-
PDF
- Split View
-
Views
-
Cite
Cite
Karl Ekwall, Gwen Cranston, Robin C Allshire, Fission Yeast Mutants That Alleviate Transcriptional Silencing in Centromeric Flanking Repeats and Disrupt Chromosome Segregation, Genetics, Volume 153, Issue 3, 1 November 1999, Pages 1153–1169, https://doi.org/10.1093/genetics/153.3.1153
Close - Share Icon Share
Abstract
In the fission yeast Schizosaccharomyces pombe genes are transcriptionally silenced when placed within centromeres, within or close to the silent mating-type loci or adjacent to telomeres. Factors required to maintain mating-type silencing also affect centromeric silencing and chromosome segregation. We isolated mutations that alleviate repression of marker genes in the inverted repeats flanking the central core of centromere I. Mutations csp1 to 13 (centromere: suppressor of position effect) defined 12 loci. Ten of the csp mutants have no effect on mat2/3 or telomere silencing. All csp mutants allow some expression of genes in the centromeric flanking repeat, but expression in the central core is undetectable. Consistent with defective centromere structure and function, chromosome loss rates are elevated in all csp mutants. Mutants csp1 to 6 are temperature-sensitive lethal and csp3 and csp6 cells are defective in mitosis at 36°. csp7 to 13 display a high incidence of lagging chromosomes on late anaphase spindles. Thus, by screening for mutations that disrupt silencing in the flanking region of a fission yeast centromere a novel collection of mutants affecting centromere architecture and chromosome segregation has been isolated.
HETEROCHROMATIN was first described in multicellular organisms as cytologically distinct chromosomal regions that remained condensed in interphase. Heterochromatin is often present at centromeres and telomeres where it can repress expression of adjacent genes in a phenomenon called position effect variegation or silencing. Even in unicellular organisms such as yeasts in which most parts of the genome are active, there are small “heterochromatic” regions; for example, near telomeres and around the silent mating-type loci (reviewed in Karpen 1994; Henikoff 1996). In the budding yeast Saccharomyces cerevisiae, the HML and HMR silent mating-type loci are normally maintained in a silent state that is under genetic control of the SIR (silent information regulators) genes. The Sir2, Sir3, Sir4, and Rap1 proteins are structural components of S. cerevisiae heterochromatin, forming large macromolecular complexes containing the HM loci and telomeres (reviewed in Laurenson and Rine 1992; Grunstein 1998).
The centromere is the chromosomal region on which the kinetochore, a large DNA-protein complex, is formed. The sister kinetochores are responsible for capturing microtubules protruding from opposite spindle poles during prometaphase and ensuring that all centromeres are bilaterally attached prior to anaphase onset. In anaphase the kinetochore coordinates the movement of chromosomes to the spindle poles (reviewed in Pluta et al. 1995). Proper centromere function is crucial for ensuring accurate segregation of chromosomes, and defects in centromere function result in chromosome loss and gain events. In humans, aneuploidy caused by chromosome missegregation is implicated in genetic disease and cancer (Lengauer et al. 1997; Cahill et al. 1998).
The fission yeast Schizosaccharomyces pombe provides an excellent model system for the study of a highly conserved process such as chromosome segregation. The genome of S. pombe is carried on three chromosomes, each with a complex centromeric region occupying 38, 65, and 97 kb of DNA (Allshire 1996). The three centromeres are arranged as symmetrical structures with a unique central core sequence 4–7 kb in length that is flanked by arrays of repeated DNA elements of variable organization (Chikashige et al. 1989; Hahnenberger et al. 1989, 1991; Clarke and Baum 1990; Murakami et al. 1991; Takahashi et al. 1992; Steiner et al. 1993; Baum et al. 1994). Both the central core and some portions of the flanking K/otr/imr-type repeats are required for full centromere function on fission yeast minichromosomes (Clarke and Baum 1990; Matsumoto et al. 1990; Hahnenberger et al. 1991; Takahashi et al. 1992; Baum et al. 1994). The repetitive organization of fission yeast centromeres is to some extent reminiscent of that described for metazoa. The centromere of the Drosophila X chromosome is contained within a 400-kb region composed of repeated simple satellite DNA interspersed with several transposable elements of various types (Murphy and Karpen 1995; Sun et al. 1997). Functional human centromeres have been deleted down to tandem arrays of alphoid satellite and can be assembled de novo on templates that are mainly composed of these repeats (Brown et al. 1994; Harrington et al. 1997; Grimes and Cooke 1998; Ikeno et al. 1998).
In Drosophila, chromosomal inversions that place the white gene (required for red eye color) into centromeric heterochromatin result in reversible repression of the white gene (reviewed in Karpen 1994; Henikoff 1996). In fission yeast a similar phenomenon occurs: the ade6+ and ura4+ marker genes are transcriptionally repressed or silenced when inserted within fission yeast centromeres, and some positions display variegated expression (Allshire et al. 1994; for review see Allshire 1996). Marker genes are also transcriptionally silenced when placed in the vicinity of the silent mat2 and mat3 matingtype loci (Thon and Klar 1992; Allshire et al. 1995; Grewall and Klar 1996; Thon and Friis 1997) or adjacent to telomeres in fission yeast (Nimmo et al. 1994, 1998). Because such changes in the position of a gene in the genome alter its expression, these phenomena have been termed position effects. Thus, fission yeast centromeres appear to share heterochromatic features in common with metazoan centromeres. This is underscored by the fact that in both Drosophila and fission yeast, factors that contribute to centromeric silencing are also required for proper chromosome segregation. The Drosophila mutations su(var)2-5 (gene encoding heterochromatin protein 1, HP1) and su(var)3-6 (gene encoding a type 2 protein phosphatase) lead to defects in chromosome segregation (Baksa et al. 1993; Kellum and Alberts 1995). Mutations in fission yeast clr4, rik1, or swi6 result in the expression of normally repressed centromeric marker genes and defective chromosome segregation (Allshire et al. 1995). The Swi6 protein localizes to centromeres, the silent mating-type loci, and telomeres (Ekwall et al. 1995) and this localization is dependent on the presence of functional Clr4p and Rik1p (Ekwall et al. 1996). Intriguingly, the fission yeast silencing proteins Clr4p and Swi6p and the Drosophila heterochromatin protein HP1 share chromodomain motifs that are thought to mediate protein-protein interactions in the formation of heterochromatin structures (Lorentz et al. 1994; Platero et al. 1995; Ivanova et al. 1998; reviewed in Cavalli and Paro 1998). Only one centromere-specific protein, Mis6p, has been reported in fission yeast (Saitoh et al. 1997). The mis6 mutant was identified using a colony-sectoring chromosome loss screen (Takahashi et al. 1994), adapted from the pioneering screen used to genetically identify several centromere proteins in budding yeast (Spencer et al. 1990).
Here, a genetic screen is described to identify fission yeast mutants defective in transcriptional silencing of marker genes inserted in the inverted repeats flanking the central core region of S. pombe centromere I. Assuming that the phenomenon of transcriptional repression within fission yeast centromeres reflects the inaccessibility of centromere architecture to the transcription machinery, such a screen may identify novel centromere structural or regulatory components. The mutants described in this study are in accordance with this idea.
MATERIALS AND METHODS
S. pombe strains and media: The genotypes for the strains are in Table 1. Supplemented yeast extract (rich) medium (YES), malt extract medium (ME), and minimal medium with glutamate as nitrogen source (PMG) have been described previously (Moreno et al. 1991; Allshire et al. 1994). Standard genetic techniques were used according to Moreno et al. (1991). The half-sectoring assay was used to measure Ch16 segregation (Allshire et al. 1995). Comparative plating and serial dilution experiments were performed as described previously (Allshire et al. 1994). Thiabendazole (TBZ; Sigma, St. Louis) was dissolved in DMSO as a stock solution at 20 mg/ml. Adenine was used at 7.5 mg/liter in low adenine indicator plates. Phloxine B (Sigma) was used at 10 mg/liter in plates.
Genetic techniques: Mutagenesis of the FY1193 strain was carried out in the following way. Cells were grown in 100 ml of YES medium to a density of ~8 × 106 cells/ml, harvested, and resuspended in two 4-ml cultures in YES. Ethyl methane-sulfonate (EMS; Sigma) was added to one of the cultures to a final concentration of 2.0%. Cells were grown at room temperature under a fume hood for 3 hr with gentle shaking and washed three times with 0.9% NaCl. Cells were diluted in YES, grown overnight at 25° (three viable cell doublings), plated on PMG plates lacking adenine (−ade plates), and incubated at 25°. The EMS-treated culture had 10- to 20-fold higher frequency (1 × 10−3) of white Ade+ than untreated cultures (1 × 10−4). Temperature-sensitive mutants were screened for by replica plating white Ade+ colonies to phloxine-containing plates at 36°. All mutants were backcrossed with a wild-type strain FY1181 three times before further phenotypic analysis.
The many crosses required to arrange the mutants into different loci and to test for allelism with preexisting silencing mutants were performed as follows. Eight times 10 mutants of opposite mating types were mixed in a 96-well microtiter dish in sterile water and spotted onto a 10 × 10-cm2 ME plate. After 3 days at 25° the cells were scraped off and suspended in a 96-well microtiter dish containing 1:60 dilution of Glusulase (New England Nuclear, Boston). The dish was sealed with mylar film (Flow) and incubated overnight at 25°. Each row of spore suspensions was serially diluted into a separate 96-well dish and spotted onto low adenine indicator plates that were incubated at 25° and onto phloxine plates that were incubated at 36° to assay for temperature-sensitive colonies.
