-
PDF
- Split View
-
Views
-
Cite
Cite
Wei-Chiang Shen, Jenny Wieser, Thomas H Adams, Daniel J Ebbole, The Neurospora rca-1 Gene Complements an Aspergillus flbD Sporulation Mutant but Has No Identifiable Role in Neurospora Sporulation, Genetics, Volume 148, Issue 3, 1 March 1998, Pages 1031–1041, https://doi.org/10.1093/genetics/148.3.1031
Close - Share Icon Share
Abstract
The Aspergillus nidulans flbD gene encodes a protein with a Myb-like DNA-binding domain that is proposed to act in concert with other developmental regulators to control initiation of conidiophore development. We have identified a Neurospora crassa gene called rca-1 (regulator of conidiation in Aspergillus) based on its sequence similarity to flbD. We found that N. crassa rca-1 can complement the conidiation defect of an A. nidulans flbD mutant and that induced expression of rca-1 caused conidiation in submerged A. nidulans cultures just as was previously observed for overexpression of flbD. Thus, the N. crassa gene appears to be a functional homologue of A. nidulans flbD and this is the first demonstration of functional complementation of an A. nidulans sporulation defect using a gene from an evolutionarily distant fungus. However, deletion of the rca-1 gene in N. crassa had no major effect on growth rate, macroconidiation, microconidiation, or ascospore formation. The only phenotype displayed by the rca-1 mutant was straight or counterclockwise hyphal growth rather than the clockwise spiral growth observed for wild type. Thus, if rca-1 is involved in N. crassa development, its role is subtle or redundant.
NEUROSPORA crassa and Aspergillus nidulans are filamentous fungi that provide simple genetic systems for the molecular genetic examination of fungal multicellular development. N. crassa produces two types of asexual spores; multinucleate macroconidia that are spherical to barrel-shaped and 5 to 10 μm in diameter, and uninucleate microconidia that are pyriform to spherical in shape and 2 to 3 μm in diameter (Springer 1993). During macroconidiation, N. crassa produces a conidiophore that is composed of simple aerial hyphae that branch symmetrically. In synchronous cultures, the growth mode of these elongating aerial hyphae switches from the filamentous form to a budding growth form about 8 hr after induction of development, leading to the production of proconidial chains. These chains of cells then separate by cleavage of a double cell wall layer that is deposited between the cells, releasing the macroconidia. In contrast, microconidia are produced from simple branched structures called microconidiophores. Microconidiophores are most often overlooked in culture because the massive canopy of macroconidiophores masks the microconidiophores that form in older cultures. The microconidiophores include specialized cells called phialides that can bud repeatedly to release chains of spores (Rossier et al. 1977; Springer 1993).
A. nidulans conidiation proceeds through formation of phialides that bud repeatedly to produce long chains of spores that are spherical in shape and ~2 μm in diameter. However, the process differs from N. crassa conidiation in that the spore-producing A. nidulans phialides are elaborated on highly organized conidiophores comprised of multiple cell types (Adams 1995). A. nidulans conidiophore development begins with formation of the stalk cell which is a specialized aerial hypha. The swollen tip of the stalk cell is called the vesicle and multiple buds are formed on the vesicle surface to produce a layer of cells called metulae which in turn bud to give rise to the phialide cells. Conidiation in a growing A. nidulans colony is governed by a well-defined genetic program that controls expression of a set of regulatory genes required for morphogenesis of the conidiophore (Adams 1995). The central regulators of conidiophore morphogenesis are brlA, abaA, and wetA and induction of development depends on activation of brlA (Adams 1995). brlA activates expression of abaA, which in turn stimulates both brlA expression to higher levels and activates wetA (Mirabito et al. 1989). Genes involved in activation of brlA were identified in screens for aconidial mutants with reduced brlA expression and include fluG, flbA, flbB, flbC, flbD, and flbE (Wieser et al. 1994). Mutations in any of the four genes flbB, flbC, flbD, or flbE result in colonies with similar phenotypes that are characterized by abundant aerial hyphae formation and some conidiation, but only after a substantial delay (Wieser et al. 1994). flbD has been characterized and shown to encode a 314 amino acid protein with two repeats of the DNA binding domain found in the Myb class of transcription factors (Wieser and Adams 1995). In plants, Myb-related proteins regulate a variety of activities related to morphogenesis, including tissue-specific production of anthrocyanin pigments (Paz-Ares et al. 1987) and trichome differentiation (Oppenheimer et al. 1991). Induced expression of flbD under control of the alcA promoter caused A. nidulans to conidiate in liquid-grown cultures where conidiation is normally suppressed, leading to the proposal that flbD regulates brlA expression and conidiophore morphogenesis.
