-
PDF
- Split View
-
Views
-
Cite
Cite
Georgette L Sass, Steven Henikoff, Pairing-Dependent Mislocalization of a Drosophila brown Gene Reporter to a Heterochromatic Environment, Genetics, Volume 152, Issue 2, 1 June 1999, Pages 595–604, https://doi.org/10.1093/genetics/152.2.595
Close - Share Icon Share
Abstract
We describe the precise positioning of a reporter gene within heterochromatin where it may be silenced. A transposition of the 59E-60A region into pericentric heterochromatin ensnares distal 59E-60A via somatic pairing. The frequency with which a brown (bw) reporter gene in 59E is silenced is influenced by chromosomal configurations. Silencing occurs only when the bw+ reporter is unpaired due to heterozygosity with a deficiency, where the frequency of bw+ reporter expression is correlated with the extent of bw gene and flanking sequence present. Surprisingly, the frequency of pairing between the transposition in heterochromatin and distal 59E observed cytologically is indistinguishable from the frequency of pairing of homologous chromosomes at 59E in wild-type larval brains, regardless of configuration. Therefore, bringing a susceptible reporter gene into close proximity with heterochromatin does not necessarily affect its expression, but local pairing changes resulting from altered chromosomal configurations can lead to silencing. We also find that an ensnared distal copy of bw that is interrupted by a heterochromatic insertion enhances silencing. This demonstrates that bw can be simultaneously acted upon by pericentric and distal blocks of heterochromatin.
THERE is an emerging picture of a compartmentalized nucleus in which individual chromosomes not only occupy specific territories but also exhibit particular configurations within that territory (reviewed in Lamond and Earnshaw 1998). This picture pertains primarily to the transcriptionally active euchromatin. However, the transcriptionally inactive and condensed heterochromatin remains poorly differentiated at the cytological level. To address this problem, investigators have used reporter genes juxtaposed to or inserted into heterochromatin as probes of the heterochromatic environment (Weiler and Wakimoto 1995; Martin and Whitelaw 1996; Wallrath 1998). Variable silencing of these reporter genes by heterochromatin establishes the importance of chromosomal context on gene expression.
Recent efforts have extended the connection between chromosomal context of a gene and its expression to include the role of its nuclear position (Wakimoto and Hearn 1990; Csink and Henikoff 1996; Dernburg et al. 1996; Brown et al. 1997; Park and Boni 1998). In the case of the mutation brownDominant (bwD), a 1-2-Mb insertion of heterochromatin into the brown (bw) coding region can mislocalize the endogenous bw+ reporter on a somatically paired homologous chromosome to the pericentric heterochromatin of the same chromosome (Csink and Henikoff 1996; Dernburg et al. 1996). A correlation between the inactivation of the bw+ gene and its association with centromeric heterochromatin indicates that heterochromatic associations effect gene silencing. Furthermore, genetic observations of a heterochromatic mini-white transgene array indicate that heterochromatic associations are responsible for silencing of reporter genes within the mislocalized heterochromatic transgene array (Dorer and Henikoff 1997). Studies in mammalian systems have also shown a correlation between heterochromatin association and gene silencing (Dobie et al. 1996; Festenstein et al. 1996, Milot et al. 1996; Brown et al. 1997). In these examples, localization of a gene to a heterochromatic “compartment” is thought to reduce accessibility or exclude factors required for expression resulting in inactivation. In each case, a precisely identified gene sequence associates with a heterochromatic compartment. Unfortunately, the definition of this compartment is so vague as to allow for the possibility that different heterochromatic loci associate with the same reporter in different cells.
In this study, we alleviate the uncertainty about the position of a mislocalized reporter with respect to target heterochromatin responsible for silencing. A reporter gene is mislocalized via somatic pairing to a precise position adjacent to heterochromatic loci that mediate silencing. A heterochromatically embedded segment that includes the bw gene behaves as a snare, allowing us to assess the degree of trans-inactivation of bw+ reporter in its normal distal position when it is somatically paired with its heterochromatic homolog. We show that in order to be trans-inactivated, the reporter must not be paired with its euchromatic homolog. Surprisingly, association between the reporter and the target heterochromatin was seen regardless of whether the gene was trans-inactivated, demonstrating that mislocalization is not the only determinant of gene inactivation.
MATERIALS AND METHODS
Fly lines and chromosomes: Flies were reared on standard cornmeal-molasses medium at a controlled temperature of 25°. Table 1 lists the Drosophila melanogaster chromosomes used in this study. The bwDX7 mutation was generated by an X-ray mutagenesis procedure in the laboratory of Thom Kaufman. Our analysis revealed that this allele should be designated as Tp(2;2)bwDX7 and the new order of chromosome 2 given as 21-41|(59E-60A)|41-59E|60A-60F (see results). Tp(2;2)bwDX7 is composed of two recombinationally separable components: a duplication of the chromosomal segment 59E-60A transposed to centromeric heterochromatin as well as a deficiency of this segment. For simplicity, we refer to the Tp(2;2)bwDX7 chromosome as TpDX7, the transposed component as DpDX7, and the deficiency component as Df DX7.
We separated the duplication and deficiency components of Tp(2;2)bwDX7 and selected derivatives that contain the duplication component and the wild-type bw region. Such DpDX7bw+ derivatives are formally designated as Dp(2;2)bwDX7, bw+ bw+ and the new order of chromosome 2 is given as 21-41|59E-60A|41-60F. We also selected the Df DX7 [Df(2R)bwDX7; 59E-60A] reciprocal recombination products: chromosomes that have a deficiency of the 59E to 60A chromosomal segment.
Cytology: Third instar larval salivary glands were dissected in 1× Ephrussi-Beadle Ringer solution, transferred to 45% acetic acid and allowed to sit for 5-10 min, and then squashed in preparation for polytene chromosome analysis. The procedure used for fluorescent in situ hybridization (FISH) was as described (Csink and Henikoff 1996; Marshall et al. 1996). For analysis of polytene chromosomes, the probes used were from an 8.4-kb bw+ genomic clone, an ∼40-kb cosmid (cPn-1111) that contains the bw gene (Dreesen et al. 1988), and cloned genomic DNA derived from the 59E region (P1 DS03480 provided by the Drosophila Genome Project). We also examined mitotic chromosomes from third instar larval neuroblasts that had been prepared for cytology as described (Csink and Henikoff 1996) with the exception that tissue was treated in 0.5% sodium citrate for 10 min prior to fixation and squashing. The satellite sequences AAGAG and AACAC (unique to the centromeric heterochromatin of 2R) were detected using the appropriate end-labeled oligonucleotides (Csink and Henikoff 1996). These same probes were used in our analysis of somatic pairing and nuclear position in interphase nuclei of third instar larval neuroblasts. In addition, cloned genomic DNA from the 23C region (P1 DS00906 provided by the Drosophila Genome Project) was used as a control for the procedure. Image accumulation and distance measurements were performed as in a previous study utilizing bwDominant (Csink and Henikoff 1998).
