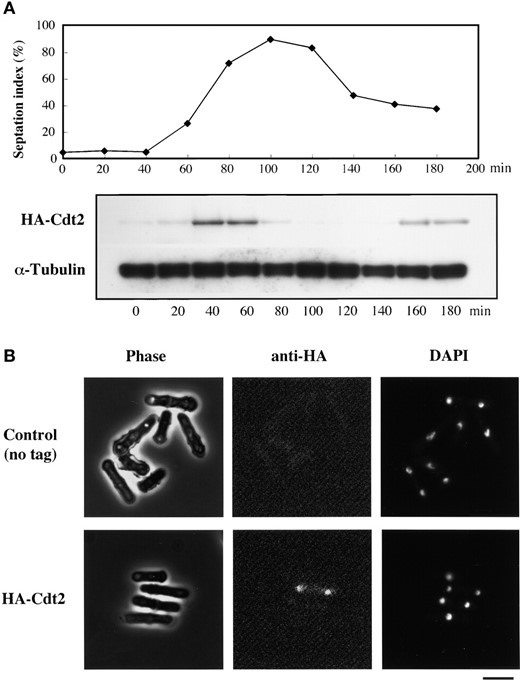
Abundance of Cdt2 in cell cycle: We presented evidence that the expression of the cdt2+ gene is regulated by the G1-S phase-specific transcription factor complex DSC1. We were next interested in examining the level of Cdt2 polypeptide throughout the cell cycle. The HA-tagged cdt2+ gene was integrated at the cdt2+ locus on chromosome I, so that a single copy of the fusion gene was expressed under the control of the authentic cdt2+ promoter (materials and methods). The HA-cdt2+ fusion fully complemented the Δcdt2 mutant (data not shown), indicating that the N-terminal fusion of the HA epitope did not affect cdt2+ function. Synchronous cell division was induced in the integrant HA-cdt2+ strain harboring cdc25-22 (SY11) and the HA-Cdt2 level was monitored by Western blotting using an anti-HA monoclonal antibody (3F10). The abundance of HA-Cdt2 oscillated, peaking at the beginning of the septation period (Figure 5A).
In vivo localization of HA-Cdt2 was studied by indirect immunofluorescence microscopy. As shown in Figure 5B, fluorescent signals of HA-Cdt2 were preferentially detected in the nucleus of binucleate cells (probably those in the G1-S phase). The fluorescence in other phases of the cell cycle decreased to the background level of a nontagged control. From the results of Western and immunofluorescence analyses, we conclude that Cdt2 is a nuclear protein that accumulates in G1-S phase.
—Mitotic and meiotic cell-cycle expression of cdt2+. (A) Transcription of cdt2+ during mitosis. Cells synchronous for mitotic division were prepared by transient temperature shifts in a cdc25-22 mutant (GG51). Samples were taken at 15-min intervals following release from restrictive temperature both for RNA preparation and to measure septation index to indicate synchrony of cell-cycle progression (top). The RNA was subjected to Northern blot analysis, with the blot consecutively hybridized with probes for cdt2+, cdc22+, and adh1+, the latter as a loading control (middle). Ratio of transcripts against adh1+ is shown (bottom). (B) Transcription of cdt2+ during meiosis. A pat1-114 homozygous diploid (JZ-670) was incubated in EMM-N for 15 hr at 25° and then shifted to 34° to induce meiosis in a synchronous fashion. At hourly intervals cell samples were taken for RNA preparation and to monitor meiotic nuclear divisions by DAPI staining (top). The RNA was subjected to Northern blot analysis, with the blot hybridized with a probe for cdt2+ (bottom). Ethidium bromide staining of ribosomal RNAs is presented as a loading control.
Δcdt2 mutants show cold-sensitive growth and deficiency in sporulation: The original sev1(cdt2)-M1 mutant was partially defective in vegetative growth and failed to sporulate. Nucleotide sequencing of this cold-sensitive cdt2-M1 allele revealed that it contained a single cytosine to thymine mutation, resulting in the insertion of an ochre nonsense codon at the 50th arginine residue. Consequently, cdt2-M1 is a nonsense allele, probably producing nonfunctional small peptides.