Much less is known about the genes controlling macroconidiation in N. crassa (Springer and Yanofsky 1989). Several mutants are known that specifically interfere with macroconidiation. acon-2 (aconidiate) and fld (fluffyoid) mutants are arrested during macroconidiation at the earliest stages of development. acon-3 and fl (fluffy) mutants are blocked in the budding stage of macroconidiation. csp-1 (conidial separation) and csp-2 are blocked at the latest stage of development, release of macroconidia. Of these, only the fl gene has been cloned (L. A. Bailey and D. J. Ebbole, unpublished results).
Here, we attempt to address the question of whether ascomycetous fungi as distantly related as A. nidulans and N. crassa share common genetic strategies for controlling asexual sporulation. We used a number of the known regulators of conidiation in A. nidulans as heterologous probes for hybridization experiments in N. crassa. flbD was the only gene that was found to cross-hybridize with N. crassa genomic DNA and we cloned and characterized an N. crassa gene with sequence similarity to A. nidulans flbD. The gene, rca-1 (regulator of conidiation in Aspergillus), functionally complemented an A. nidulans flbD mutant. However, no major role for the gene in regulation of conidiation was observed in N. crassa. A subtle but readily scorable phenotype of rca-1 mutants was a reduction in clockwise axial growth of hyphae in young colonies.
MATERIALS AND METHODS
Fungal strains, growth conditions, and genetic techniques: Restriction fragment length polymorphism (RFLP) mapping strains of N. crassa were obtained from the Fungal Genetics Stock Center (FGSC; Kansas City, KS). The rca-1 deletion mutant WC1 was crossed to the pe, fl strain FGSC 5511 to generate pe, fl and pe, fl; Δrca-1 progeny that were examined for microconidiation and spiral growth. All other fungal strains used in this study are listed in Table 1. N. crassa strains were maintained by standard procedures (Davis and de Serres 1970) on Vogel's minimal medium or supplemented media as indicated. Standard genetic techniques (Davis and de Serres 1970; Pontecorvo et al. 1953) and protoplast transformation procedures (Yelton et al. 1984; Vollmer and Yanofsky 1986) were used. All A. nidulans strains were grown at 37° in minimal medium (Käfer 1977) or complete medium (Lee and Adams 1994a). Spiral growth of N. crassa was examined as previously described (Yamashiro et al. 1996).
For induced expression in A. nidulans, alcA(p) fusions were induced by inoculating conidia (1 × 106 conidia/ml) into minimal medium containing 50 mM glucose and shaking at 300 rpm for 14 hr. Cells were harvested by centrifugation, washed with minimal medium without glucose, and transferred to medium containing 100 mM L-threonine for induction. Samples were harvested for microscopic examination and photography 14 hr after the shift to threonine medium.
Growth rates of the rca-1 and wild-type N. crassa strains were measured in race tubes as described (White and Woodward 1995). Macroconidiation of the rca-1 mutant grown on several media was compared with that of the wild-type strain. Macroconidial yield was also tested for cultures grown at 34° and 25° and cultures grown in constant light and constant dark. The number of macroconidia were quantitated from cultures
Strains used in this study
| Strain | Description or genotype | Source |
| N. crassa | ||
| 74-OR23-1A | mating type A | FGSC |
| ORS6a | mating type a | FGSC |
| FGSC7023 | a; fld, delayed conidiation | FGSC |
| FGSC5511 | a; pe, fl | FGSC |
| WC1 | Δrca-1 transformant | This study |