Pulsed-field gel electrophoresis and Southern analysis: High-molecular-weight DNA in agarose blocks for analysis by pulsed-field gel electrophoresis was prepared and analyzed as described in Allshire et al. (1995). DNA was isolated from strain FY1193 and the parental strains FY510 and FY1180 (used as controls). Upon SphI digestion of DNA and electrophoretic separation on a gel followed by Southern hybridization to ura4+ and ade6+ probes, the FY1193 isolate showed the expected
Strain list
| Strain . | Genotype . | Source or reference . |
|---|---|---|
| 972 | h− | P. Fantes, Edinburgh Univ. |
| FY86 | h+ his3 | This study |
| ED665 | h− ade6-210 leu1-32 ura4-D18 | P. Fantes, Edinburgh Univ. |
| FY501 | h+ ade6-210 leu1-32 ura4-DS/E imr1L (NcoI)::ura4+ | Allshire et al. (1995) |
| FY1180 | h+ ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ade6+ | This study |
| FY1181 | h− ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ade6+ | This study |
| FY1370 | h− csp1 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1405 | h− csp2 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1430 | h− csp3 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1376 | h− csp4 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1379 | h− csp5 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1407 | h− csp6-75 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1382 | h− csp6-95 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1384 | h− csp7 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1386 | h− csp8 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1389 | h− csp9 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1391 | h− csp10 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1393 | h− csp11 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1394 | h− csp12 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1396 | h− csp13 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1193 | h+ ade6-210 leu1-32 ura4-D18 imr1L (NcoI)::ura4+ otr1R(SphI)::ade6+ | FY501 × FY1180 |
| FY340 | h+ ade6-210 leu1-32 ura4-DS/E TM1 (NcoI)::ura4+ Random int. | Allshire et al. (1995) |
| FY973 | h− ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | Allshire et al. (1995) |
| FY1028 | h+ swi6::his1+ ade6-210 leu1-32 ura4-DS/E his1-102 | Allshire et al. (1995) |
| SPG144 | h+ clr6-1 ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ura4+ | Grewal et al. (1998) |
| 655 | h+ bub1::LEU2+ ade6-210 leu1-32 ura4-DS/E his1-102 otr1R(SphI)::ade6+ | Bernard et al. (1998) |
| FY1029 | hA rik1::LEU2+ ade6-210 leu1-32 ura4-DS/E his1-102 | Allshire et al. (1995) |
| Hu78 | h+ rik1::LEU2+ ade6-210 leu1-32 ura4- otr1R(SphI)::ade6+ | This study |
| Hu79 | h− rik1::LEU2+ ade6-210 leu1-32 ura4- otr1R(SphI)::ade6+ | This study |
| FY1470 | h+ csp1 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1473 | h− csp2 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1625 | csp3 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1477 | h− csp4 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1626 | csp5 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1627 | csp6-75 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1628 | csp6-95 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1629 | csp7 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1630 | csp8 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1488 | h+ csp9 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1491 | h− csp10 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1493 | h− csp11 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1495 | h− csp12 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| PG9 | h90 ade6-216 leu1-32 ura4-D18 mat3-M::ura4+ | Thon and Klar (1992) |
| FY1631 | h90 csp1 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1854 | h90 csp2 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1633 | h90 csp3 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1634 | h90 csp4 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1635 | h90 csp5 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1636 | h90 csp6-95 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1637 | h90 csp7 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1638 | h90 csp8 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1639 | h90 csp9 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1640 | h90 csp10 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1641 | h90 csp11 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1642 | h90 csp12 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1643 | h90 csp13 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY412 | h+ ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ | Allshire et al. (1995) |
| FY1811 | csp1 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1812 | csp2 ade6-210 leu1-32 ura4D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1813 | csp3 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1814 | csp4 ade6-210 leu1-32 ura4D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | |
| FY1856 | csp5 ade6-210 leu1-32 ura4DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1816 | csp6 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1850 | csp7 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1818 | h+ csp8 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1819 | h− csp9 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1820 | h+ csp10 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1821 | h+ csp11 ade6-210 leu1-32 ura4DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1822 | h− csp12 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1971 | h− csp13 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1469 | h− ade6-210 leu1-32 ade6-210 his1-102 (Ch16-216-LEU2+) | This study |
| FY1471 | h− csp1 ade6-210 leu132 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1472 | h+ csp2 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1474 | h+ csp3 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1476 | h+ csp4 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1478 | h+ csp5 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1480 | h+ csp6-75 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1482 | h+ csp6-95 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1484 | h+ csp7 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1486 | h+ csp8 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1487 | h+ csp9 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1489 | h− csp10 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1490 | h+ csp11 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1494 | h+ csp12 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1496 | h+ csp13 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1862 | h90 ade6-210 Jeu1-32 otr1R(SphI)::ura4+ his3-D1 his3+-tel(1L) | Nimmo et al. (1998) |
| FY1878 | csp4 ade6-210 leu1-32 otr1R(SphI)::ura4+ his3-D1 his3+-tel(1L) | This study |
| FY1524 | diploid h+/mat1-M mat2/3Δ::LEU2+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1525 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp1/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1526 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp2/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1527 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp3/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1528 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp4/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1529 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp5/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1530 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp675/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| Strain . | Genotype . | Source or reference . |
|---|---|---|
| 972 | h− | P. Fantes, Edinburgh Univ. |
| FY86 | h+ his3 | This study |
| ED665 | h− ade6-210 leu1-32 ura4-D18 | P. Fantes, Edinburgh Univ. |
| FY501 | h+ ade6-210 leu1-32 ura4-DS/E imr1L (NcoI)::ura4+ | Allshire et al. (1995) |
| FY1180 | h+ ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ade6+ | This study |
| FY1181 | h− ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ade6+ | This study |
| FY1370 | h− csp1 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1405 | h− csp2 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1430 | h− csp3 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1376 | h− csp4 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1379 | h− csp5 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1407 | h− csp6-75 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1382 | h− csp6-95 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1384 | h− csp7 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1386 | h− csp8 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1389 | h− csp9 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1391 | h− csp10 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1393 | h− csp11 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1394 | h− csp12 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1396 | h− csp13 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1193 | h+ ade6-210 leu1-32 ura4-D18 imr1L (NcoI)::ura4+ otr1R(SphI)::ade6+ | FY501 × FY1180 |
| FY340 | h+ ade6-210 leu1-32 ura4-DS/E TM1 (NcoI)::ura4+ Random int. | Allshire et al. (1995) |
| FY973 | h− ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | Allshire et al. (1995) |
| FY1028 | h+ swi6::his1+ ade6-210 leu1-32 ura4-DS/E his1-102 | Allshire et al. (1995) |
| SPG144 | h+ clr6-1 ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ura4+ | Grewal et al. (1998) |
| 655 | h+ bub1::LEU2+ ade6-210 leu1-32 ura4-DS/E his1-102 otr1R(SphI)::ade6+ | Bernard et al. (1998) |
| FY1029 | hA rik1::LEU2+ ade6-210 leu1-32 ura4-DS/E his1-102 | Allshire et al. (1995) |
| Hu78 | h+ rik1::LEU2+ ade6-210 leu1-32 ura4- otr1R(SphI)::ade6+ | This study |
| Hu79 | h− rik1::LEU2+ ade6-210 leu1-32 ura4- otr1R(SphI)::ade6+ | This study |
| FY1470 | h+ csp1 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1473 | h− csp2 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1625 | csp3 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1477 | h− csp4 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1626 | csp5 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1627 | csp6-75 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1628 | csp6-95 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1629 | csp7 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1630 | csp8 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1488 | h+ csp9 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1491 | h− csp10 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1493 | h− csp11 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1495 | h− csp12 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| PG9 | h90 ade6-216 leu1-32 ura4-D18 mat3-M::ura4+ | Thon and Klar (1992) |
| FY1631 | h90 csp1 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1854 | h90 csp2 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1633 | h90 csp3 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1634 | h90 csp4 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1635 | h90 csp5 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1636 | h90 csp6-95 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1637 | h90 csp7 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1638 | h90 csp8 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1639 | h90 csp9 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1640 | h90 csp10 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1641 | h90 csp11 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1642 | h90 csp12 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1643 | h90 csp13 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY412 | h+ ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ | Allshire et al. (1995) |
| FY1811 | csp1 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1812 | csp2 ade6-210 leu1-32 ura4D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1813 | csp3 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1814 | csp4 ade6-210 leu1-32 ura4D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | |
| FY1856 | csp5 ade6-210 leu1-32 ura4DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1816 | csp6 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1850 | csp7 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1818 | h+ csp8 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1819 | h− csp9 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1820 | h+ csp10 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1821 | h+ csp11 ade6-210 leu1-32 ura4DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1822 | h− csp12 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1971 | h− csp13 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1469 | h− ade6-210 leu1-32 ade6-210 his1-102 (Ch16-216-LEU2+) | This study |
| FY1471 | h− csp1 ade6-210 leu132 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1472 | h+ csp2 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1474 | h+ csp3 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1476 | h+ csp4 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1478 | h+ csp5 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1480 | h+ csp6-75 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1482 | h+ csp6-95 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1484 | h+ csp7 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1486 | h+ csp8 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1487 | h+ csp9 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1489 | h− csp10 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1490 | h+ csp11 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1494 | h+ csp12 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1496 | h+ csp13 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1862 | h90 ade6-210 Jeu1-32 otr1R(SphI)::ura4+ his3-D1 his3+-tel(1L) | Nimmo et al. (1998) |
| FY1878 | csp4 ade6-210 leu1-32 otr1R(SphI)::ura4+ his3-D1 his3+-tel(1L) | This study |
| FY1524 | diploid h+/mat1-M mat2/3Δ::LEU2+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1525 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp1/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1526 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp2/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1527 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp3/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1528 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp4/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1529 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp5/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1530 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp675/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
Strain list
| Strain . | Genotype . | Source or reference . |
|---|---|---|
| 972 | h− | P. Fantes, Edinburgh Univ. |
| FY86 | h+ his3 | This study |
| ED665 | h− ade6-210 leu1-32 ura4-D18 | P. Fantes, Edinburgh Univ. |
| FY501 | h+ ade6-210 leu1-32 ura4-DS/E imr1L (NcoI)::ura4+ | Allshire et al. (1995) |
| FY1180 | h+ ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ade6+ | This study |
| FY1181 | h− ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ade6+ | This study |
| FY1370 | h− csp1 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1405 | h− csp2 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1430 | h− csp3 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1376 | h− csp4 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1379 | h− csp5 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1407 | h− csp6-75 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1382 | h− csp6-95 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1384 | h− csp7 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1386 | h− csp8 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1389 | h− csp9 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1391 | h− csp10 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1393 | h− csp11 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1394 | h− csp12 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1396 | h− csp13 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1193 | h+ ade6-210 leu1-32 ura4-D18 imr1L (NcoI)::ura4+ otr1R(SphI)::ade6+ | FY501 × FY1180 |