—cdt2+ transcription in cdc10-C4. (A) RNA was prepared from cultures of wild type (GG1) and cdc10-C4 (GG250) grown at 25° and subjected to Northern blot with the blot consecutively hybridized with probes for cdt2+, cdc22+, and adh1+, the latter as a loading control. (B) Quantification of each transcript against adh1+. Ratio of transcripts against adh1+ is shown.
—DSC1 binds to MCB motifs in the cdt2+ promoter. (A) Gel retardation analysis using oligonucleotides containing MCB sequences from the cdt2+ and cdc22+ promoters, with wild-type (GG1) protein extracts. Lane F, free probe; lane P, 20 μg protein with probe. Competition reactions were performed with the same unlabeled cdt2+ and cdc22+ promoter DNA at 1/10 and 1/1 concentrations. Large arrow indicates DSC1, and small arrow indicates free probes. (B) Gel retardation analysis with protein extracts from wild-type and DSC1 mutant cells. Strains used for protein extraction are GG1 (wild type), GG219 (cdc10-129), GG154 (Δres1), GG147 (Δres2), and GG149 (Δrep2). The temperature-sensitive cdc10-129 mutant was cultured at 25° or 36°.
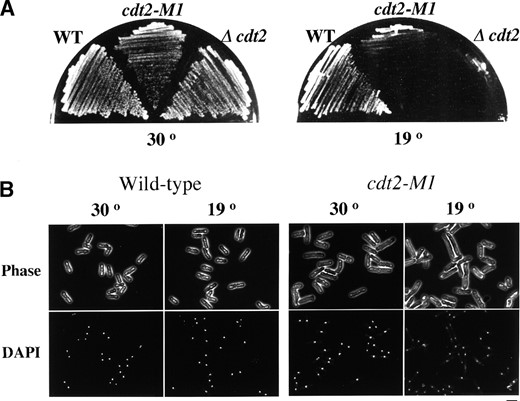
To confirm the cdt2-M1 results, we created a cdt2 null mutant in which the cdt2+ ORF was completely replaced by ura4+. This cdt2::ura4+ (designated as Δcdt2) mutant formed colonies at 30° but not at 19°, just as cdt2-M1 did (Figure 6A). The doubling time in complete medium at permissive temperature indicated that both Δcdt2 and cdt2-M1 grew more slowly than wild type (Table 2). These mutant cells were longer than wild-type cells even at the permissive temperature and elongated more remarkably at the restrictive temperature (Figure 6B). We conclude that the cdt2+ gene is dispensable under favorable growth conditions, although it is essential for proliferation and survival at low incubation temperatures.
As the cdt2-M1 nonsense mutant was sporulation deficient, we next studied in more detail the meiosis and sporulation arrest phenotypes of cdt2 mutants. After 2 days incubation at 30° on sporulation medium (MEA), progression of meiosis and sporulation was monitored by visualizing nuclei, microtubules, and spindle pole bodies using DAPI, anti-α-tubulin antibody (TAT-1), and anti-Sad1 antibody, respectively. Most of the cdt2 zygotes were still at mononucleate stage, while wild-type zygotes had completed meiosis and sporulated (Table 3). Roughly half of the Δcdt2 zygotes had one round nucleus that contained neither cytoplasmic nor spindle microtubules. The rest of the mutant zygotes were at the horse-tail stage. This observation is consistent with the previous finding that horse-tail nuclei accumulate when premeiotic DNA replication is inhibited by hydroxyurea (HU; Murakami and Nurse 1999). A proportion of mononucleate zygotes with spindle microtubules and binucleate zygotes was <10%. These results indicate that the cdt2 null mutants arrest before meiosis I.
—Cdt2 localizes to nucleus and accumulates during S phase. (A) Western analysis of HA-Cdt2. cdc25-22 HA-cdt2+ cells (SY11) were incubated in YE liquid medium at 37° for 4 hr and shifted to 25° to start a synchronous cell cycle. Western blots were probed with anti-HA antibody and anti-α-tubulin antibody, the latter as a loading control. (B) Nuclear localization of HA-Cdt2. Cultures of HA-Cdt2 (SY7) and nontagged control (TN29) strains were fixed in cold methanol and stained with anti-HA antibody and DAPI. Bar, 10 μm.