| WC11-WC15 | Δrca-1 progeny of WC1 × ORS6a | This study |
| DE41-DE45 | pe, fl; Δrca-1 progency of WC1 × FGSC5511 | This study |
| DE46-DE50 | pe, fl; Δrca-1 progeny of WC1 × FGSC5511 | This study |
| WC21 | Δrca-1; fld progeny of WC1 × fld | This study |
| A. nidulans | ||
| FGSC237 | pabaA1, yA2; trpC801, veA1 | FGSC |
| TJW29.2 | pabaA1, yΔ2; trpC::alcA(p)::flbD, veA1 | Wieser and Adams (1995) |
| RBN070 | biA1; argB2; flbD14, veA1 | Wieser et al. (1994) |
| RJW150 | yA1, pabaA1, flbA 100; argB2; veA1 | This study |
| TWC41 | pabaA1, yA2; trpC::alcA(p) ::rca-1, veA1 | This study |
| TWC3.1 | biA1; flbD14, veA1; rca-1 | This study |
| TX15:E2 | biA1; flbD14, veA1; rca-1 | This study |
| Strain | Description or genotype | Source |
| N. crassa | ||
| 74-OR23-1A | mating type A | FGSC |
| ORS6a | mating type a | FGSC |
| FGSC7023 | a; fld, delayed conidiation | FGSC |
| FGSC5511 | a; pe, fl | FGSC |
| WC1 | Δrca-1 transformant | This study |
| WC11-WC15 | Δrca-1 progeny of WC1 × ORS6a | This study |
| DE41-DE45 | pe, fl; Δrca-1 progency of WC1 × FGSC5511 | This study |
| DE46-DE50 | pe, fl; Δrca-1 progeny of WC1 × FGSC5511 | This study |
| WC21 | Δrca-1; fld progeny of WC1 × fld | This study |
| A. nidulans | ||
| FGSC237 | pabaA1, yA2; trpC801, veA1 | FGSC |
| TJW29.2 | pabaA1, yΔ2; trpC::alcA(p)::flbD, veA1 | Wieser and Adams (1995) |
| RBN070 | biA1; argB2; flbD14, veA1 | Wieser et al. (1994) |
| RJW150 | yA1, pabaA1, flbA 100; argB2; veA1 | This study |
| TWC41 | pabaA1, yA2; trpC::alcA(p) ::rca-1, veA1 | This study |
| TWC3.1 | biA1; flbD14, veA1; rca-1 | This study |
| TX15:E2 | biA1; flbD14, veA1; rca-1 | This study |
FGSC, Fungal Genetics Stock Center.
Strains used in this study
| Strain | Description or genotype | Source |
| N. crassa | ||
| 74-OR23-1A | mating type A | FGSC |
| ORS6a | mating type a | FGSC |
| FGSC7023 | a; fld, delayed conidiation | FGSC |
| FGSC5511 | a; pe, fl | FGSC |
| WC1 | Δrca-1 transformant | This study |
| WC11-WC15 | Δrca-1 progeny of WC1 × ORS6a | This study |
| DE41-DE45 | pe, fl; Δrca-1 progency of WC1 × FGSC5511 | This study |
| DE46-DE50 | pe, fl; Δrca-1 progeny of WC1 × FGSC5511 | This study |
| WC21 | Δrca-1; fld progeny of WC1 × fld | This study |
| A. nidulans | ||
| FGSC237 | pabaA1, yA2; trpC801, veA1 | FGSC |
| TJW29.2 | pabaA1, yΔ2; trpC::alcA(p)::flbD, veA1 | Wieser and Adams (1995) |
| RBN070 | biA1; argB2; flbD14, veA1 | Wieser et al. (1994) |
| RJW150 | yA1, pabaA1, flbA 100; argB2; veA1 | This study |
| TWC41 | pabaA1, yA2; trpC::alcA(p) ::rca-1, veA1 | This study |
| TWC3.1 | biA1; flbD14, veA1; rca-1 | This study |
| TX15:E2 | biA1; flbD14, veA1; rca-1 | This study |
| Strain | Description or genotype | Source |
| N. crassa | ||
| 74-OR23-1A | mating type A | FGSC |
| ORS6a | mating type a | FGSC |
| FGSC7023 | a; fld, delayed conidiation | FGSC |
| FGSC5511 | a; pe, fl | FGSC |
| WC1 | Δrca-1 transformant | This study |
| WC11-WC15 | Δrca-1 progeny of WC1 × ORS6a | This study |
| DE41-DE45 | pe, fl; Δrca-1 progency of WC1 × FGSC5511 | This study |
| DE46-DE50 | pe, fl; Δrca-1 progeny of WC1 × FGSC5511 | This study |
| WC21 | Δrca-1; fld progeny of WC1 × fld | This study |
| A. nidulans | ||
| FGSC237 | pabaA1, yA2; trpC801, veA1 | FGSC |
| TJW29.2 | pabaA1, yΔ2; trpC::alcA(p)::flbD, veA1 | Wieser and Adams (1995) |
| RBN070 | biA1; argB2; flbD14, veA1 | Wieser et al. (1994) |
| RJW150 | yA1, pabaA1, flbA 100; argB2; veA1 | This study |
| TWC41 | pabaA1, yA2; trpC::alcA(p) ::rca-1, veA1 | This study |
| TWC3.1 | biA1; flbD14, veA1; rca-1 | This study |
| TX15:E2 | biA1; flbD14, veA1; rca-1 | This study |
FGSC, Fungal Genetics Stock Center.