Genetic analysis of bwDX7: When examining the phenotype of heterozygous individuals carrying specific combinations of chromosomes, separate crosses were established to look at paternal as well as maternal inheritance of each chromosome. The parental source of the chromosome had no effect on the phenotype (data not shown). In our analysis of the phenotypic consequence of increased Heterochromatin Protein 1 (HP1), we used a heat-shock-inducible HP1 transgene (HSHP1,83C) and followed a regimen of daily 1-hr heat shocks at 37° throughout larval and pupal development (Eissenberg et al. 1992). The strategy used for design of crosses to assess the phenotypic effect of modifiers of position-effect variegation (PEV) and for scoring eye pigment variegation phenotypes was previously described (Sass and Henikoff 1998).
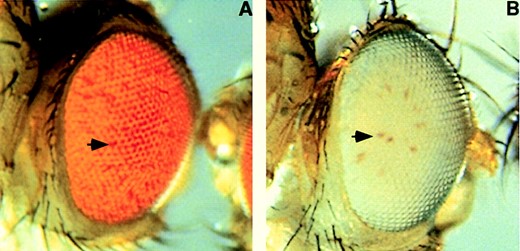
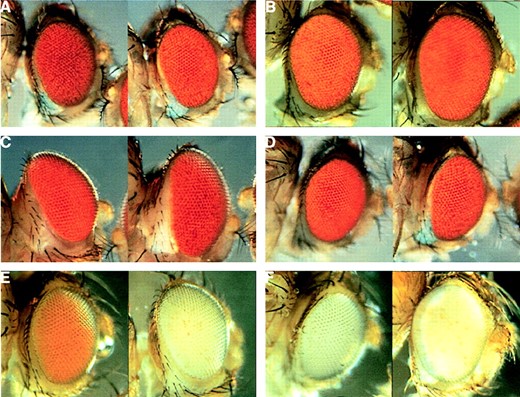
—The bwDX7 mutation exhibits atypically weak trans-inactivation. (A) Eye from a bwDX7/bw+;st individual showing weak trans-inactivation. Arrow indicates a bw-;st- ommatidium, which appears darker because absence of pigment causes an interruption in the densely packed array of pigmented cells. (B) Eye from a bwDX7/bw1;st individual showing strong cis-inactivation (i.e., silencing of the bw+ gene present on the bwDX7 chromosome). Arrow indicates a bw+;st- ommatidium; phenotypically bw-;st- ommatidia are white.
RESULTS
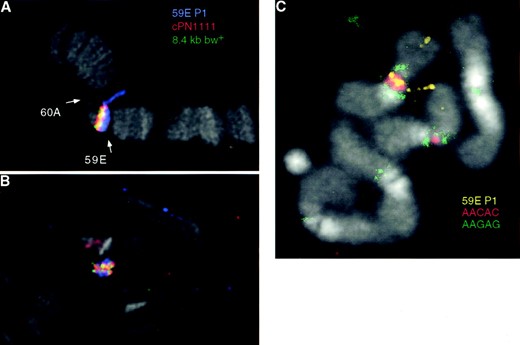
The bwDX7 mutation: The brown (bw) gene is unusual in that PEV is dominant, displaying silencing both in cis and in trans (Muller 1932). In general, the strength of the silencing in trans mirrors that of silencing in cis (Slatis 1955). However, we noticed that the homozygous lethal bwDX7 mutation generated in an X-ray mutagenesis screen displayed exceptionally weak silencing of bw in trans (Figure 1A), in spite of relatively strong cis-silencing (Figure 1B). To elucidate the basis for this atypical behavior, we examined salivary gland polytene chromosomes from individuals carrying the bwDX7 chromosome. This revealed a deletion of polytene bands 59E through 60A (Figure 2A), which uncovers the bw locus (Dreesen et al. 1988). However, the bw gene must still be present on chromosome 2, as individuals heterozygous for the bwDX7 chromosome and bw1 (a null mutation) have bw+ ommatidia (Figure 1B). Because no other aberration was detected in the euchromatin of chromosome 2, we suspected that the 59E-60A region may have been transposed to a heterochromatic location on the bwDX7 chromosome, where the coalescence and heteropycnotic appearance of the chromocenter impede cytological detection. Fluorescent in situ hybridization using probes that contained the bw region demonstrated that transposition had occurred: the region was present in the chromocenter of salivary gland polytene chromosomes from individuals carrying the bwDX7 chromosome (Figure 2B).
To map the heterochromatic location of the 59E-60A transposition, mitotic chromosomes from third instar larval neuroblasts were examined using FISH. The 59E genomic clone and the satellite sequence (AACAC)n (found only in centromeric heterochromatin of chromosome 2R) were used as probes. We found that the 59E region was present in the centromeric heterochromatin of 2R (Figure 2C). The relative positions of the 59E and AACAC signals indicate that the site of insertion is either within the AACAC block or very near it. Furthermore, since the 59E probe detected sequences at the centromere as well as the tip of 2R, we were able to confirm in mitotic chromosomes our observation in polytene chromosomes that the proximal euchromatic breakpoint of the transposition is within the region covered by the 59E genomic clone (Figure 2C). We conclude that the bwDX7 chromosome is a transposition of region 59E to 60A into 2R heterochromatin (designated TpDX7; see materials and methods).
—FISH analysis of the bwDX7 transposition. (A) Three different probes from the 59E region decorate the region in wild type, which is looped out due to deficiency of 59E-60A. 59E P1 is an ∼80-kb probe proximal to bw, and cPN111 is an ∼40-kb probe encompassing bw. (B) The 59E-60A chromosomal segment is present in the chromocenter of the same nucleus. (C) The 59E-60A chromosomal segment (yellow) maps to the pericentric heterochromatin of chromosome 2R in the region of the single AACAC satellite block (red), which is surrounded by AAGAG satellite blocks (green).