| FY340 | h+ ade6-210 leu1-32 ura4-DS/E TM1 (NcoI)::ura4+ Random int. | Allshire et al. (1995) |
| FY973 | h− ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | Allshire et al. (1995) |
| FY1028 | h+ swi6::his1+ ade6-210 leu1-32 ura4-DS/E his1-102 | Allshire et al. (1995) |
| SPG144 | h+ clr6-1 ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ura4+ | Grewal et al. (1998) |
| 655 | h+ bub1::LEU2+ ade6-210 leu1-32 ura4-DS/E his1-102 otr1R(SphI)::ade6+ | Bernard et al. (1998) |
| FY1029 | hA rik1::LEU2+ ade6-210 leu1-32 ura4-DS/E his1-102 | Allshire et al. (1995) |
| Hu78 | h+ rik1::LEU2+ ade6-210 leu1-32 ura4- otr1R(SphI)::ade6+ | This study |
| Hu79 | h− rik1::LEU2+ ade6-210 leu1-32 ura4- otr1R(SphI)::ade6+ | This study |
| FY1470 | h+ csp1 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1473 | h− csp2 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1625 | csp3 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1477 | h− csp4 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1626 | csp5 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1627 | csp6-75 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1628 | csp6-95 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1629 | csp7 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1630 | csp8 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1488 | h+ csp9 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1491 | h− csp10 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1493 | h− csp11 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1495 | h− csp12 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| PG9 | h90 ade6-216 leu1-32 ura4-D18 mat3-M::ura4+ | Thon and Klar (1992) |
| FY1631 | h90 csp1 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1854 | h90 csp2 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1633 | h90 csp3 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1634 | h90 csp4 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1635 | h90 csp5 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1636 | h90 csp6-95 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1637 | h90 csp7 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1638 | h90 csp8 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1639 | h90 csp9 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1640 | h90 csp10 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1641 | h90 csp11 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1642 | h90 csp12 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1643 | h90 csp13 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY412 | h+ ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ | Allshire et al. (1995) |
| FY1811 | csp1 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1812 | csp2 ade6-210 leu1-32 ura4D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1813 | csp3 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1814 | csp4 ade6-210 leu1-32 ura4D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | |
| FY1856 | csp5 ade6-210 leu1-32 ura4DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1816 | csp6 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1850 | csp7 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1818 | h+ csp8 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1819 | h− csp9 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1820 | h+ csp10 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1821 | h+ csp11 ade6-210 leu1-32 ura4DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1822 | h− csp12 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1971 | h− csp13 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1469 | h− ade6-210 leu1-32 ade6-210 his1-102 (Ch16-216-LEU2+) | This study |
| FY1471 | h− csp1 ade6-210 leu132 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1472 | h+ csp2 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1474 | h+ csp3 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1476 | h+ csp4 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1478 | h+ csp5 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1480 | h+ csp6-75 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1482 | h+ csp6-95 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1484 | h+ csp7 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1486 | h+ csp8 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1487 | h+ csp9 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1489 | h− csp10 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1490 | h+ csp11 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1494 | h+ csp12 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1496 | h+ csp13 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1862 | h90 ade6-210 Jeu1-32 otr1R(SphI)::ura4+ his3-D1 his3+-tel(1L) | Nimmo et al. (1998) |
| FY1878 | csp4 ade6-210 leu1-32 otr1R(SphI)::ura4+ his3-D1 his3+-tel(1L) | This study |
| FY1524 | diploid h+/mat1-M mat2/3Δ::LEU2+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1525 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp1/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1526 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp2/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1527 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp3/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1528 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp4/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1529 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp5/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1530 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp675/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| Strain . | Genotype . | Source or reference . |
|---|---|---|
| 972 | h− | P. Fantes, Edinburgh Univ. |
| FY86 | h+ his3 | This study |
| ED665 | h− ade6-210 leu1-32 ura4-D18 | P. Fantes, Edinburgh Univ. |
| FY501 | h+ ade6-210 leu1-32 ura4-DS/E imr1L (NcoI)::ura4+ | Allshire et al. (1995) |
| FY1180 | h+ ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ade6+ | This study |
| FY1181 | h− ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ade6+ | This study |
| FY1370 | h− csp1 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1405 | h− csp2 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1430 | h− csp3 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1376 | h− csp4 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1379 | h− csp5 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1407 | h− csp6-75 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1382 | h− csp6-95 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1384 | h− csp7 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1386 | h− csp8 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1389 | h− csp9 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1391 | h− csp10 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1393 | h− csp11 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1394 | h− csp12 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1396 | h− csp13 ade6-210 leu1-32 ura4-D18 or DS/E otr1R(SphI)::ade6+ | This study |
| FY1193 | h+ ade6-210 leu1-32 ura4-D18 imr1L (NcoI)::ura4+ otr1R(SphI)::ade6+ | FY501 × FY1180 |
| FY340 | h+ ade6-210 leu1-32 ura4-DS/E TM1 (NcoI)::ura4+ Random int. | Allshire et al. (1995) |
| FY973 | h− ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | Allshire et al. (1995) |
| FY1028 | h+ swi6::his1+ ade6-210 leu1-32 ura4-DS/E his1-102 | Allshire et al. (1995) |
| SPG144 | h+ clr6-1 ade6-210 leu1-32 ura4-D18 otr1R(SphI)::ura4+ | Grewal et al. (1998) |
| 655 | h+ bub1::LEU2+ ade6-210 leu1-32 ura4-DS/E his1-102 otr1R(SphI)::ade6+ | Bernard et al. (1998) |
| FY1029 | hA rik1::LEU2+ ade6-210 leu1-32 ura4-DS/E his1-102 | Allshire et al. (1995) |
| Hu78 | h+ rik1::LEU2+ ade6-210 leu1-32 ura4- otr1R(SphI)::ade6+ | This study |
| Hu79 | h− rik1::LEU2+ ade6-210 leu1-32 ura4- otr1R(SphI)::ade6+ | This study |
| FY1470 | h+ csp1 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1473 | h− csp2 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1625 | csp3 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1477 | h− csp4 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1626 | csp5 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1627 | csp6-75 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1628 | csp6-95 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1629 | csp7 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1630 | csp8 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1488 | h+ csp9 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1491 | h− csp10 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1493 | h− csp11 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| FY1495 | h− csp12 ade6-210 leu1-32 ura4-DS/E otr1R(SphI)::ura4+ his1-102 | This study |
| PG9 | h90 ade6-216 leu1-32 ura4-D18 mat3-M::ura4+ | Thon and Klar (1992) |
| FY1631 | h90 csp1 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1854 | h90 csp2 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1633 | h90 csp3 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1634 | h90 csp4 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1635 | h90 csp5 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1636 | h90 csp6-95 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1637 | h90 csp7 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1638 | h90 csp8 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1639 | h90 csp9 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1640 | h90 csp10 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1641 | h90 csp11 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1642 | h90 csp12 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1643 | h90 csp13 ade6-210 leu1-32 ura4-D18 mat3-M::ura4+ otr1R(SphI)::ade6+ | This study |
| FY412 | h+ ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ | Allshire et al. (1995) |
| FY1811 | csp1 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1812 | csp2 ade6-210 leu1-32 ura4D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1813 | csp3 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1814 | csp4 ade6-210 leu1-32 ura4D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | |
| FY1856 | csp5 ade6-210 leu1-32 ura4DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1816 | csp6 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1850 | csp7 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1818 | h+ csp8 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1819 | h− csp9 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1820 | h+ csp10 ade6-210 leu1-32 ura4-DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1821 | h+ csp11 ade6-210 leu1-32 ura4DS/E CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1822 | h− csp12 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1971 | h− csp13 ade6-210 leu1-32 ura4-D18 CC2 (SphI)::ura4+ otr1R(SphI)::ade6+ | This study |
| FY1469 | h− ade6-210 leu1-32 ade6-210 his1-102 (Ch16-216-LEU2+) | This study |
| FY1471 | h− csp1 ade6-210 leu132 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1472 | h+ csp2 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1474 | h+ csp3 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1476 | h+ csp4 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1478 | h+ csp5 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1480 | h+ csp6-75 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1482 | h+ csp6-95 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1484 | h+ csp7 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1486 | h+ csp8 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1487 | h+ csp9 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1489 | h− csp10 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1490 | h+ csp11 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1494 | h+ csp12 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1496 | h+ csp13 ade6-210 leu1-32 ade6-210 ura4-D18 otr1R(SphI)::ura4+ his1-102 (Ch16-216-LEU2+) | This study |
| FY1862 | h90 ade6-210 Jeu1-32 otr1R(SphI)::ura4+ his3-D1 his3+-tel(1L) | Nimmo et al. (1998) |
| FY1878 | csp4 ade6-210 leu1-32 otr1R(SphI)::ura4+ his3-D1 his3+-tel(1L) | This study |
| FY1524 | diploid h+/mat1-M mat2/3Δ::LEU2+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1525 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp1/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1526 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp2/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1527 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp3/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1528 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp4/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1529 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp5/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
| FY1530 | diploid h+/mat1-M mat2/3Δ::LEU2+ csp675/+ ade6-210/216 leu1-32/+ ura4/ura4-D18 | This study |
digestion patterns for a strain carrying both ura4+ and ade6+ within cen1 (data not shown).
Cell-cycle analysis and synchronous cultures: Cultures (500 ml) of csp3 and csp6 cells were grown at 25° to a density of ~5 × 106 cells/ml. The cells were harvested, resuspended in 10 ml of YES, and subjected to mild sonication, two times for 10 sec, to separate newly septated cells. The cells were harvested and layered onto a 40-ml 10–40% d-lactose gradient following the procedure of Edwards and Carr (1997). The gradient was centrifuged in a swing out rotor at 1000 rpm (220 × g) for 3 min at 25°. The centrifugation resulted in a large pellet and a layer of cells in the gradient midzone. Cells from the top of the gradient were collected using a syringe. Each 1.0-ml fraction was examined under the microscope. The first three 1.0-ml fractions, containing mainly small cells, were pooled and layered onto a second d-lactose gradient. The procedure was repeated and produced only a small pellet; the pooled top three 1.0-ml fractions contained ~2 × 107 small, nonseptated cells. These cells were inoculated into a 50-ml YES culture that was shifted to the restrictive temperature of 36° and subjected to viability measurements, FACS analysis, and immunofluorescence microscopy. A small portion of the culture was shaken at 25° to assay for synchrony. The frequency of septated cells peaked after 3 hr (63 and 68% for csp6 and csp3 cells, respectively) in the 25° cultures, indicating their synchronous nature.
Immunofluorescence: Cell growth, fixation in 3.8% para-formaldehyde, staining, detection of Swi6p and α-tubulin using TAT1 monoclonal antibody (Woods et al. 1989), and collection of images and spindle length measurements were described by Ekwall et al. (1995, 1996). Affinity-purified Sad1p antibodies, kindly provided by Iain Hagan, University of Manchester, were used to visualize spindle pole bodies (Hagan and Yanagida 1995). Fluorescent in situ hybridization (FISH) used to detect centromere-repeated DNA was described by Ekwall et al. (1996). Approximately 500 cells were examined for each cell culture. Mitotic spindle length (the pole to pole distance) was measured, using the Zeiss HOME system, to classify cells as either early (<5 μm) or late (>5 μm) anaphase cells, and then lagging centromere FISH signals were scored (see also Table 3).
RNA analysis: Northern analysis of RNA extracted from cells was as described by Allshire et al. (1994, 1995). Cells were grown at 25° before the analysis. The Northern blot was probed with a radiolabeled ura4-DS/E DNA fragment and exposed to a phosphorimaging screen. Reverse transcriptase PCR (RT-PCR) analysis was performed according to Ekwall et al. (1997).