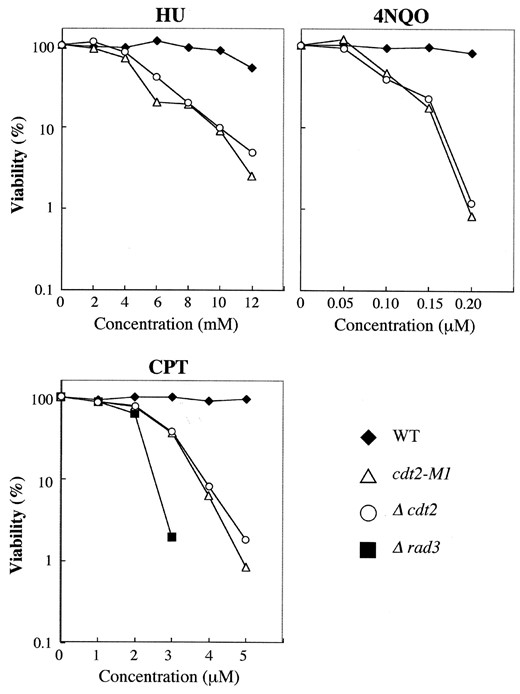
cdt2 mutants are sensitive to HU, 4NQO, and CPT: As the cdt2+ gene is expressed at the G1-S phase under the control of DSC1, this suggests that Cdt2 protein may have a role in DNA replication, or S-phase progression. Generally, DNA replication mutants in fission yeast are supersensitive to inhibitors of DNA synthesis, and to DNA-damaging agents. To see if this was also the case for cdt2+, Δcdt2, cdt2-M1, and Δrad3 cells were incubated at 30° on YE plates containing various concentrations of HU, a potent inhibitor of ribonucleotide reductase, camptothecin (CPT), a DNA topoisomerase poison, or 4-nitroquinoline-1-oxide (4NQO), a base-modifying agent. As shown in Figure 7, the viability of both cdt2 mutants was considerably reduced in a dose-dependent manner relative to wild type, although a loss of viability of Δrad3 was more conspicuous. It is concluded that cdt2 mutants are medially sensitive to these drugs.
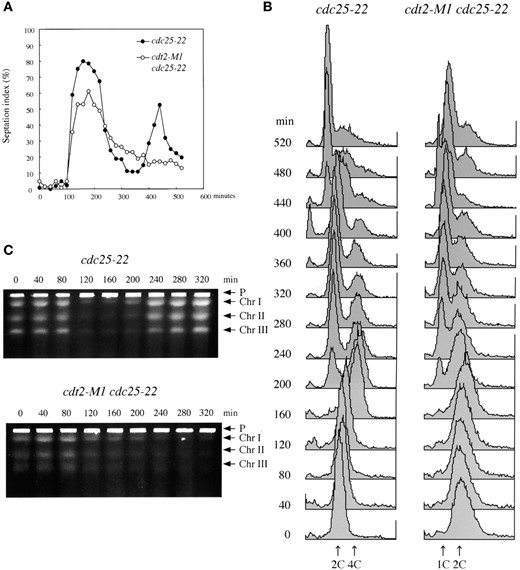
cdt2 mutants are defective in DNA replication: To examine whether Cdt2 is involved in the normal progression of S phase, flow cytometry and PFGE analyses were carried out with synchronously dividing mitotic cultures of both wild-type and mutant strains. cdt2+ cdc25-22 (SY8) and cdt2-M1 cdc25-22 (SY9) strains were transiently arrested in G2 at 37° for 4 hr before shifting the cultures to 19°, which is the permissive temperature for cdc25-22, but the restrictive temperature for cdt2-M1. At 40-min intervals, cell samples were taken and prepared for flow cytometry and PFGE. In the wild-type culture the frequency of septated cells (corresponding to the G1-S phase) fluctuated periodically after temperature shift, with two peaks around 180 and 450 min (Figure 8A). In contrast, the cdt2-M1 culture showed only a single peak of septation at a time similar to the first peak in wild-type cells (Figure 8A). This result strongly suggested a cell-cycle arrest or delay in cdt2-M1 cells at 19°.