grown in 16 × 150 mm slants (6 ml medium), 125 ml flasks (50 ml medium), and 150 mm × 300 mm bottles (50 ml medium). For synchronous macroconidiation studies, mycelial pads were harvested by filtration from overnight cultures grown in minimal medium. Filter papers were placed onto minimal agar medium with the mycelial pads exposed to air. Strains were induced to produce microconidia as described (Pandit and Maheshwari 1993). Colonies of rca-1 mutant and wild-type strains were examined with a stereomicroscope at ×70 magnification to examine production of microconidiophores. pe, fl strains were point inoculated in the center of 7-cm petri dishes and microconidia were harvested after 7 days of growth. Microconidial yield was then quantitated by counting with a hemacytometer.
The location of rca-1 on the N. crassa genetic map was determined using the standard RFLP mapping strains (Metzenberg and Grotelueschen 1995). A polymorphism was detected using cosmid X15:E2 as a probe after digestion of chromosomal DNA of the parent strains with SalI. Analysis of the RFLP pattern of SalI-digested progeny DNA was used to determine the genetic location of rca-1 by comparison to RFLP patterns of known markers.
Nucleic acid procedures: Heterologous hybridization using A. nidulans genes was performed at 42°. Following overnight hybridization, blots were washed with 2× SSC (0.3 M sodium chloride, 0.03 M sodium citrate), 0.1% sodium dodecyl sulfate at room temperature for 15 min and then washed once with the same solution at 50° for 30 min.
The locations of degenerate primers based upon conserved amino acid sequences are shown in Figure 1. The primer sequences are 5′-GGNCCNTGG[A/G][T/C]NCCNGA[A/G] GA[T/C]CA-3′ and 5′-[A/G]TTCCA[A/G/C][C/T][A/G] [A/G]TT[C/T]TTNA[C/T]NGC[A/G]TT[A/G]TC-3′. The PCR amplification employed the “Touchdown” PCR strategy (Roux 1994). The annealing temperature decreased by 1° from 55° to 37° every 3 cycles followed by 20 cycles at 37°. Amplification was performed in a Perkin-Elmer DNA Thermal Cycler (Perkin-Elmer Corp., Norwalk, CT).
An N. crassa cosmid library (Orbach and Sachs 1991) and a cDNA library constructed by Dr. R. H. Garrett were screened using the PCR product. Several positive cosmid clones were identified and one (X15:E2) was chosen for further analysis. A 9.5-kb BamHI fragment containing rca-1 was subcloned into pBluescript SK− to produce plasmid pWC1. Cosmid X15:E2 was cotransformed with pSALargB into RBN070, an A. nidulans argB− flbD− strain, to produce strain TX15:E2. The 9.5-kb BamHI fragment was also cloned into pPK1 (Wieser and Adams 1995) to create pWC3 and used for transformation of RBN070 (to generate TWC3.1) and an A. nidulans argB− flbA− mutant, RJW150 (J. Wieser and T. H. Adams, unpublished data). A cDNA clone was identified by plaque hybridization (pWC2).
DNA sequence analysis was carried out using SequiTherm Cycle Sequencing Kits (Epicentre Technologies Corp., Madison, WI). DNA sequences of genomic and cDNA clones were confirmed on both strands. The nucleotide sequence was analyzed using the BESTFIT (Devereux et al. 1984) program and the databases were searched using the BLAST search algorithm (Altschul et al. 1990). The DNA sequence of rca-1 has been deposited in GenBank with accession AF006202.
RNA was isolated from synchronously developing cultures as described (Sachs and Yanofsky 1991). RNA blots were hybridized with gel-purified insert of pWC2 or conidiation-specific genes (Sachs and Yanofsky 1991). Probes were radiolabeled by the random priming method (Sambrook et al. 1989).