RESULTS
Construction of FY1193, the tester strain: To distinguish cis-acting or trans-acting mutations, a strain that has the ura4+ and ade6+ marker genes inserted both on the left and right sides of centromere I within the inner and outer repeats, respectively, was constructed (Figure 1A). Trans-acting mutations are likely to affect both marker genes while cis-acting mutations or rearrangements are likely to affect only one of the marker genes. Silencing at both marker genes responds in a manner similar to that of clr4, rik1, and swi6 mutations (Allshire et al. 1995). The doubly marked strain was constructed by crossing the parental strains FY501 and FY1180. FY501 contains a ura4+ marker inserted in the left side of the NcoI site of the imr1L/B′ region of cen1 (cen1 imr1L(NcoI)::ura4+; for brevity shortened to cen1L-ura4+). Because ura4+ is repressed at this site, these cells grow poorly on plates lacking supplementing uracil. The FY1180 strain carries a strongly silenced copy of theade6+ gene inserted in the SphI site on the right side of the otr1R/K″ of cen1 (cen1 otr1R(SphI)::ade6+; for brevity shortened to cen1Rade6+). Strong repression at this site results in red rather than white colonies on indicator plates containing low supplementing adenine and little growth on plates lacking adenine. The two sites of insertion in strains FY501 and FY1180 lie ~15 kb apart (Figure 1A). Consistent with the observation that recombination is suppressed across fission yeast centromeres (Chikashige et al. 1989), only three very weakly Ade+ and Ura+ recombinant colonies were isolated from ~1 × 105 spores on selective plates after prolonged (2 wk) incubation. No rearrangements of cen1 were detected in these rare recombinant strains when FY1193 and others were checked by Southern analyses (data not shown). As expected the FY1193 strain formed red colonies on the low adenine plates (wt; Figure 1B) and grew very poorly on both −adenine and −uracil plates (not shown). Typically, spontaneous white Ade+ colonies appeared on the −adenine plates at frequencies of 1 × 10−4. Upon replating, these colonies rapidly returned to the red wild-type state. This suggested that selecting stable white Ade+ colonies in the primary screen would reveal mutants defective in centromeric silencing.
Screening for mutants affecting centromeric silencing: Two types of mutants that alleviate centromeric silencing were selected from the screen. First, because it was expected that mutations in certain centromere proteins might be lethal, temperature-sensitive (ts) mutants, which formed white Ade+ colonies at 25° but were inviable at 36°, were isolated. Second, nontemperature-sensitive (non-ts) mutants, which formed white Ade+ colonies, were identified. Screening against mutants that affect mat2/3 silencing avoids the reisolation of mutants such as clr4, rik1, and swi6 that affect silencing at all tested heterochromatic loci (Allshire et al. 1995). Therefore, mutants allowing some expression of centromeric marker genes but that maintained repression of silent mating-type genes were selected.
The strain FY1193 was mutagenized and 2200 white Ade+ colonies were tested for growth at 36°. Forty-eight mutants unable to grow at 36° were identified. The white and ts phenotypes cosegregated through three successive backcrosses with only 7 of these mutants (data not shown). These 7 were crossed in all pairwise combinations and grouped as six different loci by a recombination test based on temperature sensitivity and colony color. In this test, isogenic control crosses of white colonies of variegating mutants produced predominately (>90%) white spore colonies and 100% ts colonies after sporulation, whereas unlinked mutations produced ~25% red, non-ts wild-type spore colonies. Because the
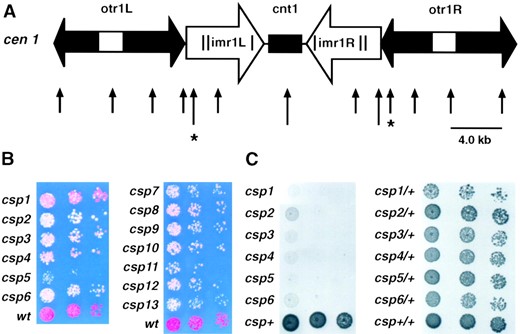
Silent centromere I marker gene insertions utilized in screen and the effect of csp1–13 mutations on cen1-Rade6+ repression. (A) Map of S. pombe centromere I DNA structure showing the insertion sites for cen1Lura4+ (cen1-im-r1L(NcoI)::ura4+; left asterisk) and for cen1Rade6+ (cen1-otr1R- (SphI)::ade6+) and cen1Rura4+ (cen1-otr1R(SphI)::ura4+) marker genes (right asterisk). NcoI sites are indicated by long arrows and SphI restriction sites are indicated by short arrows. The map is drawn approximately to scale. (B) Colony color assays of wild-type (wt) and csp mutant strains bearing cen1Rade6+ after 3 days of growth at 25°. Cells from white colonies, grown on low adenine YES indicator plates at 25°, were serially diluted in fivefold steps and spotted onto low adenine YES indicator plates at 25°. Mutant strain backgrounds were as indicated. The strains were FY1370, 1405, 1430, 1376, 1379, 1382, 1384, 1386, 1389, 1391, 1393, 1394, 1396, and wt is FY1181. (C) Comparative plating assay of haploid and heterozygous diploid temperature-sensitive csp mutants. Cells were serially diluted in fivefold steps and spotted onto YES plates for haploid strains (left) and minimal medium plates lacking adenine for diploid strains (right). Haploid strains are FY1370, 1405, 1430, 1376, 1379, 1382, and wt is FY1181, diploid strains are FY1524–1530, wt is FY1524. Plates were photographed after 3 days of growth at 36°.
primary phenotype was the suppression of the position effect imposed on genes placed within centromeres, the six loci identified were named csp1–6 (centromere: suppressor of position effect). One allele of csp1–5 and two alleles of csp6 (−75 and −95) were isolated.
To isolate mutants with strong defects in centromeric silencing that maintain mating-type silencing, non-ts mutant colonies were subjected to iodine staining to identify those defective in mating-type silencing (Thon and Klar 1992; Grewal et al. 1998). The FY1193 strain (h+N) has stable P mating-type gene expression from an unswitchable mat1:2 locus and two silent M loci (Beach and Klar 1984). Defective mating-type silencing allowing M expression would result in a precocious sporulation phenotype (haploid meiosis) due to the simultaneous expression of M and P genes. Such colonies stain dark with iodine vapors because iodine detects starch that accumulates in spores. Out of the 2152 non-ts white isolates 387 were screened in this way and 146 stained dark with iodine vapors. The remaining nonstaining 241 isolates were then tested for expression of the ura4+ gene residing on the left side of cen1. Cells lacking functional ura4+ gene product are unable to grow in the absence of supplementing uracil but are unaffected by 5-fluoro-orotic acid (FOA), which blocks the growth of Ura+ cells (Boeke et al. 1984, 1987). Because of the repressed state of the ura4+ gene inserted within cen1 in FY1193, this strain grows well on plates containing FOA; however, even slight expression of this cen1Lura4+ marker results in sensitivity to FOA (Allshire et al. 1995). Therefore, only those non-ts mutants that were strongly sensitive to FOA were further analyzed. Only seven of the 241 Ade+, iodine-negative colonies were supersensitive to FOA. Crossing these seven mutants (designated csp7, 8, 9, 10, 11, 12, and 13) in all pairwise combinations defined six loci using the recombination analysis based on colony color (as explained above). The csp8 mutation was found to be very tightly linked (<0.2 cM) and probably allelic to csp10. Thus, nonts mutations in six loci that are required to maintain silencing of both cen1Lura4+ and cen1Rade6+ were uncovered.
The degree of cen1Rade6+ expression in 13 of the csp mutants defined by the screen is shown by the colony color assay in Figure 1B. It is evident that all csp mutants result in a higher incidence of white cen1Rade6+-expressing colonies than wild type. However, although only white, Ade+ colonies were originally picked in the primary screen, some red or pink colonies develop on plating of each csp mutant. This suggests that there is still some variegation of marker gene expression. In this regard, the non-ts mutants, csp7–13, tended to variegate less than the ts class, csp1–6 (Figure 1B and data not shown).
To test dominance or recessiveness of the csp mutations, heterozygous diploids (h−/h+ ade6-210/216 ura4-D18/ura4-D18 csp/csp+ cen1Rura4+) were constructed and assayed for growth on FOA plates and for temperature sensitivity. All seven heterozygous diploid strains created with csp7–13 grew well on FOA, indicating that these csp mutants are recessive with respect to centromere-silencing defects if Csp+ function is required for silencing in diploids (data not shown). Heterozygous diploids were also created with the ts mutants csp1–6, and these grew as well as the wild-type control at 36° indicating that csp1–6 are recessive with respect to temperature sensitivity (Figure 1C). Assuming that Csp+ function is required for growth and silencing in diploids, it appears that the ts csp mutations are also recessive because these csp+/csp− diploids grow at the restrictive temperature of 36° (Figure 1C).
Allelism of the csp mutations: The seven non-ts mutants, csp7, 8, 9, 10, 11, 12, and 13, were crossed with the six ts mutants csp1, 2, 3, 4, 5, and 6. In all cases, red wild-type (Csp+) recombinants were produced with frequencies expected for unlinked loci (data not shown). Thus, a total of 12 csp loci were identified. Each of the 12 csp loci was then tested for linkage to the previously known more general silencing mutants clr1, clr2, clr3, clr4, rik1, and swi6. Consistent with previously reported phenotypes (Allshire et al. 1995), the clr1, clr2, clr3, clr4, rik1, and swi6 produced a variegating white or pink colony color when combined with the cen1Rade6+ insertion. Crosses were performed between csp1–13 and clr1, clr2, clr3, clr4, rik1, and swi6 strains carrying the cen1Rade6+ insertion. All combinations (apart from csp13 × rik1) produced ~25% red wild-type (Csp+) colonies from spores. This indicated that 11 of the 12 csp loci were distinct from clr1, clr2, clr3, clr4, rik1, and swi6. The csp13 × rik1 cross produced only a low frequency of red wild-type (Csp+) spore colonies, indicating that csp13 is ~1.6 cM from rik1 but distinct from it. The bub1+ gene encodes a mitotic centromere protein that is required for proper chromosome segregation (Bernard et al. 1998). Because both the bub1 and rik1 genes are tightly linked to ade6 on chromosome III (Bernard et al. 1998; Egel et al. 1989), we also tested linkage between csp13 and bub1 and found that these two loci were separated by ~15 cM. Allelism was tested for all csp mutants with clr6+, which encodes a histone deacetylase protein required for centromere silencing and chromosome segregation (Grewal et al. 1998). In crosses of csp1–13 × clr6, recombinants were produced with frequencies that indicated that these mutations represent distinct genes (data not shown).
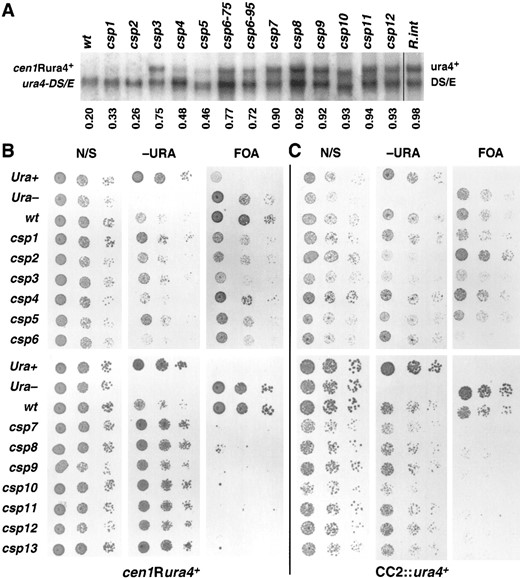
Further characterization of centromeric marker gene expression: The ura4-DS/E deletion allele produces a shorter message than the ura4+ locus, allowing accurate quantification of full-length ura4+ expression from ura4+ inserted into heterochromatin (Allshire et al. 1994). By this assay the ura4+ gene in the cen1 flanking repeats is very strongly repressed (Allshire et al. 1995). We similarly assayed expression of cen1Rura4+ in the csp mutant cells grown under nonselective growth conditions (Figure 2A). A positive control was provided by a strain (FY340) carrying a copy of ura4+ integrated at an unknown site, which produced a ura4+ to ura4-DS/E transcript ratio of 0.98 (Figure 2A, lane 14, R.int). In contrast, transcripts from the cen1Rura4+ gene were barely detectable relative to the ura4-DS/E control (Figure 2A, lane 1, wt). In the csp mutants, however, the cen1R ura4+ gene was expressed at different levels ranging from 0.26 to 0.94 (Figure 2A, lanes 2–13). This indicates that csp7–12 abolish centromeric silencing in the flanking repeat region of cen1 whereas csp1–6 have variable effects.