—Phenotypes of cdt2 mutants. (A) Cold-sensitive growth of cdt2 mutants. Wild-type (C996-11D), cdt2-M1 (SY3), and Δcdt2 (SY6) cells were incubated on YE plates at 30° for 2 days or at 19° for 3 days. (B) Cell morphology of a cdt2 mutant at the restrictive temperature of 19°. Cells of wild-type strain (C996-11D) and cdt2-M1 mutant (SY3) were cultured in YE liquid medium at 19° for 24 hr, fixed with 70% ethanol, and stained with DAPI. Bar, 10 μm.
The flow cytometry data of DNA content are presented in Figure 8B. Wild-type haploid cells in G2/M had a 2C DNA content, with those completing S phase in the next cell cycle displaying a 4C DNA peak. This was because septated cells enter S phase before cell separation. The transition from 2C to 4C occurred at 80-160 min in cdt2+ cells (Figure 8B, left) during which septation peaked. After cell separation, a proportion of cells containing 2C DNA content increased again. In cdt2-M1 cultures, however, an apparent peak shift from 2C to 4C could not be observed. The major peak of 2C DNA content was still observed even when the septation index reached maximum. After cell separation, cells containing 1C DNA content appeared after 200 min (Figure 8B, right). These results indicate that the cdt2-M1 mutant is defective in the initiation and/or progression of S phase at 19°.
To examine DNA replication in cdt2-M1, progression of DNA replication was also analyzed by PFGE in the same cultures. Figure 8C shows a clear resolution of the three fission yeast chromosomes by PFGE in the 0 time points for both cdt2+ and cdt2-M1 cells. Replication intermediates of chromosomal DNA are unable to enter gels during PFGE. In cdt2+ cells, three distinct DNA bands of chromosomes in gels became transiently faint in S phase and then appeared again as they finished DNA replication (Figure 8C, top). In contrast, in cdt2-M1 cells the intensity of chromosomal DNA bands in gels constantly diminished only after 120 min (Figure 8C, bottom). These data indicate that although cdt2-M1 mutant cells are able to initiate DNA replication, the progression of DNA replication is strongly retarded.
Growth property of cdt2 mutants
| . | Doubling time . | |
|---|---|---|
| Strain . | hr . | % . |
| cdt2+ (C996-11D) | 2.54 | 100 |
| cdt2-M1 (SY3) | 4.07 | 160 |
| Δcdt2 (SY6) | 4.41 | 174 |
| . | Doubling time . | |
|---|---|---|
| Strain . | hr . | % . |
| cdt2+ (C996-11D) | 2.54 | 100 |
| cdt2-M1 (SY3) | 4.07 | 160 |
| Δcdt2 (SY6) | 4.41 | 174 |
Cells were incubated in YE liquid medium at 30°.
Growth property of cdt2 mutants
| . | Doubling time . | |
|---|---|---|
| Strain . | hr . | % . |
| cdt2+ (C996-11D) | 2.54 | 100 |
| cdt2-M1 (SY3) | 4.07 | 160 |
| Δcdt2 (SY6) | 4.41 | 174 |
| . | Doubling time . | |
|---|---|---|
| Strain . | hr . | % . |
| cdt2+ (C996-11D) | 2.54 | 100 |
| cdt2-M1 (SY3) | 4.07 | 160 |
| Δcdt2 (SY6) | 4.41 | 174 |
Cells were incubated in YE liquid medium at 30°.