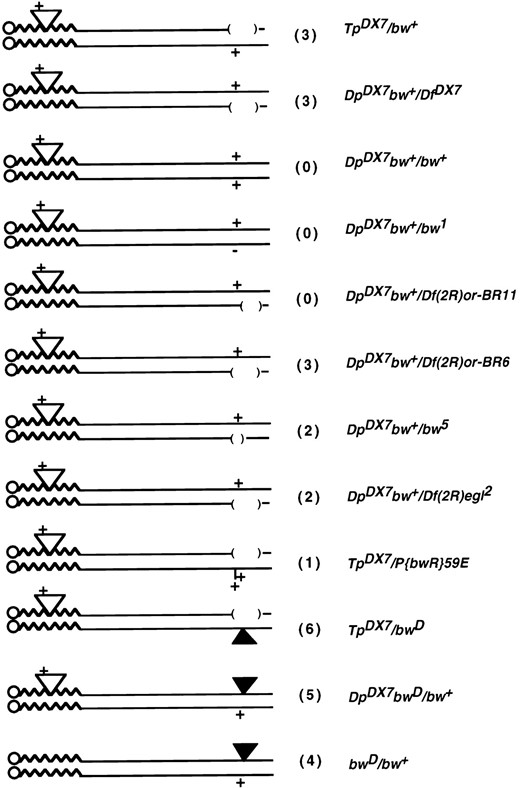
DpDX7 silences an unpaired reporter: In the heterozygote TpDX7/bw+ there are two copies of the bw region—a copy on the transposed segment, which is embedded in pericentric heterochromatin, and a reporter, which is in its normal distal position on the wild-type homologous chromosome. (Heterozygous combinations used in this study are depicted schematically in Figure 3.) In Drosophila, homologous chromosomes are paired somatically as well as meiotically (Metz 1916). Therefore, in the case of TpDX7/bw+, interhomolog pairing of the bw regions would trans-inactivate the bw+ gene on the wild-type chromosome.
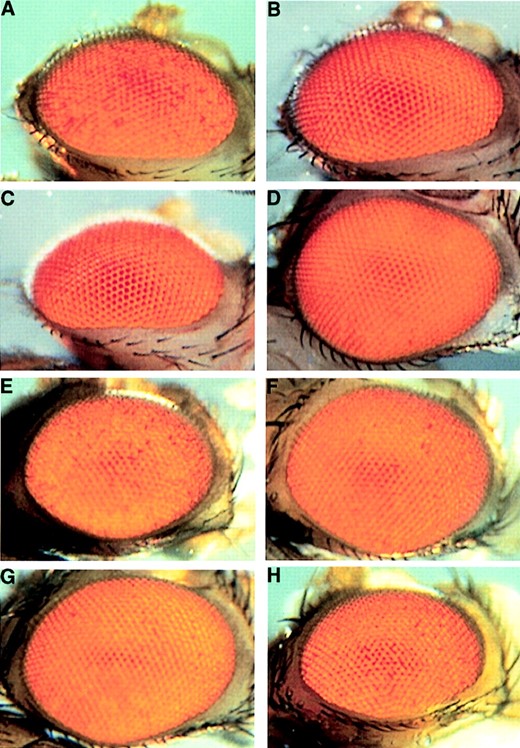
We wondered if intrahomolog pairing of bw regions (i.e., between the transposition located in pericentric heterochromatin and the distal reporter on the same chromosome) would also result in trans-inactivation. To address this issue, we used recombinant derivatives of TpDX7 (Table 1). The phenotype of TpDX7/bw+ individuals is indistinguishable from that of DpDX7bw+/Df DX7 (Figure 4A). This indicates that inactivation of a wild-type bw gene mediated by DpDX7 (the chromosomal segment transposed to heterochromatin) occurs similarly whether it is on the same chromosome or on the homologous chromosome. Furthermore, this implies that the region included in DpDX7 is large enough to efficiently pair with homologous sequences at the tip of 2R.
The recombinant derivatives of TpDX7 also revealed that both DpDX7 and Df DX7 must be present for trans-inactivation to occur: DpDX7bw+/bw+ individuals are wild type and do not exhibit bw inactivation (Figure 4B). There are at least two possible reasons for this requirement. Reduction of bw+ function might be necessary to reveal trans-inactivation by DpDX7. This predicts that any null mutation of bw will expose trans-inactivation in combination with DpDX7bw+. We tested this hypothesis by crossing DpDX7bw+ to four bw alleles. Flies of all allelic combinations failed to show variegation, including the null mutants bw1 (Figure 4C) and bw2b (data not shown). Although the molecular defect of three of these alleles is unknown, the bw1 allele makes normal amounts of a truncated transcript (Dreesen et al. 1988). Therefore, the failure to see trans-inactivation in genotypes with two copies of the endogenous bw locus is not because an increased amount of bw protein conceals the phenotype, but rather silencing simply does not occur.
An alternative possibility is that a deficiency of or near bw in trans to the reporter might be necessary to expose the reporter, enhancing its susceptibility to pair with the transposed segment and be trans-inactivated. To test this, we made individuals heterozygous for DpDX7bw+ and each of several different deficiencies in this region and examined their eye phenotypes. We chose deficiencies that had been generated in a bw1 null background, allowing us to assess the phenotypic inactivation of the distal bw+ gene in DpDX7bw+. Deficiencies of regions either proximal or distal to bw do not show trans-inactivation in combination with DpDX7bw+ (data not shown and Figure 4D, respectively). However, one deficiency, Df(2R)or-BR6, did show bw+ inactivation when heterozygous with DpDX7bw+ (Figure 4E). This deficiency removes the bw locus, as though deletion of the bw+ gene itself is necessary for trans-inactivation of the reporter.
—Diagrammatic depiction of heterozygous combinations of 2R homologs referred to in the text. Heterochromatin is depicted as a wavy line, the centromere as a circle, DpDX7 as an open triangle, bwD as a filled triangle, deficiencies as parentheses, and a bw+ transgene as a vertical line. Genotypes are categorized by their degree of silencing (in parentheses), where category 0 appears wild type and category 6 appears almost completely unpigmented. The actual position of the bw+ gene within DpDX7 component has not been determined.