Analysis of silencing in the central core of cen2: Marker genes are repressed or repressible at all centromere insertion sites assayed (Allshire et al. 1995). To test if the csp mutants affect silencing in distinct centromeric regions, a plating assay was used to compare silencing of ura4+ in the flanking repeats (cen1Rura4+) with silencing at the SphI site in the central core of cen2 (CC2::ura4+; Allshire et al. 1994). Relative growth on minimal nonselective (N/S), selective (−URA), and counterselective (FOA) plates is shown (Figure 2, B and C). As expected from the Northern analyses above (Figure 2A), csp+ cells carrying cen1Rura4+ grew poorly, forming only small colonies on −URA plates relative to a fully Ura+ strain. Conversely, csp+ cells grew as well on FOA plates as a strain lacking functional ura4+, due to repression of cen1Rura4+ imposed by wild-type centromere structure. In contrast mutants csp7–13 bearing cen1Rura4+ formed large colonies on plates lacking uracil and were strongly inhibited for growth on FOA (Figure 2B). The ts mutants csp1, 2, 3, 5, and 6, with the insertion cen1Rura4+, were inhibited only partially for growth on FOA plates, whereas the csp4 cen1Rura4+ strain retained FOA resistance (Figure 2B).
Silencing of the CC2::ura4+ marker behaves in a very similar manner to insertions in the central core of cen1 (Allshire et al. 1994, 1995). Wild-type cells bearing the CC2::ura4+ marker grew well on FOA, and the csp1, 3, 4, and 5 mutations had little or no effect (Figure 2C, top). The csp2 mutant appears to enhance CC2::ura4+ repression because the csp2 CC2::ura4+ strain grew better than wild type on FOA and less well on −URA plates. The csp6–13 mutations appear to alleviate repression of CC2::ura4+ because growth on FOA was inhibited (Figure 2C). Despite this FOA sensitivity no increase in ura4+ expression could be detected relative to ura4-DS/E by RT-PCR (data not shown). This suggests that an undetectable increase in CC2::ura4+ expression causes the FOA-sensitive phenotype in csp6–13. The relatively poor growth of csp6–13 mutant and wild-type cells on −URA plates is also consistent with repression being maintained at CC2::ura4+. A similar pattern of derepression of markers within centromeres was previously observed with clr4, rik1, and swi6 mutants (Allshire et al. 1995). We conclude that mutants csp7–13 have major defects in silencing over the flanking centromeric repeats and
Relative effects of csp1–csp13 on centromeric silencing in the flanking repeat and central core regions. (A) Northern analysis of RNAs from strains with a fully expressed functional ura4+ integrated in the genome (R.int, FY340) and of wild-type (wt, FY973) and csp mutant strains (FY1470, 1473, 1625, 1477, 1626, 1627, 1628, 1629, 1630, 1488, 1491, 1493, and 1495) with ura4+ inserted within the flanking otr1R repeats of centromere I (cen1Rura4+). All strains contain the ura4-DS/E deletion allele at the endogenous ura4 locus. The cen1R-ura4+/ura4-DS/E ratio is indicated below each lane. (B and C) Photographs of comparative plating assays displaying the centromeric silencing phenotypes of csp mutants. The insertion sites of ura4+ marker genes used in the strains are: (B) otr1R(SphI)::ura4+ (cen1R-ura4+) strains (FY1470, 1473, 1625, 1477, 1626, 1628, 1629, 1630, 1488, 1491, 1493, 1495, and 1496), wt is FY973 and (C) CC2 (SphI)::ura4+ (CC2:: ura4+) strains (FY1811, 1812, 1813, 1814, 1856, 1816, 1850, 1818, 1819, 1820, 1821, 1822, 1971), wt is FY412. Positive control (Ura+), 972; negative control (Ura−), ED665. Cells were serially diluted in fivefold steps and spotted onto nonselective (N/S) plates, plates lacking uracil (−URA), and plates containing FOA. Plates were photographed after 3 days of growth at 25°.
only slight defects in central core silencing, whereas mutants csp1–6 are only partially defective in silencing over the flanking centromeric repeats with little or no effect on the central core.
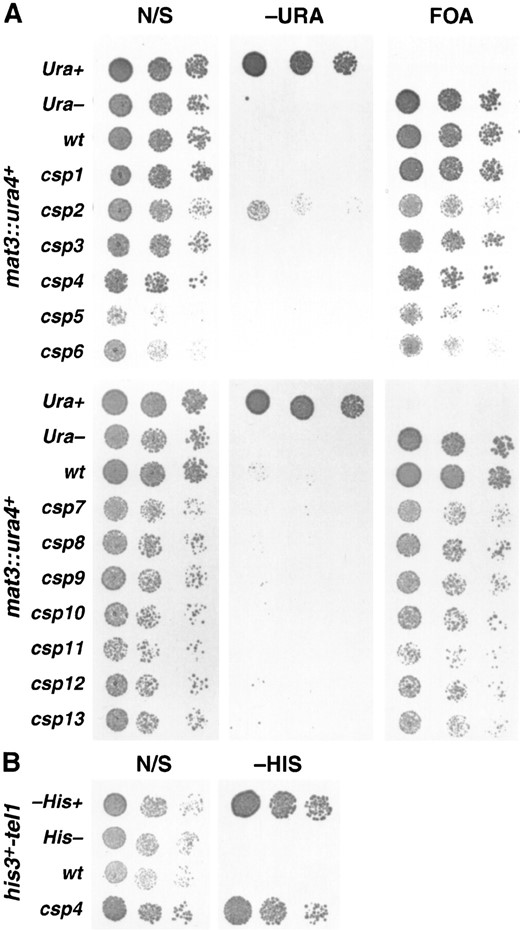
Effects on mating type and telomeric silencing: The csp mutants were also assayed for effects on mating-type mat3(EcoRV)::ura4+ silencing (Thon and Klar 1992). Wild-type csp+ mat3::ura4+ cells do not grow on −URA plates and form large colonies on FOA plates (Figure 3A). Most mutants grew almost as well as wild type on FOA plates. Apart from csp2, all of the csp mutants behaved as wild-type cells and were unable to grow on −URA plates. Partial growth of csp2 cells on −URA plates indicated some increase in mat3::ura4+ expression. In mating assays a csp2 mutant with a homothallic h90 mating-type configuration also displayed a reduced sporulation phenotype (gray rather than black iodine staining; data not shown). Increased expression of the mat3-M locus in clr mutant h90 strains leads to reduced (gray) iodine staining (Ekwall and Ruusala 1994). These observations are therefore consistent with the csp2 mutant having a partial defect in silencing of the mat3-M mating-type cassette. Thus, 12 of the 13 csp mutants tested have defective centromere silencing and maintain a silent mating-type region.
Wild-type cells with the his3+ marker gene placed adjacent to the left telomere on chromosome I (his3+-tel1L) are unable to grow on plates lacking histidine (Nimmo et al. 1998). To assay for telomeric silencing phenotypes, the csp mutants were separately crossed with a wild-type strain bearing this very repressed telomeric his3+ marker, and the phenotypes of resulting progeny were examined. Testing of csp1–13 revealed that only csp4 alleviated telomeric silencing. In plating
Relative effects of csp1–csp13 on silencing of ura4+ adjacent to the mat3 locus and his3+ adjacent to a telomere. (A) A comparative plating assay showing mating-type silencing phenotypes of the csp mutants. The ura4+ marker gene insertion site is adjacent to mat3. Strains are FY1631, 1854, 1633–1643; wt is PG9. Positive control (Ura+), 972; negative control (Ura−), ED665. Cells were serially diluted in five-fold steps and spotted onto nonselective (N/S) plates, plates lacking uracil (−URA), and plates containing FOA. Plates were photographed after 3 days growth at 25°. (B) Comparative plating assay of his3+tel1 wild-type and csp4 strains. The positive control (His+) strain is 972, the wt strain is FY1862, the csp4 strain is FY1878, and the negative control (His−) is FY86. Cells were serially diluted in fivefold steps and spotted onto nonselective (N/S) plates, and plates lacking histidine (−HIS). Plates were photographed after 3 days of growth at 25°.
assays, csp4 mutant cells overcome telomeric silencing, allowing expression of his3+-tel1L and growth on selective plates lacking histidine (−HIS) to a similar extent as a completely His+ strain (Figure 3B). Thus, of the 13 csp mutants examined only csp4 displays defective telomeric silencing and only csp2 alleviates mating-type silencing. We conclude that csp1, 3, 5, 6, 7, 8, 9, 10, 11, 12, and 13 specifically affect silencing at centromeres and mainly within the flanking inverted repeat regions.
Chromosome loss phenotypes and sensitivity to TBZ: Mutants such as swi6, clr4, and rik1, which display strong defects in silencing over the centromeric repeats of cen1, are sensitive to microtubule-destabilizing drugs and exhibit elevated chromosome loss rates (Allshire et al. 1995; Ekwall et al. 1996). To investigate possible effects of the csp mutants on centromere function, the rate of loss of the nonessential 530-kb minichromosome Ch16 (Niwa et al. 1989) was measured in all csp mutants. Ch16 carries the ade6-216 allele that complements the ade6-210 allele. Ch16 was crossed into all csp mutant strains bearing ade6-210 to allow the stability of Ch16 to be accurately assessed (Allshire et al. 1995). Because colonies maintaining Ch16 are white and colonies without Ch16 are red, a minichromosome loss event in the first division on a low adenine indicator plate results in a half-red-/half-white-sectored colony. The Ch16 loss rate in the temperature-sensitive mutant backgrounds (csp1–6) was measured at permissive (25°) and semipermissive (28° and 32°) temperatures (Table 2). In csp+ cells Ch16 was lost in <0.1% of cell divisions at all three temperatures. All of the mutants csp1–6 clearly had elevated rates of Ch16 loss. The loss rate of Ch16 was high in csp1, 4, and csp6-95, even at the permissive temperature, whereas csp2, 3, 5, and csp6-75 displayed a more pronounced effect at the semipermissive temperatures. At 28° the csp5 mutant had the highest loss rate (9.7–14.3%), 140-fold higher than that of wild-type cells. Chromosome loss in the other mutants, csp7–13, was assayed at 25° and all displayed elevated Ch16 loss (Table 2). The rate of loss in these mutants appeared to vary from colony to colony, ranging from a 2- to 100-fold increase over the wild-type loss rates. Thus, there is a good correlation between the inability in csp mutants to maintain normal centromeric architecture, as assayed by transcriptional silencing, and defective centromere function, as revealed by augmented chromosome loss rates (compare Table 2 and Figure 2A).