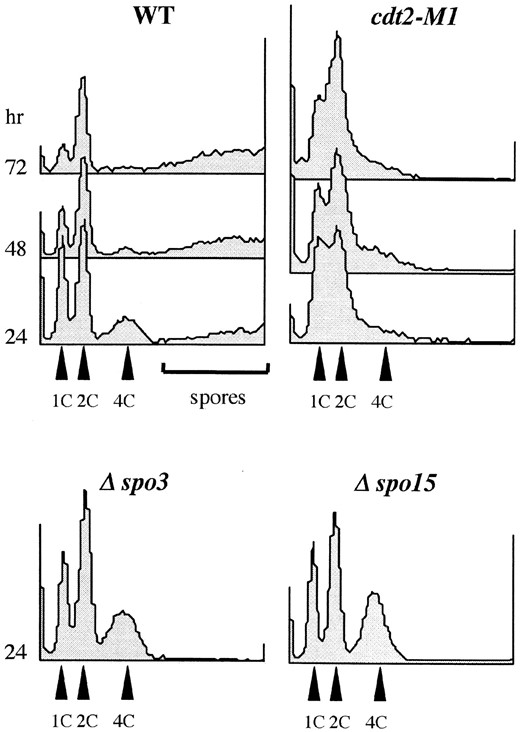
Finally, we explored premeiotic DNA replication with propidium-iodide-stained cdt2 mutant cells by flow cytometry. Homothallic haploid strains harboring cdt2+, cdt2-M1, Δspo3, or Δspo15 alleles were incubated on the MEA sporulation medium. Haploid cells conjugated to form zygotes, which then underwent meiosis. After 24 hr of incubation, zygotic cells containing 4C DNA content appeared, indicating that these cells completed premeiotic DNA replication (Figure 9). Because wild-type zygotes formed spores, which gave abnormally intense signals, the 4C peak gradually decreased. Δspo3 and Δspo15 mutants are defective in spore formation, although their premeiotic DNA synthesis and meiotic divisions progress normally (Ikemoto et al. 2000; Nakamura et al. 2002). These mutant zygotes, which were not converted to asci, exhibited a discrete 4C peak. In the cdt2 mutant cultures, however, the 4C peak was absent even after 72 hr of incubation. These observations strongly suggest that premeiotic DNA synthesis did not occur in the cdt2 mutants.
cdt2-M1 shows synthetic lethality with DNA replication checkpoint mutants: Completion of DNA replication and DNA damage repair is monitored by a checkpoint mechanism in which several checkpoint rad genes are involved (reviewed by Caspari and Carr 1999). When DNA is unreplicated or damaged, the cell cycle is blocked by this mechanism. The significance of this checkpoint for cell survival is evident because exposure of the checkpoint mutants to HU causes lethality (Al-Khodairy and Carr 1992). Our PFGE data indicated that replication intermediates accumulated in cdt2 mutant cells incubated at 19°. These intermediates may be detected by the DNA replication checkpoint, resulting in cell-cycle arrest. If the checkpoint genes were impaired by mutation, cdt2 mutants would be predicted to be lethal. To test this possibility, the cdt2-M1 mutant was crossed with Δrad1, Δrad3, Δrad17, Δrad26, or Δcds1 checkpoint mutants. The heterozygous diploids were then sporulated and tetrads were dissected. Among segregants, no double mutants were found, indicating that the cdt2-M1 mutant was synthetically lethal in combination with all the DNA replication checkpoint mutations tested (data not shown). We also constructed a cdt2-M1 Δchk1 double mutant, the latter mutation being defective in the DNA damage checkpoint. A cdt2-M1 Δchk1 double mutant could form colonies (data not shown). These results support the notion that the cdt2 mutants are defective in DNA replication.