Df(2R)or-BR6 is comparable in size to Df DX7. The large size of these deficiencies might contribute to trans-inactivation by destabilizing somatic pairing in the region. Therefore, we examined the effect of a smaller deficiency of the bw gene on inactivation by DpDX7. Df(2R)bw5 (bw5) is a deficiency with its proximal breakpoint in the coding region of the bw gene, deleting the 5′ end (A. K. Csink and P. B. Talbert, unpublished data) and extending distally to 59F1, (Dreesen et al. 1988). DpDX7bw+/bw5 heterozygotes show weak trans-inactivation relative to DpDX7bw+/Df DX7 individuals (Figure 4F). Inactivation was similarly weak in DpDX7bw5/bw+ heterozygotes (data not shown). The greater silencing seen with Df DX7 than with bw5 might be caused either by the larger size of the Df DX7 deficiency or by the absence of the bw+ gene in Df DX7.
To determine whether deficiency size or deletion of the bw sequence is important for trans-inactivation of the reporter, we used Df(2R)egl2, which removes the same region as Df DX7 (59E-60A1, Flybase). The presence of the bw gene in this deficiency cannot be determined phenotypically because it was generated in a bw1 background. Trans-inactivation in DpDX7bw+/Df(2R)egl2 individuals is weaker than in DpDX7bw+/Df DX7 (Figure 4G). Because these deficiencies uncover the same region, we conclude that their different phenotypic effects are attributable to proximal sequences, such as the bw gene, deleted from Df DX7 that may still be present in Df(2R)egl2. Consistent with this interpretation, Df(2R)egl2 and bw5 have breakpoints near bw and display similar trans-inactivation when heterozygous with DpDX7bw+, even though Df(2R)egl2 is about twice the size of bw5. Larger deletions might enhance trans-inactivation by better exposing the reporter as a consequence of freeing up flanking sequences, which would generate a loop. As a result, the exposed region would pair better with the heterochromatically embedded transposition, thus subjecting the reporter to trans-inactivation.
If the role of flanking sequence is to interact with the transposition, then a transgene that lacks flanking sequences would escape trans-inactivation. Indeed, ectopic transgene insertions are not inactivated at all when heterozygous with TpDX7 (data not shown). Similar observations have been made for other variegating bw mutations (Dreesen et al. 1991). Lack of transgene trans-inactivation is not due to inherent resistance of ectopic copies of bw+ to silencing by DpDX7, because a transgene insertion into 59E1-2, the band that contains brown, is trans-inactivated (Figure 4H). In this case, the transgene is surrounded by 59E-60A flanking sequences, which can pair with the heterochromatically embedded transposition. Sequences important for trans-inactivation have been previously mapped to this transgene segment, close to the 5′ end of the brown gene (Dreesen et al. 1991; Martin-Morris et al. 1993). These observations with transgenes emphasize the crucial role played by sequences flanking brown in pairing with the transposition and subjecting the reporter to trans-inactivation.
PEV modifiers reveal differences between cis- and trans-inactivation for bwDX7: To further characterize the bwDX7 mutation, we tested the effect of known modifiers of PEV in combination with TpDX7. Suvar3-7 and Suvar2-5 are mutations in genes that encode protein components of heterochromatin (Weiler and Wakimoto 1995). Both suppressed trans-inactivation in TpDX7/bw+ individuals (data not shown). Thus, decreasing the dose of a specific heterochromatic protein relieves the trans-inactivation of bw+ imposed by the bwDX7 transposition. In contrast, Evar3-93D and Trithorax-like failed to enhance (data not shown). These results with bwDX7 are similar to those reported previously for the bwD mutation (Sass and Henikoff 1998).
Chromosomes used in this study
| Name . | Description |
|---|---|
| TpDX7 | Tp(2;2)bwDX7; transposition of chromosomal segment 59E to 60A into 2R centromeric heterochromatin |
| DpDX7bw+ | Dp(2;2)bwDX7, bw+ bw+; derivative contains both the bwDX7 duplication and the endogenous 59E to 60A region |
| DfDX7 | Df(2R)bwDX7; derivative that carries only the deficiency of 59E to 60A |
| DpDX7bw5 | Dp(2;2)bwDX7, bw+ bw5; derivative contains both the bwDX7 duplication and the bw5 mutation |
| DpDX7bwD | Dp(2;2)bwDX7, bw+ bwD ; derivative contains both the bwDX7 duplication and the bwD mutation |
| bw+ | Wild-type chromosome from Amherst strain |
| bwD | Null allele of bw caused by a >1-Mb heterochromatic insertion |
| bw1 | Insertion of ̃8 kb into the bw gene creating a null mutation |
| bw5 | Df(2R)bw5; deficiency from 5′ region of bw to 59F1 |
| bw2b | Null allele of bw |
| bw75 | Hypomorphic allele of bw |
| bw81 | Hypomorphic allele of bw |
| Df(2R)Pu-D17 | Deficiency that removes polytene bands 57B5 to 58B1-58B2 |
| Df(2R)or-BR11 | Deficiency that removes polytene bands 59F6-59F8 to 60A8-60A16 |
| Df(2R)or-BR6 | Deficiency that removes polytene bands 59D5 to 60B3-60B8 |
| Df(2R)egl2 | Deficiency that removes polytene bands 59E-60A1 |
| P{bw+R}59E | Insertion of a plasmid containing an 8.4-kb bw genomic fragment into 59E |
| YSX ∙ YL, In(1)EN | Attached X and Y chromosome used in the Y chromosome effect study |
| Su(var)2-502 | Mutation in the gene encoding Heterochromatin Protein 1 |
| Su(var)3-7 | Mutation in a gene encoding a heterochromatic protein |
| E(var)3-93D | Mutation in a gene encoding a protein containing a BTB domain |
| Trithorax-like | Mutation in a gene encoding the GAGA protein |