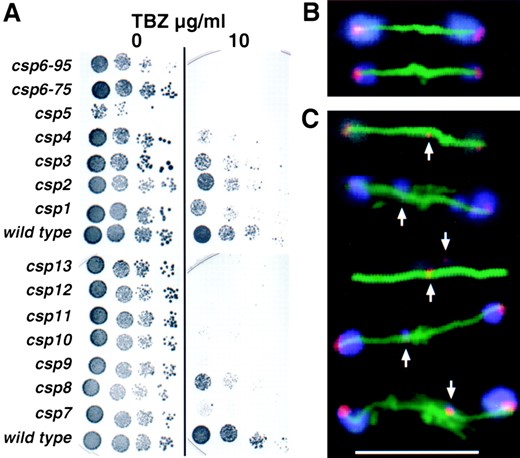
To further characterize the csp mutants, sensitivity to TBZ (Kilmartin 1981) was assessed. Wild-type and mutant cells were assayed on plates containing different concentrations of TBZ at 25° (Figure 4A). The mutants csp2 and 3 responded as wild-type cells, while csp5, 6, 7, 9, 10, 11, 12, and 13 were clearly supersensitive to TBZ at 25° (Figure 4A). The mutants csp1–4, and 8 were only partially sensitive to TBZ, because they display some growth under these conditions. Thus, csp5–13 appear to behave similarly to clr4, rik1, and swi6 mutants (Ekwall et al. 1996) with regard to centromere silencing, chromosome loss, and TBZ-sensitivity phenotypes.
Anaphase defects in csp7–12: In addition to the phenotypes mentioned above, swi6, clr4, and rik1 have a
The loss rate of the 530-kb nonessential linear minichromosome Ch16 in csp1–13 mutants at different temperatures
| Strain . | Genotype . | Ch16 loss (%) 25° . | Ch16 loss (%) 28° . | Ch16 loss (%) 32° . |
|---|---|---|---|---|
| FY1469 | csp+ | 0.064 | <0.10 | <0.10 |
| FY1471 | csp1 | 2.0, 4.0 | 1.8, 3.4 | Microcolonies |
| FY1472 | csp2 | <0.3 | 0.28, 0.53 | 1.5, 2.0 |
| FY1474 | csp3 | 0.3, 0.3 | 0.80, 1.6 | Dead |
| FY1476 | csp4 | 0.89, 3.1 | 1.4, 2.7 | Microcolonies |
| FY1478 | csp5 | 2.2, 3.2 | 9.7, 14.3 | Dead |
| FY1480 | csp6-75 | 0.57, 0.97 | 0.34, 0.81 | 1.0, 3.5 |
| FY1482 | csp6-95 | 2.0, 3.1 | 1.5, 3.8 | Dead |
| FY1484 | csp7 | 2.3, 10.0 | ND | ND |
| FY1486 | csp8 | 0.93, 3.9 | ND | ND |
| FY1487 | csp9 | 0.19, 0.21 | ND | ND |
| FY1489 | csp10 | 0.76, 11.0 | ND | ND |
| FY1490 | csp11 | 0.39, 2.3 | ND | ND |
| FY1494 | csp12 | 0.89, 2.9 | ND | ND |
| FY1496 | csp13 | 0.91, 1.3, 3.5 | ND | ND |
| Strain . | Genotype . | Ch16 loss (%) 25° . | Ch16 loss (%) 28° . | Ch16 loss (%) 32° . |
|---|---|---|---|---|
| FY1469 | csp+ | 0.064 | <0.10 | <0.10 |
| FY1471 | csp1 | 2.0, 4.0 | 1.8, 3.4 | Microcolonies |
| FY1472 | csp2 | <0.3 | 0.28, 0.53 | 1.5, 2.0 |
| FY1474 | csp3 | 0.3, 0.3 | 0.80, 1.6 | Dead |
| FY1476 | csp4 | 0.89, 3.1 | 1.4, 2.7 | Microcolonies |
| FY1478 | csp5 | 2.2, 3.2 | 9.7, 14.3 | Dead |
| FY1480 | csp6-75 | 0.57, 0.97 | 0.34, 0.81 | 1.0, 3.5 |
| FY1482 | csp6-95 | 2.0, 3.1 | 1.5, 3.8 | Dead |
| FY1484 | csp7 | 2.3, 10.0 | ND | ND |
| FY1486 | csp8 | 0.93, 3.9 | ND | ND |
| FY1487 | csp9 | 0.19, 0.21 | ND | ND |
| FY1489 | csp10 | 0.76, 11.0 | ND | ND |
| FY1490 | csp11 | 0.39, 2.3 | ND | ND |
| FY1494 | csp12 | 0.89, 2.9 | ND | ND |
| FY1496 | csp13 | 0.91, 1.3, 3.5 | ND | ND |
Cells from white, Ch16-containing colonies (n = 2,3), grown on low adenine plates at 25°, were plated at the temperatures indicated, and the fraction of half-sectored colonies (which represents the loss rate per cell division) was determined. Colonies, 300–1600 in total and from 1 to 55 half-sectored colonies were scored for each sample (ND, not determined).
The loss rate of the 530-kb nonessential linear minichromosome Ch16 in csp1–13 mutants at different temperatures
| Strain . | Genotype . | Ch16 loss (%) 25° . | Ch16 loss (%) 28° . | Ch16 loss (%) 32° . |
|---|---|---|---|---|
| FY1469 | csp+ | 0.064 | <0.10 | <0.10 |
| FY1471 | csp1 | 2.0, 4.0 | 1.8, 3.4 | Microcolonies |
| FY1472 | csp2 | <0.3 | 0.28, 0.53 | 1.5, 2.0 |
| FY1474 | csp3 | 0.3, 0.3 | 0.80, 1.6 | Dead |
| FY1476 | csp4 | 0.89, 3.1 | 1.4, 2.7 | Microcolonies |
| FY1478 | csp5 | 2.2, 3.2 | 9.7, 14.3 | Dead |
| FY1480 | csp6-75 | 0.57, 0.97 | 0.34, 0.81 | 1.0, 3.5 |
| FY1482 | csp6-95 | 2.0, 3.1 | 1.5, 3.8 | Dead |
| FY1484 | csp7 | 2.3, 10.0 | ND | ND |
| FY1486 | csp8 | 0.93, 3.9 | ND | ND |
| FY1487 | csp9 | 0.19, 0.21 | ND | ND |
| FY1489 | csp10 | 0.76, 11.0 | ND | ND |
| FY1490 | csp11 | 0.39, 2.3 | ND | ND |
| FY1494 | csp12 | 0.89, 2.9 | ND | ND |
| FY1496 | csp13 | 0.91, 1.3, 3.5 | ND | ND |
| Strain . | Genotype . | Ch16 loss (%) 25° . | Ch16 loss (%) 28° . | Ch16 loss (%) 32° . |
|---|---|---|---|---|
| FY1469 | csp+ | 0.064 | <0.10 | <0.10 |
| FY1471 | csp1 | 2.0, 4.0 | 1.8, 3.4 | Microcolonies |
| FY1472 | csp2 | <0.3 | 0.28, 0.53 | 1.5, 2.0 |
| FY1474 | csp3 | 0.3, 0.3 | 0.80, 1.6 | Dead |
| FY1476 | csp4 | 0.89, 3.1 | 1.4, 2.7 | Microcolonies |
| FY1478 | csp5 | 2.2, 3.2 | 9.7, 14.3 | Dead |
| FY1480 | csp6-75 | 0.57, 0.97 | 0.34, 0.81 | 1.0, 3.5 |
| FY1482 | csp6-95 | 2.0, 3.1 | 1.5, 3.8 | Dead |
| FY1484 | csp7 | 2.3, 10.0 | ND | ND |
| FY1486 | csp8 | 0.93, 3.9 | ND | ND |
| FY1487 | csp9 | 0.19, 0.21 | ND | ND |
| FY1489 | csp10 | 0.76, 11.0 | ND | ND |
| FY1490 | csp11 | 0.39, 2.3 | ND | ND |
| FY1494 | csp12 | 0.89, 2.9 | ND | ND |
| FY1496 | csp13 | 0.91, 1.3, 3.5 | ND | ND |
Cells from white, Ch16-containing colonies (n = 2,3), grown on low adenine plates at 25°, were plated at the temperatures indicated, and the fraction of half-sectored colonies (which represents the loss rate per cell division) was determined. Colonies, 300–1600 in total and from 1 to 55 half-sectored colonies were scored for each sample (ND, not determined).
specific defect during mitosis that is manifest as a high incidence of lagging centromeres and chromosomes on late anaphase spindles (Ekwall et al. 1995, 1996). To determine if csp7–12 have similar defects, cells were grown at 32° and shifted to 18° for about four cell doublings prior to fixation. Growth at 18° increases the frequency of lagging chromosomes in clr4, rik1, and swi6 mutants (Ekwall et al. 1995, 1996). Fixed cells were incubated with anti-α-tubulin monoclonal antibody to decorate cytoplasmic and spindle microtubules and allow staging of cells in the cell cycle. Fluorescent in situ hybridization with labeled centromeric DNA allowed detection of all three centromeres (see materials and methods). No lagging centromere signals were detected upon examination of 33 late anaphase spindles in wild-type cells (Table 3). In csp7–12 cells 11–58% of late anaphase cells had one or more lagging centromere signals upon examination of ~20 late anaphase cells for each mutant (Table 3). Representative examples of centromere and chromosome behavior on wild-type and csp9 late anaphase spindles are shown in Figure 4, B and C. Similar defective anaphase configurations were observed in csp7, 8, 10, 11, and 12 (Table 3 and data not shown). Limited analyses of csp13 also revealed the presence of lagging chromosomes in late anaphase (data not shown).
The lagging chromosome phenotype in csp7–13 may result from a failure to establish the normal bipolar attachment of sister kinetochores in prometaphase, which might in turn lead to delayed anaphase onset. If this were the case, then cells at metaphase/early anaphase might accumulate in cultures of these csp mutants. However, the fraction of cells with short spindles (<5 μm) was similar or only slightly increased over that observed in wild-type cells (Table 3). The fraction of late anaphase cells (spindle length >5 μm) was also slightly increased in csp7–12 mutants. Thus, the csp mutants may be slightly defective in spindle elongation. Alternatively lagging or defective centromeres may activate a checkpoint surveillance mechanism, causing these cells to slow down or pause in late anaphase. The lagging phenotype is discussed in Ekwall et al. (1996) and interactions between the spindle checkpoint and swi6 have recently been investigated (Bernard et al. 1998).
Localization of the Swi6 protein in csp mutants: The phenotypes displayed by the csp7–13 mutants resembled very closely the defects observed in clr4, rik1, and swi6. An additional striking phenotype of clr4 and rik1 mutant cells is the failure to localize the Swi6 protein to centromere regions (Ekwall et al. 1996). It was therefore of interest to determine if any of the newly isolated csp mutants also affect the subnuclear localization of Swi6p and if there are any genetic interactions between the csp mutants and swi6. Cultures of csp1–13 cells were fixed and Swi6 was localized using anti-Swi6 polyclonal antibodies according to Ekwall et al. (1995). All of the csp mutants retained a normal Swi6 immunolocalization pattern with two to four spots per haploid nucleus and usually one spot of signal was larger than the others (data not shown).