Terminal morphology of cdt2 mutants in sporulation
| Morphology of zygote . | Frequency (%) . | ||||
|---|---|---|---|---|---|
| No. of nuclei . | Nucleus . | Microtubule (MT) . | Wild type . | cdt2-M1 . | Δcdt2 . |
| 1 | Horse-tail | Cytoplasmic | 0.4 | 33 | 30.7 |
| 1 | Round | Cytoplasmic | 0 | 10 | 11.9 |
| 1 | Round | No MT | 5.7 | 52 | 47.5 |
| 1 | Round | Spindle | 0 | 2 | 2 |
| 2 | Round | No MT | 1.7 | 3 | 7.9 |
| 3 or 4 | Round | No MT | 3 | 0 | 0 |
| 4 | Four-spored asci | 89.1 | 0 | 0 | |
| Morphology of zygote . | Frequency (%) . | ||||
|---|---|---|---|---|---|
| No. of nuclei . | Nucleus . | Microtubule (MT) . | Wild type . | cdt2-M1 . | Δcdt2 . |
| 1 | Horse-tail | Cytoplasmic | 0.4 | 33 | 30.7 |
| 1 | Round | Cytoplasmic | 0 | 10 | 11.9 |
| 1 | Round | No MT | 5.7 | 52 | 47.5 |
| 1 | Round | Spindle | 0 | 2 | 2 |
| 2 | Round | No MT | 1.7 | 3 | 7.9 |
| 3 or 4 | Round | No MT | 3 | 0 | 0 |
| 4 | Four-spored asci | 89.1 | 0 | 0 | |
Cells were incubated on MEA sporulation medium at 30° for 2 days. Strains C996-11D, SY3, and SY6 were used.
Terminal morphology of cdt2 mutants in sporulation
| Morphology of zygote . | Frequency (%) . | ||||
|---|---|---|---|---|---|
| No. of nuclei . | Nucleus . | Microtubule (MT) . | Wild type . | cdt2-M1 . | Δcdt2 . |
| 1 | Horse-tail | Cytoplasmic | 0.4 | 33 | 30.7 |
| 1 | Round | Cytoplasmic | 0 | 10 | 11.9 |
| 1 | Round | No MT | 5.7 | 52 | 47.5 |
| 1 | Round | Spindle | 0 | 2 | 2 |
| 2 | Round | No MT | 1.7 | 3 | 7.9 |
| 3 or 4 | Round | No MT | 3 | 0 | 0 |
| 4 | Four-spored asci | 89.1 | 0 | 0 | |
| Morphology of zygote . | Frequency (%) . | ||||
|---|---|---|---|---|---|
| No. of nuclei . | Nucleus . | Microtubule (MT) . | Wild type . | cdt2-M1 . | Δcdt2 . |
| 1 | Horse-tail | Cytoplasmic | 0.4 | 33 | 30.7 |
| 1 | Round | Cytoplasmic | 0 | 10 | 11.9 |
| 1 | Round | No MT | 5.7 | 52 | 47.5 |
| 1 | Round | Spindle | 0 | 2 | 2 |
| 2 | Round | No MT | 1.7 | 3 | 7.9 |
| 3 or 4 | Round | No MT | 3 | 0 | 0 |
| 4 | Four-spored asci | 89.1 | 0 | 0 | |
Cells were incubated on MEA sporulation medium at 30° for 2 days. Strains C996-11D, SY3, and SY6 were used.
Overexpression of cdt2+ causes a cell-cycle delay: As cdt2 mutants are defective in S-phase progression, we next examined whether overexpression of wild-type cdt2+ affected cell-cycle progression. When cdt2+ was overexpressed using the strong nmt1 promoter in wild-type cells, growth was delayed (data not shown). Cells containing overexpressed cdt2+ exhibited a discrete 1C peak, as shown by flow cytometry (data not shown), indicating that overexpression of cdt2+ causes cell-cycle delay in G1 or in early S phase. These observations are consistent with the suggestion that Cdt2 has a role in G1-S progression.
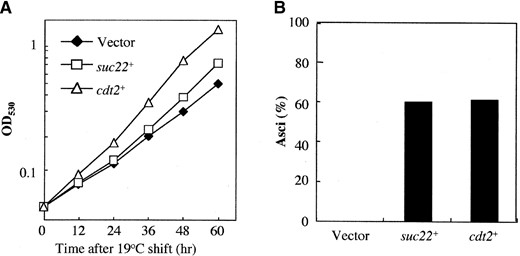
cdt2+ genetically interacts with suc22+: To understand how Cdt2 functions in DNA replication, we isolated multicopy suppressors of the cdt2-M1 allele. An S. pombe cDNA library was introduced into cdt2-M1 cells and several Spo+ transformants were isolated. Sequencing of isolated clones defined two genes: One was cdt2+ itself, whereas the other clone contained suc22+, which encodes the small subunit of ribonucleotide reductase (Fernandez-Sarabia et al. 1993). Overexpression of suc22+ in cdt2-M1 cells completely suppressed the sporulation deficiency while only partly rescuing the cold sensitivity (Figure 10). S. pombe ribonucleotide reductase is composed of Suc22 (the catalytic subunit) and Cdc22 (the regulatory subunit). We found that overexpression of cdc22+ did not complement the sporulation deficiency of cdt2-M1 cells (data not shown).