| P{neoR}HSHP1.83C | HP1 transgene under the control of the Hsp70 promoter |
| Name . | Description |
|---|---|
| TpDX7 | Tp(2;2)bwDX7; transposition of chromosomal segment 59E to 60A into 2R centromeric heterochromatin |
| DpDX7bw+ | Dp(2;2)bwDX7, bw+ bw+; derivative contains both the bwDX7 duplication and the endogenous 59E to 60A region |
| DfDX7 | Df(2R)bwDX7; derivative that carries only the deficiency of 59E to 60A |
| DpDX7bw5 | Dp(2;2)bwDX7, bw+ bw5; derivative contains both the bwDX7 duplication and the bw5 mutation |
| DpDX7bwD | Dp(2;2)bwDX7, bw+ bwD ; derivative contains both the bwDX7 duplication and the bwD mutation |
| bw+ | Wild-type chromosome from Amherst strain |
| bwD | Null allele of bw caused by a >1-Mb heterochromatic insertion |
| bw1 | Insertion of ̃8 kb into the bw gene creating a null mutation |
| bw5 | Df(2R)bw5; deficiency from 5′ region of bw to 59F1 |
| bw2b | Null allele of bw |
| bw75 | Hypomorphic allele of bw |
| bw81 | Hypomorphic allele of bw |
| Df(2R)Pu-D17 | Deficiency that removes polytene bands 57B5 to 58B1-58B2 |
| Df(2R)or-BR11 | Deficiency that removes polytene bands 59F6-59F8 to 60A8-60A16 |
| Df(2R)or-BR6 | Deficiency that removes polytene bands 59D5 to 60B3-60B8 |
| Df(2R)egl2 | Deficiency that removes polytene bands 59E-60A1 |
| P{bw+R}59E | Insertion of a plasmid containing an 8.4-kb bw genomic fragment into 59E |
| YSX ∙ YL, In(1)EN | Attached X and Y chromosome used in the Y chromosome effect study |
| Su(var)2-502 | Mutation in the gene encoding Heterochromatin Protein 1 |
| Su(var)3-7 | Mutation in a gene encoding a heterochromatic protein |
| E(var)3-93D | Mutation in a gene encoding a protein containing a BTB domain |
| Trithorax-like | Mutation in a gene encoding the GAGA protein |
| P{neoR}HSHP1.83C | HP1 transgene under the control of the Hsp70 promoter |
Except as noted, chromosomes and mutations are described in FlyBase.
Chromosomes used in this study
| Name . | Description |
|---|---|
| TpDX7 | Tp(2;2)bwDX7; transposition of chromosomal segment 59E to 60A into 2R centromeric heterochromatin |
| DpDX7bw+ | Dp(2;2)bwDX7, bw+ bw+; derivative contains both the bwDX7 duplication and the endogenous 59E to 60A region |
| DfDX7 | Df(2R)bwDX7; derivative that carries only the deficiency of 59E to 60A |
| DpDX7bw5 | Dp(2;2)bwDX7, bw+ bw5; derivative contains both the bwDX7 duplication and the bw5 mutation |
| DpDX7bwD | Dp(2;2)bwDX7, bw+ bwD ; derivative contains both the bwDX7 duplication and the bwD mutation |
| bw+ | Wild-type chromosome from Amherst strain |
| bwD | Null allele of bw caused by a >1-Mb heterochromatic insertion |
| bw1 | Insertion of ̃8 kb into the bw gene creating a null mutation |
| bw5 | Df(2R)bw5; deficiency from 5′ region of bw to 59F1 |
| bw2b | Null allele of bw |
| bw75 | Hypomorphic allele of bw |
| bw81 | Hypomorphic allele of bw |
| Df(2R)Pu-D17 | Deficiency that removes polytene bands 57B5 to 58B1-58B2 |
| Df(2R)or-BR11 | Deficiency that removes polytene bands 59F6-59F8 to 60A8-60A16 |
| Df(2R)or-BR6 | Deficiency that removes polytene bands 59D5 to 60B3-60B8 |
| Df(2R)egl2 | Deficiency that removes polytene bands 59E-60A1 |
| P{bw+R}59E | Insertion of a plasmid containing an 8.4-kb bw genomic fragment into 59E |
| YSX ∙ YL, In(1)EN | Attached X and Y chromosome used in the Y chromosome effect study |
| Su(var)2-502 | Mutation in the gene encoding Heterochromatin Protein 1 |
| Su(var)3-7 | Mutation in a gene encoding a heterochromatic protein |
| E(var)3-93D | Mutation in a gene encoding a protein containing a BTB domain |
| Trithorax-like | Mutation in a gene encoding the GAGA protein |
| P{neoR}HSHP1.83C | HP1 transgene under the control of the Hsp70 promoter |
| Name . | Description |
|---|---|
| TpDX7 | Tp(2;2)bwDX7; transposition of chromosomal segment 59E to 60A into 2R centromeric heterochromatin |
| DpDX7bw+ | Dp(2;2)bwDX7, bw+ bw+; derivative contains both the bwDX7 duplication and the endogenous 59E to 60A region |
| DfDX7 | Df(2R)bwDX7; derivative that carries only the deficiency of 59E to 60A |
| DpDX7bw5 | Dp(2;2)bwDX7, bw+ bw5; derivative contains both the bwDX7 duplication and the bw5 mutation |
| DpDX7bwD | Dp(2;2)bwDX7, bw+ bwD ; derivative contains both the bwDX7 duplication and the bwD mutation |
| bw+ | Wild-type chromosome from Amherst strain |
| bwD | Null allele of bw caused by a >1-Mb heterochromatic insertion |
| bw1 | Insertion of ̃8 kb into the bw gene creating a null mutation |
| bw5 | Df(2R)bw5; deficiency from 5′ region of bw to 59F1 |
| bw2b | Null allele of bw |
| bw75 | Hypomorphic allele of bw |
| bw81 | Hypomorphic allele of bw |
| Df(2R)Pu-D17 | Deficiency that removes polytene bands 57B5 to 58B1-58B2 |
| Df(2R)or-BR11 | Deficiency that removes polytene bands 59F6-59F8 to 60A8-60A16 |
| Df(2R)or-BR6 | Deficiency that removes polytene bands 59D5 to 60B3-60B8 |
| Df(2R)egl2 | Deficiency that removes polytene bands 59E-60A1 |
| P{bw+R}59E | Insertion of a plasmid containing an 8.4-kb bw genomic fragment into 59E |
| YSX ∙ YL, In(1)EN | Attached X and Y chromosome used in the Y chromosome effect study |
| Su(var)2-502 | Mutation in the gene encoding Heterochromatin Protein 1 |
| Su(var)3-7 | Mutation in a gene encoding a heterochromatic protein |
| E(var)3-93D | Mutation in a gene encoding a protein containing a BTB domain |
| Trithorax-like | Mutation in a gene encoding the GAGA protein |
| P{neoR}HSHP1.83C | HP1 transgene under the control of the Hsp70 promoter |
Except as noted, chromosomes and mutations are described in FlyBase.