In order to delete rca-1 from the N. crassa genome, pWC1 was digested with MluI, digested with exonuclease III, and treated with S1 nuclease to create blunt ends. The 1.5-kb HpaI fragment from pCB1004 (Carroll et al. 1994) was added and the mixture was ligated and transformed into E. coli. Several colonies were screened and pWC5 was selected for further analysis. The 5′- and 3′-deletion end points of the fragment in pWC5 were determined by sequencing. pWC5 was used directly for transformation of N. crassa 74-OR23-1A. Transformants were screened for resistance to hygromycin following serial passage from conidia and 34 independent stable transformants were recovered. The presence of rca-1 sequences was screened by amplification of DNA directly from conidia (Xu and Hamer 1995) with rca-1 internal primers. Transformants that failed to yield an rca-1 amplification product were further characterized by Southern blot analysis and one isolate was identified that contained a single copy replacement of rca-1 with the hygB gene. The Δrca-1 strain was backcrossed to ORS6a and hygromycin-resistant progeny were examined by Southern blot analysis to verify the gene replacement event. The BamHI fragment of pWC5 was cloned into pPK1 to generate pWC6. pWC6 was used as a control plasmid for transformation experiments with A. nidulans flbD mutants.
To express rca-1 in A. nidulans, an alcA(p)::rca-1 fusion was prepared by inserting the cDNA insert from pWC2 into pBN55 (Lee and Adams 1996) to create pWC13. pWC13 was transformed into A. nidulans FGSC237 to produce strain TWC41.
Microscopy and photography: Photomicrographs of hyphal development were taken with an Olympus BH2 microscope (Olympus Corp., Lake Success, NY) using differential interference contrast optics. The micrographs of whole colonies were obtained using an Olympus SZ-11 stereomicroscope.
RESULTS
Identification and cloning of an N. crassa flbD homolog: We examined the possibility that N. crassa had homologs of the A. nidulans developmental regulatory genes fluG, flbA, flbC, flbD, brlA, and abaA by probing N. crassa genomic DNA with gene-specific fragments under low stringency hybridization conditions (see materials and methods). The only reproducible signal detected was with a flbD gene-specific probe although the hybridization signal was weak. Because the N-terminal portion of A. nidulans FlbD shares significant identity with the DNA-binding domain of the Myb family of transcription factors (Wieser and Adams 1995) we attempted to isolate an N. crassa flbD-like gene by PCR amplification using degenerate oligonucleotide primers based on conserved residues between FlbD and the DNA-binding domain in other Myb-like proteins (Figure 1). Direct sequencing of the PCR product indicated that the amplified sequence was unique and had sequence similarity to Myb-like transcription factors (Figure 2). This PCR product was also used as a hybridization probe to identify cosmid X15:E2 from a cosmid library of N. crassa genomic DNA. The cosmid clone was used as a hybridization probe with a standard set of RFLP mapping strains (Metzenberg and Grotelueschen 1995) to map the gene to the right arm of N. crassa chromosome V in the 20 map unit region between leu-5 and al-3 (Perkins et al. 1982). This map location is not near any known genes that specifically influence conidiation.
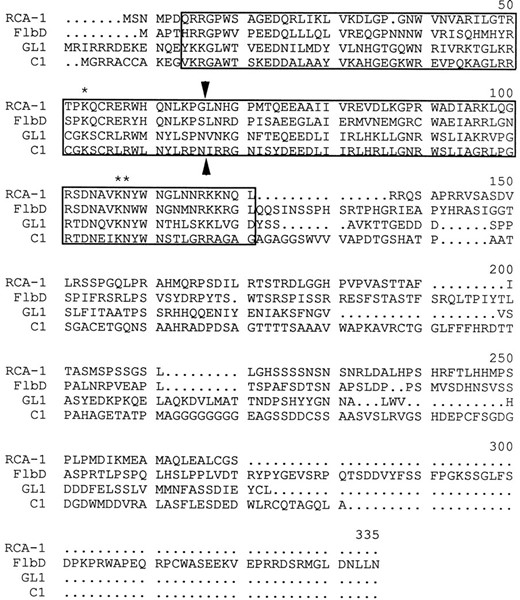
The DNA sequence of the rca-1 genomic region and the predicted RCA-1 amino acid sequence. The Myb-like DNA binding domain is underlined. The ends of the cDNA clone (pWC2) are indicated by large dots above the nucleotides. The 5′ and 3′ deletion junctions produced by exonuclease III digestion from the MluI site (bold type) are indicated by boxed nucleotides. The hygromycin phosphotransferase gene replaces the deleted region in the gene replacement vector pWC5. The locations of the degenerate oligonucleotides used for amplification are shown by dashed underlines.