DISCUSSION
Cell differentiation is promoted by a set of genes that are not required for proliferation. Sporulation is an important differentiation process in unicellular micro-organisms such as budding and fission yeasts. Genetic studies on sporulation-deficient mutants of S. pombe have defined at least 20 spo genes. Recently, we found that spo14+ and spo20+ encode essential components of a general protein secretion pathway, both of which are essential for proliferative growth (Nakase et al. 2001; Nakamura-Kubo et al. 2003). In the present study we describe a genetic screen for sev genes that are required for both sporulation and vegetative growth. We defined five sev loci, two of which have been cloned and their products identified. It appears that these sev gene products have roles in a wide range of cellular processes. Further genome-wide screening of sev genes may shed light on the problem of how growth-indispensable and sporulation-specific genes share functions for cell differentiation.
—HU, 4NQO, and CPT sensitivity of cdt2 mutants. Wild-type (C996-11D), cdt2-M1 (SY3), Δcdt2 (SY6), and Δrad3 (SY12) cells were spread onto YE plates containing various concentrations of HU, 4NQO, or CPT. After 5 days of incubation at 30°, colony numbers were counted. Viability was calculated as a percentage of control cells that were not treated with drugs. Viability of Δrad3 was <0.1% on YE plates containing 2 mm HU, 0.05 μm 4NQO, or 4 μm CPT.
—Flow cytometric and PFGE analyses of DNA replication in synchronous culture. cdt2+ cdc25-22 (SY8) and cdt2-M1 cdc25-22 (SY9) cultures were transiently incubated at 37° for 4 hr before shifting to 19° to restart the cell cycle from G2 phase. At 40-min intervals, samples were taken for septation index (A), flow cytometry (B), and PFGE (C). “P” in C indicates the sample plug.
The sev1+ gene is identical to cdt2+, which has previously been identified as a target of the transcription factor DSC1 (Hofmann and Beach 1994). In the present study, we provide direct and indirect evidence that Cdt2 is involved in DNA replication, like other targets of DSC1: (1) cdt2+ is transcribed preferentially at the G1-S boundary and Cdt2 protein accumulates in S phase; (2) cdt2+ expression is affected by cdc10-C4, a mutant of a component of DSC1, in vivo, and a cdt2+ promoter fragment binds to DSC1 in vitro; (3) cdt2-M1 mutant cells prevent an increase in DNA content after M phase; (4) S-phase progression is inhibited in cdt2 mutant; (5) transcription of cdt2+ is stimulated in the sexual cycle before meiosis I; (6) premeiotic DNA replication is inhibited and meiosis arrests before first division in cdt2 mutants; (7) cdt2 and DNA replication checkpoint mutation are synthetically lethal; (8) cdt2 mutant cells are sensitive to HU; (9) cdt2 mutations are suppressed by overexpression of suc22+, which encodes the small subunit of ribonucleotide reductase; and (10) overexpression of cdt2+ inhibits the G1-to-S-phase transition. Combined, these observations suggest that Cdt2 has an important regulatory role relating DNA replication during S phase of both the mitotic and the meiotic life cycles in fission yeast.