We increased the levels of HP1 (encoded by Suvar2-5) by using an HP1 transgene inducible by heat shock (Eissenberg et al. 1992). Trans-inactivation in TpDX7/bw+ individuals carrying this transgene is strongly enhanced when they are heat shocked throughout development (Figure 5A). Enhancement with increased HP1 was also seen in DpDX7bw5/bw+ flies (Figure 5B). We tested whether induction of the transgene might induce variegation in genotypes that showed complete suppression, like DpDX7bw+/bw+. There was no effect (data not shown). This extends our previous assertion that inactivation of bw+ by the bwDX7 transposition requires, and is not simply enhanced by, a deficiency of the bw locus.
We also examined chromosomal modifiers of PEV. Y chromosome dosage modifies PEV, with XXY females showing suppressed variegation and X0 males showing enhancement (Spofford 1976). We examined the effects of changing Y dosage on trans-inactivation of the bw+ reporter. X.Y/X;TpDX7/bw+ showed suppressed variegation, as expected (Figure 5C). However, the absence of the Y chromosome in X/0;TpDX7/bw+ males did not result in enhancement, rather it weakly suppressed silencing (Figure 5D). We also examined Df(2R)M41A10, which enhances the phenotypes of both white (w) and bw PEV mutations (Lindsley et al. 1960). We found that there was no effect of Df(2R)M41A10 on bw inactivation in TpDX7/Df(2R)M41A10 individuals (data not shown). These findings led us to investigate what effect Y chromosome dosage would have on the cis-inactivation of bw+ within the bwDX7 transposition. Cis-inactivation can be seen in TpDX7/bw1 heterozygotes. Like other bw variegating mutations, cis-inactivation of bw+ in DpDX7 is strong (Figure 1B). Females of the genotype X.Y/X;TpDX7/bw1 showed suppressed cis-inactivation, as expected (Figure 5E). We also found that X0 males showed enhanced cis-inactivation (Figure 5F). Thus, cis-inactivation in DpDX7 is enhanced with loss of heterochromatin from the genome, whereas trans-inactivation is not. Perhaps only direct changes in the concentration of specific heterochromatin components, such as HP1, can modify trans-inactivation in TpDX7/bw+.
bwD enhances silencing in bwDX7: In addition to investigating modification by changes in the amount of heterochromatin in the genome as a whole, we examined the effects of heterochromatin within the bw gene itself using the heterochromatic insertion allele bwD. TpDX7/ bwD heterozygotes were more strongly silenced than TpDX7/bw1 heterozygotes (data not shown). Such enhancement is opposite from what is expected for addition of extra heterochromatin to the genome and indicates that the large bwD heterochromatic insertion can directly contribute to silencing of the copy of bw+ in the transposition. This long-range interaction between a proximal copy of bw and distal heterochromatin presumably results from pairing between DpDX7 and sequences flanking bwD. Our interpretation is supported by examination of the phenotypes of other configurations involving bwDX7 and bwD. For instance, DpDX7bwD/bw+ shows stronger trans-inactivation than bwD/bw+ (735 vs. 1346 pigmented ommatidia for 20 individuals of each genotype, P < 0.0001), in spite of the fact that it contains an additional copy of bw+ that must be silenced. To account for this difference, we suppose that bwD and 2R heterochromatin surrounding bwDX7 act additively to enhance silencing of both the reporter and the bw+ copy in the transposition.
—Analysis of genetic requirements for trans-inactivation. (A) Eye from a DpDX7bw+/Df DX7 individual. The phenotype is indistinguishable from that of TpDX7/bw+ (Figure 1A), showing that trans-inactivation is not affected whether pairing between the bw+ snare and the bw+ reporter is interchromosomal or intrachromosomal. (B) Eye from a DpDX7bw+/bw+ individual. There is no detectable trans-inactivation. (C) Eye from a DpDX7bw+/bw1 individual, demonstrating that activity of the bw gene on the homolog is not necessary for a wild-type phenotype. (D) Eye from a DpDX7bw+/Df(2R)or-BR11 individual, demonstrating that deletion of sequences distal to the bw gene does not reveal trans-inactivation. (E) Eye from a DpDX7bw+/Df(2R)or-BR6 individual, demonstrating that a deletion of comparable size to Df DX7, which also removes the bw gene, results in trans-inactivation that is indistinguishable from that seen for TpDX7/bw+ individuals. Trans-inactivation of the bw+ reporter in DpDX7bw+ is suppressed when heterozygous to either (F) bw5 or (G) Df(2R)egl2. (H) Eye from a TpDX7/ P{bw+R}59E individual, showing trans-inactivation of a mislocalized transgene at 59E.
Pairing and long-range associations involving bwDX7: Trans-inactivation at the bw locus has been proposed to be a consequence of somatic pairing of homologous chromosomes, followed by recruitment of the paired bw locus into a heterochromatic compartment of the nucleus, where the bw gene cannot be expressed (Csink and Henikoff 1998). Recruitment to a heterochromatic compartment is thought to be mediated by ectopic heterochromatin near the bw locus on one of the homologs. Trans-inactivation of a bw+ gene by the bwD mutation is correlated with its nuclear localization to centromeric heterochromatin (Csink and Henikoff 1996; Dernburg et al. 1996). This model for trans-inactivation predicts that those cells that continue to express the bw gene are those in which bw has not been relocated to a heterochromatic compartment.
While the heterochromatic insertion in bwD appears to relocate the endogenous bw gene to a heterochromatic compartment by heterochromatin-mediated associations, DpDX7 relocates the endogenous bw region to a compartment by homologous pairing forces. Based on the bwD model, the very low amount of trans-inactivation in TpDX7/bw+ individuals might be due to a decrease in the frequency of somatic pairing between the bw+ reporter on the homolog and the snare-like transposition. This would account for reduced trans-inactivation, because the endogenous bw+ reporter could escape association with a heterochromatic compartment.