Further analysis of cosmid X15:E2 showed that the putative flbD-like gene was located on a 9.5-kb BamHI fragment that was subcloned and used as a template for additional sequencing. Sequencing primers were synthesized based on the sequence of the PCR fragment and additional primers were used to complete the sequence of the 2500-bp region containing rca-1. As shown in Figures 1 and 2, this sequence predicts a 229-codon open reading frame that includes a region with high similarity to FlbD and other proteins with Myb-like DNA binding domains. A cDNA clone (pWC2) was also sequenced that initiated 121 nt upstream of the ATG initiation codon and extended 318 nt downstream of the predicted termination codon. The identity of the cDNA and genomic sequence indicates that the gene lacks introns (Figure 1).
Alignment of the putative DNA binding domain sequence of A. nidulans FlbD and N. crassa RCA-1 revealed 75% similarity and 57% identity when analyzed by BEST-FIT alignment (Devereux et al. 1984). In contrast, only 42% similarity and 21% identity was observed in the region outside the predicted DNA binding domain (Figure 2). The fungal proteins align most closely with plant Myb-like proteins such as maize C1 and Arabidopsis GL1 (Figure 2; Paz-Ares et al. 1987; Oppenheimer et al. 1991). RCA-1 has 37% identity to the DNA binding domain region of both of the plant proteins. There is little sequence similarity of the fungal and plant proteins
Comparison of deduced amino acid sequences of N. crassa RCA-1, A. nidulans FlbD, Arabidopsis GL1, and Maize C1 proteins. The Myb-like DNA binding domains of these proteins are boxed. The conserved residues in c-Myb that make specific contacts with the nucleotides of the AACNG sequence are marked by asterisks. Arrowheads mark the boundary between the imperfect repeats of the Mby-like DNA binding domains.
outside of the DNA binding domain. All of these proteins can be characterized as having two closely related imperfect repeats of Myb-like DNA binding domains followed by 100 to 200 amino acid segments that are hydrophilic and serine/threonine-rich (Figure 2).
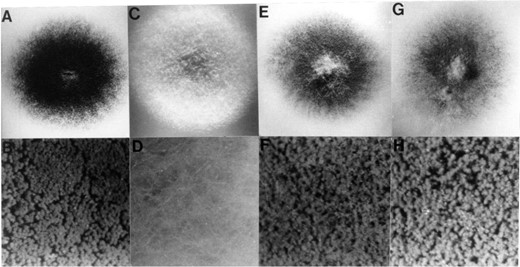
N. crassa rca-1 can complement an A. nidulans flbD mutant: The similarity in the DNA binding domains suggested that RCA-1 and FlbD proteins may be transcription factors that recognize similar DNA sequences. To test this idea, an A. nidulans flbD mutant strain was transformed with the N. crassa rca-1 gene. The 9.5-kb BamHI fragment with the N. crassa rca-1 gene was subcloned into the A. nidulans argB-containing vector pPK1 and both this plasmid (pWC3) and cosmid X15:E2 were used to transform an A. nidulans flbD mutant strain. In addition, an internal deletion within the 9.5-kb BamHI fragment was created by exonuclease III digestion to eliminate the rca-1 coding region (Figure 1) and this plasmid was used in transformation experiments as a control. For each of the constructs containing the intact rca-1 gene, most of the transformants conidiated like wild type (Figure 3). In contrast, all transformants using the rca-1 disruption plasmid had the delayed conidiation phenotype normally observed for the A. nidulans flbD mutant strain (Wieser and Adams 1995). Southern blot analysis was used to verify that only those A. nidulans transformants that displayed a wild-type conidiation phenotype had acquired the N. crassa rca-1 DNA (data not shown). Finally, to test whether complementation of the A. nidulans flbD mutation with N. crassa rca-1 was specific, we used the same rca-1 containing plasmids in transformation experiments with an A. nidulans flbA
N. cassa rca-1 complements the A. nidulans flbD mutant. A. nidulans wild-type strain (A and B), flbD mutant (C and D), and flbD mutant cotransformed with cosmid X15:E2 (E and F) or transformed with the 9.5 kb BamHI fragment rca-1 subclone (G and H) were point inoculated on complete medium and grown for 3 days. B, D, F, and H show a ×30 magnification of the colonies.
mutant strain (Lee and Adams 1994b). All of the transformants had a fluffy autolytic phenotype identical to the flbA mutant parent (data not shown).