The cdt2+ gene encodes a nuclear protein containing 490 amino acid residues. No known proteins have a high degree of sequence similarity with Cdt2. Cdt2 contains five WD repeats as well as a potential PEST sequence in the N-terminal region. The WD repeats are thought to participate in protein-protein interactions. The original sev1 mutant (cdt2-M1) has a nonsense mutation near its N terminus, producing a predicted truncated protein of only 49 amino acids. The cdt2-M1 mutant and Δcdt2 cells lacking the entire ORF are able to grow at 30° with an extended doubling time, indicating that Cdt2 is not absolutely required for DNA replication. This growth defect is more severe at low incubation temperatures. We speculate that Cdt2 functions to assist the formation of protein complex required for DNA replication. Higher incubation temperature may facilitate interaction between protein components without Cdt2. The PEST sequence has been often found in unstable proteins. HA-Cdt2, expressed from its native chromosomal locus, is almost exclusively present in binucleate cells and scarcely detected in mononucleate cells. This observation suggests that HA-Cdt2 accumulates during S phase and decreases in G2 phase, implying that Cdt2 is unstable. The PEST sequence present in Cdt2 may contribute to such protein instability.
—Premeiotic DNA replication in cdt2 mutants. Wild-type (C996-11D), cdt2-M1 (SY3), Δcdt2 (SY6), Δspo3 (MKD3), and Δspo15 (SI51) mutants were incubated on MEA medium for 2 days. Cells were collected and subjected to flow cytometry. 1C, G1-arrested vegetative cells; 2C, zygotes before premeiotic S phase; and 4C, zygotes after premeiotic S phase. Note that no discrete 4C peak was detected in cdt2 mutants.
—Suppression of cdt2 mutants by suc22+. (A) Growth defect of cdt2 mutant partially rescued by a suc22+-carrying plasmid. Δcdt2 (SY6) transformed with pREP41, pREP41 (suc22+), or pAL (sev1+)1 was incubated in liquid EMM medium without thiamine. pAL(sev1+)1 carries the cdt2+ gene. Growth was measured by optical density at 530 nm (OD530). Mean OD530 values of three independent cultures run in parallel are presented. Differences in OD530 at 60 hr between different plasmids are statistically significant. (B) Sporulation defect in cdt2 mutants was completely recovered by suc22+. Strain SY6 (Δcdt2) transformed with pREP41, pREP41 (suc22+), or pAL(sev1+)1 was incubated on thiamine-free SSA sporulation medium at 30° for 2 days. Frequency of asci was counted under a phase-contrast microscope.
Ribonucleotide reductase is an essential enzyme required for the synthesis of dNTPs, and thus its activity is indispensable for DNA replication and DNA repair (Jordan and Reichard 1998). From yeasts to mammals, this enzyme is composed of two subunits: a small catalytic subunit and a large regulatory subunit. In fission yeast, cdc22+ and suc22+ encode a large and a small subunit of ribonucleotide reductase, respectively (Fernandez-Sarabia et al. 1993). We found that cdt2 mutations are rescued by suc22+ when overexpressed, but not by cdc22+. Furthermore, cdt2 mutants are sensitive to HU, a potent inhibitor of ribonucleotide reductase. These findings imply that Cdt2 regulates the activity of Suc22 during the G1-S phase. This hypothesis could be tested by measuring dNTP levels in the cdt2 mutants. We note that the requirement for Cdt2 during sporulation is higher than that for cell growth, because cdt2 mutants display sporulation defects even at the permissive temperature for growth. The suppression of cdt2 defects by suc22+ is more remarkable in recovery of sporulation than suppression of cold-sensitive mitotic growth. Presumably, these results suggest a closer relationship between Cdt2 and Suc22 in premeiotic S phase than in the mitotic cell cycle.
Acknowledgement
We thank A. M. Carr of the University of Sussex and H. Okayama of the University of Tokyo for S. pombe strains, S. L. Forsburg of The Salk Institute for Biological Studies for plasmids, O. Niwa of Kazusa DNA Research Institute for anti-Sad1 antibody, and K. Gull of the University of Manchester for anti-α-tubulin antibody, TAT-1. We also thank M. Kishida of Osaka Prefecture University for PFGE analysis. This study was supported by Grant-in-Aid for Scientific Research on Priority Areas “Genome Biology” to C.S. Work in the laboratory of C.J.M. was supported by the Biotechnology and Biological Sciences Research Council, Wellcome Trust, and The Royal Society. H. Al-A. was supported by the Saudi Arabian Cultural Bureau.
Footnotes
Communicating editor: P. Russell
LITERATURE CITED