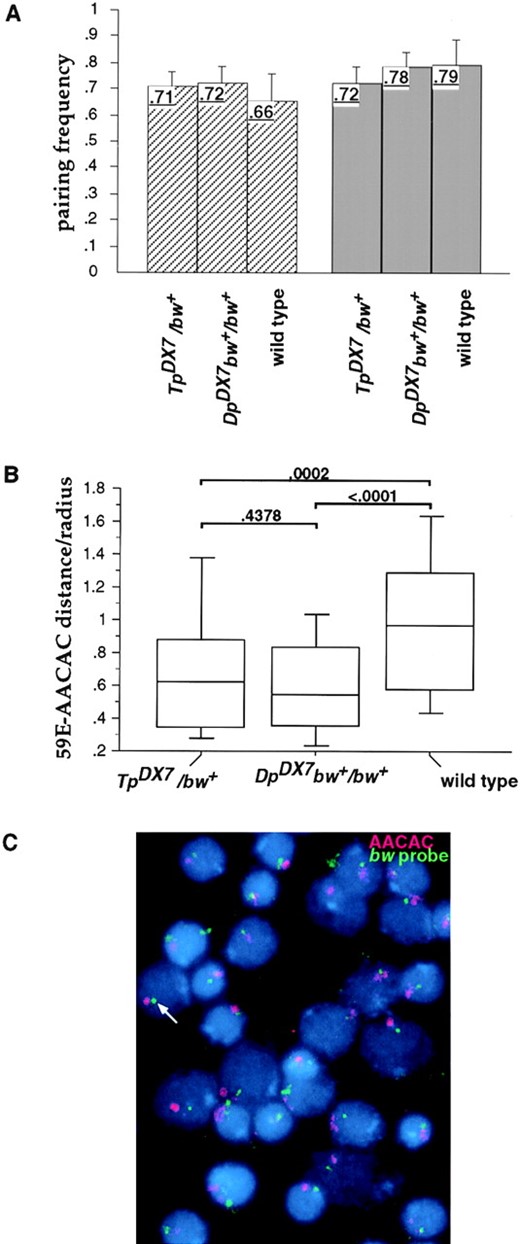
To directly test this model, we measured the frequency of somatic pairing between DpDX7 and the distal bw locus using FISH with a probe to 59E. There was no significant difference in the frequency of pairing of the bw region in third instar larval neuroblasts of TpDX7/bw+, DpDX7bw+/bw+, and wild-type individuals (Figure 6A). In all three genotypes somatic pairing was seen as a single hybridization signal in 70-80% of the nuclei. This result is surprising because individuals of the genotype TpDX7/ bw+ exhibit trans-inactivation, but DpDX7bw+/bw+ individuals are wild type. Importantly, this frequency of somatic pairing is not significantly different from that reported for bwD/bw+ individuals (Csink and Henikoff 1998), a genotype in which bw is trans-inactivated in ∼98% of the pigment cells. That is, somatic pairing is indistinguishably high among genotypes that span a range of trans-inactivation from 0 (DpDX7bw+/bw+) to ∼98% (bwD/ bw+). We conclude that differences in trans-inactivation do not result from differences in the frequency of somatic pairing between the bw+ reporter on the homolog and the heterochromatically embedded transposition.
—Analysis of modification of the bwDX7 phenotype by modifiers of PEV. Eyes of heat-shocked individuals with (left side in A and B) or without (right side) an HP1 transgene controlled by a heat-shock promoter. Increased HP1 enhances trans-inactivation of (A) TpDX7/bw+ and (B) DpDX7bw5/bw+. (C) Suppression of trans-inactivation in TpDX7/bw+ by addition of a Y chromosome: eye of XXY female (left) and eye of XX female (right). (D) Lack of enhancement of trans-inactivation in TpDX7/bw+ with removal of a Y chromosome: eye of X0 male (left) and eye of XY male (right). (E) Suppression of cis-inactivation in TpDX7/bw+ by addition of a Y chromosome: eye of XXY female (left) and eye of XX female (right). (F) Enhancement of cis-inactivation in TpDX7/bw+ with removal of a Y chromosome: eye of X0 male (left) and eye of XY male (right). Flies are st+, except for st- flies shown in E and F.
Because the frequency of somatic pairing of DpDX7 and the endogenous bw region appeared normal, we considered the possibility that the paired locus may be pulled away from the centric heterochromatin. We examined the relative positions of the bw region and pericentric heterochromatin on interphase chromosomes of third instar larval neuroblasts using FISH. The bw region and centromeric heterochromatin of chromosome 2 were detected with probes to 59E and the satellite sequence AACAC, respectively (Figure 6). The nuclear distance between 59E and AACAC in wild-type, TpDX7/bw+, and DpDX7bw+/bw+ individuals was determined. These sites were significantly closer together in DpDX7-bearing cells than in wild type (P < 0.001, Figure 6B). DpDX7 relocates the bw locus to centromeric heterochromatin with a mean distance between 59E and AACAC that is comparable to that measured in bwD-bearing cells (Csink and Henikoff 1998). Furthermore, there is no effect of Df DX7 on the 59E-AACAC distance (P = 0.47, Figure 6B). Therefore, the differences observed in trans-inactivation do not correspond to detectable alterations in interphase configurations.
DISCUSSION
Chromatin associations fall into two general categories: homologous pairing and heterochromatic coalescence. Homologous pairing is the force underlying numerous phenomena, referred to variously as transvection (Lewis 1954), trans-sensing (Henikoff 1996), and topology effects (Morris et al. 1998). Although the mechanism of homologous pairing is not understood, the determinants responsible for these phenomena are often well mapped, typically within gene regulatory regions (Jack and Judd 1979; Geyer et al. 1990; Gindhart and Kaufman 1995; Kapoun and Kaufman 1995, Sigrist and Pirrotta 1997). In contrast, determinants for coalescence of heterochromatin have not been localized. For example, the chromocenter of polytene chromosomes involves coalescence of extensive regions, and multiple unknown determinants must be involved. This uncertainty hampers attempts to characterize phenomena that involve heterochromatic coalescence such as nuclear compartmentalization, which is a likely causal factor in PEV (Weiler and Wakimoto 1995). Our study addresses this problem by providing a single site at which compartmentalization and reporter gene silencing can be studied. We utilized a homologous copy of the bw gene region transposed to pericentric heterochromatin to precisely position a bw reporter within heterochromatin. Unexpectedly, we found that when the bw region is seen to be mislocalized to heterochromatin in this way, silencing may or may not result.