Wild-type A. nidulans does not normally conidiate in submerged liquid culture (Figure 4A) (Timberlake 1990). A striking property of the A. nidulans flbD gene is that its overexpression from the highly inducible alcA promoter during growth in submerged culture can cause inappropriate conidiation (Figure 4B). The cDNA corresponding to rca-1 was placed under the control of the A. nidulans alcA promoter and the fusion construct was used to transform A. nidulans. As observed with the A. nidulans flbD gene (Wieser and Adams 1995), overexpression of rca-1 by induction of the alcA promoter activated A. nidulans sporulation in liquid culture (Figure 4C).
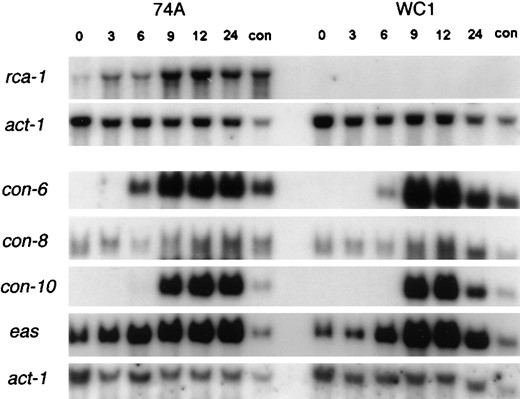
N. crassa rca-1 is present throughout the life cycle: Although the A. nidulans flbD gene functions specifically during sporulation and has no apparent role during vegetative growth, it was previously shown that flbD mRNA was present at relatively constant levels in vegetatively growing and developing A. nidulans cultures (Wieser and Adams 1995). Similarly, we found that rca-1 mRNA was present in both mycelial and developing cultures of N. crassa (Figure 5). However, over a time course of synchronized conidial development, we observed a slight elevation of rca-1 mRNA accumulation relative to expression of actin mRNA (Figure 5). Finally, the Δrca-1 strain (see below) lacked rca-1 mRNA as measured by northern blot analysis (Figure 5).
rca-1 disruption has no detectable effect on the sporulation in N. crassa: N. crassa wild-type 74-OR23-1A was
Expression of rca-1 in A. nidulans using the alcA promoter. A. nidulans strains TTA11 (panel A; wild type), TJW29.1 [panel B; alcA(p)::flbD], and TWC41 [panel C; alcA(p)::rca-1] were grown in liquid medium containing glucose for 14 hr and then shifted to liquid minimal medium with threonine and allowed to grow. Photographs were taken14 hr after the shift.
Northern analysis of N. crassa wild-type (WT) strain 74-OR23-1A and the Δrca-1 strain WC1. Total RNA was extracted from the wild-type and Δrca-1 strains at different developmental stages after induction of macroconidiation. Time after induction (hr) is indicated for each lane. RNA from 7-day-old macroconidia (con) was also isolated. The top two panels show a single blot of RNA from WT and Δrca-1 strains sequentially probed with rca-1 and actin (act-1). The lower five panels represent a second blot of the same RNA samples probed sequentially with con-6, con-8, con-10, eas, and act-1 clones.
transformed with the plasmid containing the rca-1 deletion construct, pWC6, and hygromycin-resistant transformants were selected. Primers that could amplify the rca-1 gene were used to screen transformants for the absence of the endogenous rca-1 gene from 34 transformants. The resulting candidate strains were further screened by Southern blot analysis and one isolate was identified for which the rca-1 gene region had been replaced by the hygromycin phosphotransferase gene. This isolate was backcrossed to the wild-type strain, ORS6a, and hygromycin-resistant progeny were examined by Southern blot analysis to confirm the gene replacement event (data not shown). The degenerate primers initially used to amplify the rca-1 gene were again used to test chromosomal DNA from the deletion strain to verify the absence of the rca-1 DNA binding domain in the genome (data not shown).
A number of conditions were examined in an attempt to discern a phenotype for the Δrca-1 mutant. Mycelial growth rate was measured in race tubes with Vogel's minimal agar medium, minimal medium with 2% peptone, or synthetic crossing medium and was always indistinguishable from the parental wild-type strain (not shown). Quantitation of macroconidial yield for Δrca-1 (WC1) or wild-type (74-OR23-1A) strains grown in flasks for 7 days with: constant light, constant dark, Vogel's minimal medium agar with no added carbon source, minimal agar medium with 1.5% sucrose, minimal medium with 2% peptone, or synthetic crossing medium, did not reveal any striking differences between strains (not shown). The WC1 strain appeared to produce slightly fewer macroconidia on average than the parent strain on synthetic crossing medium. The yields for 74-OR23-1A and WC1 were 7.1 ± 0.3 × 107 and 5.3 ± 0.6 × 107 macroconidia from growth in 125 ml flasks(>95% confidence level). However, the 34% reduction in macroconidia of WC1 relative to 74-OR23-1A observed in this experiment did not provide a visual phenotype.