Genetic interactions: To test for possible genetic interactions with swi6, all of the csp mutants were crossed into a swi6 null background (swi6Δ::his1+). Only csp4
Sensitivity of csp7–13 mutants to TBZ and abnormal anaphase in csp9 cells. (A) Growth of csp cells in the presence of the microtubule-destabilizing drug thiabendazole. Top: serially diluted (fivefold dilutions) temperature-sensitive csp strains (FY1370, 1405, 1430, 1376, 1379, 1407, and 1382) were plated. wt, FY1181. Plates were photographed after 3 days of growth at 25°. Bottom: the nontemperature-sensitive csp7–13 strains (FY1384, 1386, 1389, 1391, 1393, 1394, and 1396) were serially diluted and plated. wt, FY1181. Plates were photographed after 5 days growth at 25°. (B and C) Immunofluorescent microscopy of microtubules (green), DNA (blue), and centromere FISH (red). (B) csp+ (FY1469). (C) csp9 mutant (FY1487). Arrowheads indicate lagging chromosomes with centromere FISH signals in the midzone of the spindle. A very late anaphase cell (or telophase) with a lagging centromere FISH signal is depicted at the bottom. Similar defects were detected in 11–28% of anaphases in csp7–12 mutant cells (see Table 4). Bar, 10 μm.
displayed strong synergy in combination with swi6Δ, leading to reduced viability at 25°, at which both single mutants are viable (Figure 5). In addition, all of the double combinations of csp7–13 with each other or with a rik1 null allele were viable (data not shown). Genetic interactions between temperature-sensitive csp mutants were also tested. The following crosses were analyzed by tetrad dissection: csp6 × csp1, 2, 3, 4, and 5 and csp3 × csp1, 4, and 5. No csp4 csp6, csp5 csp6, or csp3 csp5 double mutants were recovered upon dissection of five or six tetrads. In these crosses tetratype (T) asci are expected to produce one inviable, two viable ts, and one csp+ spore. Nonparental ditype (NPD) asci should produce only two viable spores, both of which were csp+. The number of asci obtained were as follows: for csp4 × csp6, one PD and five T; for csp5 × csp6, two PD, one T, and two NPD; and for csp3 × csp5, one PD, four T, and one NPD. Thus, these mutant combinations are lethal or defective in spore germination.
Cell-cycle defects in csp3 and csp6: Growth of the temperature-sensitive csp mutants at 25° followed by a shift to the restrictive temperature of 36° for several
Centromere detection and spindle length measurements in csp7–12 mutants
| Strain . | Genotype . | Total number of cells examined . | Cells in early anaphasea . | Cell in late anaphaseb . | Cells in late anaphase with lagging centromeres . |
|---|---|---|---|---|---|
| FY1469 | csp+ | 874 | 40 (5.5)c | 33 (3.6)c | 0 (<3.0)d |
| FY1484 | csp7 | 507 | 30 (5.9)c | 23 (4.5)c | 9 (39)d |
| FY1486 | csp8 | 375 | 26 (6.9)c | 18 (4.8)c | 2 (11)d |
| FY1487 | csp9 | 323 | 17 (5.3)c | 22 (6.8)c | 10 (45)d |
| FY1489 | csp10 | 323 | 27 (8.4)c | 20 (6.2)c | 10 (50)d |
| FY1490 | csp11 | 431 | 37 (8.6)c | 21 (4.9)c | 8 (38)d |
| FY1494 | csp12 | 418 | 21 (5.0)c | 26 (6.2)c | 15 (58)d |
| Strain . | Genotype . | Total number of cells examined . | Cells in early anaphasea . | Cell in late anaphaseb . | Cells in late anaphase with lagging centromeres . |
|---|---|---|---|---|---|
| FY1469 | csp+ | 874 | 40 (5.5)c | 33 (3.6)c | 0 (<3.0)d |
| FY1484 | csp7 | 507 | 30 (5.9)c | 23 (4.5)c | 9 (39)d |
| FY1486 | csp8 | 375 | 26 (6.9)c | 18 (4.8)c | 2 (11)d |
| FY1487 | csp9 | 323 | 17 (5.3)c | 22 (6.8)c | 10 (45)d |
| FY1489 | csp10 | 323 | 27 (8.4)c | 20 (6.2)c | 10 (50)d |
| FY1490 | csp11 | 431 | 37 (8.6)c | 21 (4.9)c | 8 (38)d |
| FY1494 | csp12 | 418 | 21 (5.0)c | 26 (6.2)c | 15 (58)d |
The fraction of abnormal anaphase cells with lagging centromeres was determined using the Zeiss HOME microscope system. Cells were grown in log-phase at 18°, fixed, stained with anti-α-tubulin antibodies and subjected to centromere FISH. Lagging centromeres were defined as one or more centromere FISH signals in the midzone, more than 1.5 μm from one end of a >5 μm spindle.
Sindle length <5 μm.
Spindle length >5 μm.
Percentage of total cells.
Percentage lagging.
Centromere detection and spindle length measurements in csp7–12 mutants
| Strain . | Genotype . | Total number of cells examined . | Cells in early anaphasea . | Cell in late anaphaseb . | Cells in late anaphase with lagging centromeres . |
|---|---|---|---|---|---|
| FY1469 | csp+ | 874 | 40 (5.5)c | 33 (3.6)c | 0 (<3.0)d |
| FY1484 | csp7 | 507 | 30 (5.9)c | 23 (4.5)c | 9 (39)d |
| FY1486 | csp8 | 375 | 26 (6.9)c | 18 (4.8)c | 2 (11)d |
| FY1487 | csp9 | 323 | 17 (5.3)c | 22 (6.8)c | 10 (45)d |
| FY1489 | csp10 | 323 | 27 (8.4)c | 20 (6.2)c | 10 (50)d |
| FY1490 | csp11 | 431 | 37 (8.6)c | 21 (4.9)c | 8 (38)d |
| FY1494 | csp12 | 418 | 21 (5.0)c | 26 (6.2)c | 15 (58)d |
| Strain . | Genotype . | Total number of cells examined . | Cells in early anaphasea . | Cell in late anaphaseb . | Cells in late anaphase with lagging centromeres . |
|---|---|---|---|---|---|
| FY1469 | csp+ | 874 | 40 (5.5)c | 33 (3.6)c | 0 (<3.0)d |
| FY1484 | csp7 | 507 | 30 (5.9)c | 23 (4.5)c | 9 (39)d |
| FY1486 | csp8 | 375 | 26 (6.9)c | 18 (4.8)c | 2 (11)d |
| FY1487 | csp9 | 323 | 17 (5.3)c | 22 (6.8)c | 10 (45)d |
| FY1489 | csp10 | 323 | 27 (8.4)c | 20 (6.2)c | 10 (50)d |
| FY1490 | csp11 | 431 | 37 (8.6)c | 21 (4.9)c | 8 (38)d |
| FY1494 | csp12 | 418 | 21 (5.0)c | 26 (6.2)c | 15 (58)d |
The fraction of abnormal anaphase cells with lagging centromeres was determined using the Zeiss HOME microscope system. Cells were grown in log-phase at 18°, fixed, stained with anti-α-tubulin antibodies and subjected to centromere FISH. Lagging centromeres were defined as one or more centromere FISH signals in the midzone, more than 1.5 μm from one end of a >5 μm spindle.
Sindle length <5 μm.
Spindle length >5 μm.
Percentage of total cells.
Percentage lagging.
Genetic interaction between csp4 and swi6. Growth of primary restreak of colonies generated by spores dissected from a tetratype ascus resulting from crossing csp4 (FY1477) × swi6::his1+ (FY1028) strains.
hours prior to fixation revealed various spindle defects and chromosome missegregation events upon immunostaining with anti-α-tubulin antibody and 4′,6-diamidino-2-phenylindole (DAPI). The two most striking examples were csp3 and csp6, which displayed clear cell-cycle-arrest phenotypes at the restrictive temperature (described below). This, coupled with the observation that csp3 and csp6 had the most obvious centromeric silencing defects of the ts class (see Figure 2A), led us to focus on their analysis. Synchronous cultures of csp3 and csp6 inoculated with small G2 cells were shifted to the restrictive temperature of 36°, and samples were taken and fixed at regular intervals. These samples were stained with anti-α-tubulin antibody and antibodies against Sad1p, a spindle pole body (SPB) component (Hagan and Yanagida 1995). The growth of csp3 cells was clearly arrested at 36° because no increase in cell number was observed over 10 hr, and after 6 hr cell viability had decreased to 50% (Figure 6A). Simultaneous with the decrease in viability, cells with a bent shape appeared in the culture (Figure 6B), rising to a maximum of ~70% of cells after 10 hr. These bent cells frequently contained very unusual, strongly staining bundles of cytoplasmic microtubules not normally observed in wild-type cells. The Sad1p marker for the SPB was associated with these microtubule bundles and often localized close to the cell cortex. After 7 hr 10% of these bent cells underwent a defective mitosis in which condensed chromosomes appeared to be stuck in the midzone of an anaphase spindle (Figure 6C). Hence, the csp3 mutation affected both interphase cytoplasmic microtubule organization and chromosome segregation on spindles.
Synchronous cultures of csp6 were unable to divide at the restrictive temperature and viability decreased to ~60% after 6 hr (Figure 7A). csp6 cells underwent normal spindle elongation and mitosis at the permissive temperature (Figure 7B), but mitosis failed to be completed at the restrictive temperature. After 10 hr, cells with condensed chromosomes and a V-shaped spindle accumulated in 18% of cells (Figure 7C). Frequently, only one SPB could be detected at the base of the V-shape spindle by anti-Sad1p staining. After 10–12 hr there was a sharp drop in cell viability simultaneous with the disappearance of V-shaped spindles and the appearance of cells that had undergone a lethal cytokinesis culminating in the “cut” phenotype (cell untimely torn; see Yanagida 1995). In most cases cut cells divided aberrantly, giving rise to nucleated and anucleate progeny, or, alternatively, chromosomal DNA appeared to be bisected by the septum. These phenotypes are reminiscent of the phenotypes previously described for the cut7 and sad1 mutants (Hagan and Yanagida 1990, 1992, 1995). From these analyses it appears that csp6 cells arrest during prometaphase perhaps as a result of defective centromere function.