The expectation that mislocalization in larval brains would be correlated with silencing of an adult eye color gene is based upon cytological and genetic observations with the bwD heterochromatic insertion into the bw gene (Henikoff 1996). Heterochromatic associations are seen in multiple tissues and are responsive to chromosomal rearrangements and to suppressors or enhancers of PEV. Moreover, phenomena dependent upon somatic pairing, such as the zeste-white effect, are also visualized in the adult eye, even though white expression begins during pupal stages. These observations suggest that the nuclear organization seen in larval brains should be indicative of the state of the bw gene in the eye. However, we observed differences in levels of bw gene silencing despite similar large-scale chromosomal configurations. We conclude that these differences arise from local interactions at the bw reporter that occur within the heterochromatic “compartment” of the nucleus.
—Cytological analysis of bw pairing in larval neuroblasts. (A) Bar graph (with 95% confidence intervals) showing the mean pairing frequency of the bw region (light bars) and the 23C control region (dark bars). For each squash examined, the number of nuclei with a single hybridization signal was divided by the number of nuclei with one or two signals, and the mean frequency of single hybridization signals from multiple squashes is shown for each genotype (upper left region of each bar). More than two signals within a nucleus were only rarely detected, with frequencies that did not significantly differ between genotypes. (B) Box plots depicting the distribution of measurements of the nuclear distance between the bw region, detected by the 59E P1 probe, and pericentric heterochromatin, detected by the AACAC probe. Each measured distance was divided by the nuclear radius. Measurements were made using the same images collected for analysis of pairing frequency. The bottom of the box shows the 25th percentile, the top shows the 75th percentile, the horizontal line shows the median, and the tails show the 10th (lower) and 90th percentiles. These measurements were subjected to the Mann-Whitney U-test and the resulting P values are given over brackets. (C) A typical image (from DpDX7/bw+) used to obtain the data for pairing frequency and nuclear distance measurements. The bw region probe is in green and a single signal is indicated (arrow); the pericentric AACAC probe is in red.
Our genetic analysis of bwDX7 provides insight as to what might be happening on a local level to the bw+ reporter brought into the vicinity of centromeric heterochromatin. Silencing by heterochromatin surrounding the transposition is only revealed when the bw reporter lacks a normal pairing partner. When somatic pairing between the bw reporter and its pairing partner on the homologous chromosome is reduced by a deficiency, the reporter becomes more strongly silenced by heterochromatin surrounding the transposition. This effect must occur at a local level, because the frequency of somatic pairing detected by FISH is the same as wild-type whether or not the deficiency is present.
We consider two models to explain how local changes in homologous pairing at a bw gene in proximity to centromeric heterochromatin can result in differences in silencing. In the euchromatic bubble model, pairing between DpDX7 and the endogenous 59E-60A region creates a euchromatic environment that buffers the effects of heterochromatin. When the bw+ reporter is ensnared in the absence of a third copy of bw on the homologous chromosome, the reporter is susceptible to silencing by the surrounding heterochromatic environment. But in the presence of a third copy, the increased amount of euchromatin facilitates formation of a “bubble” that provides a buffer against silencing of the reporter. This buffering might result from heightened gene activity. This model is consistent with the observation that increasing the levels of HP1 enhances the phenotype of two-copy individuals, where the weak euchromatic environment would be unstable, but not three-copy individuals, where the stronger euchromatic environment would be stable. However, the euchromatic bubble model is not consistent with the similar phenotypes observed for two deficiencies, bw5 and Df(2R)egl2, that are very different in size.
In the local unpairing model, the bw+ reporter escapes trans-inactivation when it unpairs from its homolog present in the heterochromatically embedded transposition. Increased unpairing in the vicinity of the reporter would occur when an additional bw gene region that is present on the paired homolog competes for the reporter. This model is supported by the observation that two-copy (TpDX7/bw+) individuals exhibit a dilute background level of bw+ pigmentation (e.g., Figure 4A) that is equivalent to that seen for single-copy flies [e.g. Df(bw)/bw+; Slatis 1955; P. Talbert, personal communication]: Local unpairing would result in escape of the bw reporter from trans-inactivation, leaving behind the silenced bw+ copy in the transposition. The euchromatic bubble model does not predict this phenotype, because all copies in a bubble should contribute gene product.
Local unpairing is an attractive model to account for our results because it has been implicated in other examples of transvection and trans-inactivation. For instance, it has been suggested that transvection at the yellow locus occurs by local unpairing of the promoter, which allows increased access to transcription factors (Morris et al. 1999). “Breathing” of the yellow promoter in the unpaired state would allow it to capture an enhancer in trans. Similarly, breathing of the brown promoter in the unpaired state would allow its capture by the transposition, subjecting it to heterochromatic silencing. Previously, a local unpairing model was proposed for trans-inactivation of a bw+ transgene reporter: silencing weakens in proportion to the amount of transgene sequence that is deleted from the paired heterochromatin-linked copy (Dreesen et al. 1991). In this case, deletion was hypothesized to cause the formation of an unpaired loop containing the reporter, allowing it to escape silencing by heterochromatin. Deletions of only a few kilobases were effective, revealing that local changes in pairing could modify silencing. In the present study, where a bw+ reporter was mislocalized to a compartment some tens of megabases away, we nevertheless found that local pairing interactions determine the silencing phenotype.
Local interactions do not entirely account for silencing of the reporter. Previous studies documented a long-range enhancement of silencing by bwD, interpreted as coalescence of bwD with 2R heterochromatin, and coalescence was confirmed in cytological studies (Henikoff 1996). However, it was not clear that bwD could contribute to silencing beyond its participation in coalescence. Here we have shown that bwD can indeed contribute to silencing, because silencing was enhanced when this heterochromatic element was ensnared by the transposition. This result is remarkable considering that bwD enhanced trans-inactivation even though the transposition contains an extra copy of bw to be silenced. Thus, silencing appears to result from a combination of large-scale heterochromatic and local pairing interactions.
Acknowledgement
Zheng Fan performed data collection on nuclei. We thank Kami Ahmad for insightful discussions. This work was supported by the Howard Hughes Medical Institute.
Footnotes
Communicating editor: T. C. Kaufman
LITERATURE CITED