DISCUSSION
Heterochromatin and centromere function: We have described a screen that identified mutations at 12 csp loci that allow expression of marker genes inserted in the inverted repeat region surrounding the central core of S. pombe centromere I. Mutations at all 12 loci result in chromosome-loss phenotypes, many of the mutants also show distinct defects in chromosome segregation upon cytological examination. This reinforces the idea that the packaging of the repetitive structures at fission yeast centromeres into silent chromatin provides an important function for promoting the assembly of a fully functional kinetochore (Allshire et al. 1995). The intimate relationship between heterochromatin structures and centromere function is not unique to S. pombe. In Drosophila, centromere function maps to heterochromatin (Murphy and Karpen 1995; Sun et al. 1997), and genes are repressed when placed within or close to this centromeric heterochromatin. Many Su(var) mutations alleviate repression close to Drosophila centromeric heterochromatin, and some of these also impair centromere function (Wines and Henikoff 1992; Baska et al. 1993). Several of these gene products, such as HP1 and the interacting protein SU(VAR)3-7, localize to centromeric heterochromatin (Cleard et al. 1997). The assembly of active centromeres in both S. pombe and Drosophila is subject to epigenetic regulation (Karpen and Allshire 1997). Thus, parallels are emerging with respect
Cytological abnormalities detected in csp3 cells. (A) Synchronized csp3 cells were incubated at permissive and restrictive temperatures. Left: Viability (red and black squares) and cell number (green and blue circles) in the cultures at 25° and 36°. Right: Fraction of bent (red and black squares) and defective anaphase cells (green circles) detected in the synchronized csp3 cultures at 25° and 36°. (B and C) Immunofluorescent microscopy images of synchronized csp3 cells at the restrictive temperature, fixed and stained for α-tubulin (green), DNA (blue), and spindle pole bodies (red). (B) Bent and pear-shaped cells with staining of strong bundles of microtubules accumulated after incubation at 36° for 10 hr. The middle cell is bent and septated. (C) Two defective anaphase cells with lagging chromosomes detected in csp3 cells incubated at 36° for 8 hr. Bar, 10 μm.
to the involvement of heterochromatin structures in centromere function in S. pombe and Drosophila.
A large number of loci affect fission yeast centromeric silencing: It is clear that mutant isolation by the screen described has not reached saturation. Single alleles were isolated for 10 of the 12 csp loci identified and only two alleles were isolated at the csp6 (csp6-75 and csp-95) and csp8/10 loci (assuming that these are allelic). Thus, it is likely that many more csp loci remain to be identified. This highlights another similarity with Drosophila heterochromatin structure; 120 modifiers of position-effect variegation have been estimated, 75 of which are predicted to be suppressors of variegation (Reuter and Wolff 1981; Reuter and Spierer 1992). In fission yeast, mutations in 28 loci that affect various types of silencing have so far been identified. These are (1) the 6 general loci clr1, 2, 3, 4, rik1, and swi6, which alleviate silencing to some extent at mat2/mat3, centromeres, and telomeres (Thon and Klar 1992; Thon et al. 1994; Ekwall and Ruusala 1994); (2) a group of 3 esp loci and clr6 that act synergistically with swi6 and clr1 (Thon and Friis 1997; Grewal et al. 1998); (3) the 12 csp loci defined in this study; (4) 3 loci, lot2, lot3, and taz1 that specifically alleviate telomeric silencing (Nimmo et al. 1998); and (5) 3 loci (cep1, 2, and 3) that enhance repression in the central core of cen1 (Javerzat et al. 1999). Clearly gene silencing both in Drosophila and in S. pombe is under complex genetic control.
Relationship between csp mutants and clr4, rik1, and swi6: The more general silencing mutants, clr4, rik1, and swi6, display strong defects in silencing over the centromeric repeats of cen1, are sensitive to microtubule-destabilizing drugs, and exhibit chromosome-loss phenotypes (Allshire et al. 1995). Clr4 and Rik1 are required for proper localization of Swi6 to centromeres (Ekwall et al. 1996). The normal localization of Swi6
Cytological abnormalities detected in csp6-95 cells. (A) Synchronized csp6-95 cells were incubated at permissive and restrictive temperatures. Left: Viability (red and black squares) and cell number (green and blue circles) in the cultures at 25° and 36°. Right: Fraction of V-shape spindle (black squares) and cut cells (red squares) detected in the synchronized csp6-95 culture at 36°. (B and C) Immunofluorescence microscopy images of synchronized csp6-95 cells at the restrictive temperature, fixed and stained for α-tubulin (green), DNA (blue), and spindle pole bodies (red). (B) Two normal anaphase csp6-95 cells from a synchronized culture incubated at the 25°. (C) Prometaphase arrested csp6-95 cells with condensed chromosomes and V-shaped spindles after 6 hr incubation of the synchronized culture at 36°, the restrictive temperature. Bar, 10 μm.
in the csp mutants leaves many possibilities open. The csp mutants might act in a different pathway from clr4+, rik1+, and swi6+ and affect a structure distinct from that disrupted by clr4, rik1, and swi6. Alternatively, the csp+ gene products may represent additional components of the same pathway or complex, but do not affect Swi6 localization. The first possibility clearly predicts that genetic interactions should be detected between the csp mutants and clr4, rik1, or swi6.
Surprisingly, only csp4 was found to be synthetically lethal when combined with a swi6 null, and none of the mutants csp7–13 showed any obvious synergistic phenotypes when combined with each other or csp7–12 when combined with a rik1 null. Some combinations of temperature-sensitive mutants were not recovered: for example, csp3 and 5 and csp4 and 6. This analysis suggests that the csp4+ gene product may act in a parallel pathway to affect structures distinct from that affected by swi6+ and that the csp4+ and csp6+, and csp3+ and csp5+ gene products, respectively, may affect or be part of parallel structures or pathways.
The csp+ gene products could represent additional components of a putative Clr4/Rik1/Swi6 complex not required for Swi6 localization. However, it seems unlikely that all the csp+ gene products in addition to clr4+, rik1+, and swi6+ act through the same pathway.
If we assume that the phenotypes associated with the csp alleles obtained in this study are hypomorphic or null, then one possibility is that csp1–13 act via parallel but redundant pathway(s) from clr4, rik1, and swi6 and that there are several redundant centromeric proteins or even complexes performing a role similar to that of clr4, rik1, and swi6. It will clearly be very informative to identify and determine the localization of the csp+ gene products in wild-type and clr4, rik1, and swi6 mutant backgrounds and to test for physical interactions between these gene products.
Cell-cycle defects in temperature-sensitive csp mutants: Two of the temperature-sensitive csp mutants, csp3 and csp6, displayed dramatic mitotic defects. In both mutants growth is arrested at the restrictive temperature. Cells with V-shaped spindles accumulate in csp6 cultures. A similar phenotype was reported for mutations in the cut7+ gene, encoding a kinesin-related motor protein, and mutations in the sad1+ and cut12+ genes, which encode components of the SPB (Hagan and Yanagida 1990, 1992, 1995; Bridge et al. 1998). In cut7 cells, the V-shaped spindle has been interpreted as a failure of this kinesin to mediate the interdigitation of microtubules from each pole and push the two poles apart to form a bipolar spindle (Hagan and Yanagida 1995). Defective centromere function in csp6 mutants might result in a similar phenotype if aberrant microtubule attachments to the kinetochore interfere with the establishment of a bipolar spindle. It is also possible that csp6+ encodes a protein that acts at centromeres and has an independent role in SPB separation. The cytological defects show that the csp3 and csp6 mutants must have defects in addition to those displayed by csp7–13, since mitosis is completely defective at the restrictive temperature. Assuming that the phenotypes of the alleles obtained in this study are representative of gene function, it appears that the csp3+ and csp6+ gene products have more pleiotropic effects than those of csp7+–13+. Alternatively, the csp3 and csp6 mutations may affect structures distinctly different from centromeric chromatin and more closely connected with the kinetochore and its associated motors.
Relationship between csp mutants and acetylation of histones in centromeric chromatin: The phenotype of defective centromeric flanking repeat silencing and lagging chromosomes in late anaphase as seen in csp7–13 and previously reported in clr4, rik1, and swi6 mutants (Ekwall et al. 1995, 1996) is also observed in other situations. The flanking centromeric repeats are packaged in nucleosomes that are underacetylated on the N-terminal lysine residues of the core histones H3 and H4. These can be forced into a hyperacetylated state by treating cells with trichostatin A, an inhibitor of histone deacetylase activity (Ekwall et al. 1997). Thus, disturbances in the integrity of centromere repeat chromatin either by trans-acting mutation or by altering the state of histone acetylation at centromeres causes very similar phenotypes. Indeed, some of the csp mutants might encode components of a histone deacetylase activity required to maintain underacetylated chromatin over these flanking repeats. The clr6+ gene encodes a histone deacetylase, and clr6 mutants also alleviate centromeric silencing and cause chromosome missegregation (Grewal et al. 1998). However, in test crosses none of the csp mutants were found to be allelic with clr6.
Auxiliary role of flanking repeats in centromere function? The relative contribution of the central core and the flanking repeat regions to fission yeast centromere structure and function is not completely known. The inverted flanking centromeric repeats and associated factors may contribute only partially to centromere function. In this case the crippled centromere activity observed in clr4, rik1, swi6, csp, and other situations might represent the full phenotype for disruption of functions associated with these elements. This possibility is supported by the observation that the inverted repeat structure surrounding the central domain is dispensable on circular minichromosomes. Minimal centromere constructs lacking an inverted repeat structure are subject to epigenetic regulation, forming functional centromeres in only some transformants. However, once the functional state is established it remains stable in subsequent divisions (Steiner and Clarke 1994; Baum et al. 1994; Ngan and Clarke 1997). We do not know the nature of the csp7–13 alleles; however, an auxiliary role for the centromeric flanking repeats could explain why clr4Δ, rik1Δ, swi6Δ, csp7, 8, 9, 10, 11, 12, and 13 do not display more severe chromosome segregation phenotypes. This would also provide an explanation for the lack of synergistic phenotypes when csp7–13 are combined with each other or rik1Δ. It seems plausible that the essential functions associated with the central domain remain predominantly intact in these mutants and are sufficient to allow reasonable chromosome segregation. This is consistent with central core (CC2) silencing being relatively unaffected in these mutants (Figure 2C). This is further supported by the observation that the Mis6 protein associates predominantly with the central core region and that mis6 mutations disrupt centromere function resulting in unequal chromosome segregation in most divisions (Saitoh et al. 1997). Further investigations will unravel the role played by these new csp gene products in fission yeast centromere structure and function.
Acknowledgement
We are most grateful to past and present members of the Allshire laboratory for advice and useful discussions during the course of this work. We thank Alison Pidoux, Janet Partridge, Kristin Scott, Stefan Hermann, and Pernilla Bjerling for valuable comments on the manuscript and Paul Perry for excellent assistance with microscopy and imaging. Iain Hagan generously provided us with Sad1p antibodies and Peter Fantes, Shiv Grewal, and Jean-Paul Javerzat provided various strains. K.E. was funded by European Molecular Biology Organization, Human Frontiers Scientific Program, European Commission fellowships, and subsequently Medicinska Forskningsradet project grants 11821 and 12562. Core funding for centromere research in the Allshire laboratory is provided by The Medical Research Council of Great Britain.
Footnotes
Communicating editor: G. R. Smith
LITERATURE CITED
Author notes
Present address: Department of Biosciences at Novum, Karolinska Institutet, S-141 57 Huddinge, Sweden.
Present address: MRC Collaborative Centre Scotland, Crewe Rd. South, Edinburgh EH4 2SP, Scotland.