Strain WC1 was backcrossed to wild type and we observed 1:1 segregation of hygromycin resistance. We noted that the behavior of the Δrca-1 mutant was similar to that of wild type as either the male or female parent in meiotic crosses. Hygromycin-resistant progeny WC11, WC13, and WC15 were tested for macroconidiation at different times after inoculation of flasks (Table 2). Macroconidiation occurred by 47 hr after inoculation of flasks with 74-OR23-1A (wild type) and each of three different Δrca-1 progeny examined. By 66 hr after inoculation of flasks, the Δrca-1 strains had produced from 36 to 61% of the macroconidia made by wild type (Table 2) and this difference was statistically significant (95% confidence level). However, by 96 hr, macroconidial yields of mutant and wild-type strains were indistinguishable (Table 2). To examine more carefully the possibility that the Δrca-1 mutation affected the timing of conidiation, synchronous sporulation was induced (Springer and Yanofsky 1989) and the timing of appearance of the first macroconidiophores was observed to be the same for all strains (not shown). Thus, we conclude that the Δrca-1 mutation has no distinguishable effect on the timing of conidiation but may have minor effects on conidial yield. This conclusion was tested further by growing cultures in different containers including 16 × 150 mm tubes or 150 × 300 mm bottles (not shown). In all cases the intrinsic variation of the experiments limited our ability to distinguish whether small variation between strains had any biological significance.
Environmental conditions including starvation in submerged culture have been shown to induce sporulation in wild-type strains (Plesofsky-Vig et al. 1983; Springer 1993). We therefore examined macroconidiation by the Δrca-1 strain in liquid culture but again saw no apparent change in timing when compared to a wild-type strain grown under the same conditions. We also examined the effect of the Δrca-1 mutation on macroconidiation of strains grown in flasks at either 25° or 34° to see if the mutant phenotype could be enhanced by temperature sensitivity, but no significant effect was seen (not shown).
Because we were unable to detect any major differences in macroconidiation between wild type and the Δrca-1 mutant, we examined the possibility that a minor developmental phenotype for Δrca-1 would be amplified in a strain that already had a partial defect in macroconidiation. We therefore crossed the Δrca-1 mutant with a fluffyoid (fld) mutant strain and examined the progeny.
Timing and extent of macroconidiation by wild-type and Δrca-1 strains
| Time . | 74-OR23-14 . | ORS6a . | WC11 . | WC13 . | WC15 . |
|---|---|---|---|---|---|
| 47 | 0.12 ± 0.06a | 0.22 ± 0.05 | 0.051 ± 0.009 | 0.10 ± 0.03 | 0.11 ± 0.04 |
| 66 | 3.8 ± 1.0 | 3.1 ± 0.6 | 1.7 ± 0.02 | 1.4 ± 0.1 | 1.9 ± 0.9 |
| 96 | 9.5 ± 3.7 | 8.7 ± 1.0 | 7.2 ± 2.0 | 7.1 ± 1.4 | 8.3 ± 0.6 |
| Time . | 74-OR23-14 . | ORS6a . | WC11 . | WC13 . | WC15 . |
|---|---|---|---|---|---|
| 47 | 0.12 ± 0.06a | 0.22 ± 0.05 | 0.051 ± 0.009 | 0.10 ± 0.03 | 0.11 ± 0.04 |
| 66 | 3.8 ± 1.0 | 3.1 ± 0.6 | 1.7 ± 0.02 | 1.4 ± 0.1 | 1.9 ± 0.9 |
| 96 | 9.5 ± 3.7 | 8.7 ± 1.0 | 7.2 ± 2.0 | 7.1 ± 1.4 | 8.3 ± 0.6 |
Total yeild of conidia (× 10−7) from flasks of Vogel's minimal medium containing 1.5% sucrose were inoculated and grown at 25° for the time (hr) indicated prior to harvesting.
Standard deviation of triplicate samples.
Timing and extent of macroconidiation by wild-type and Δrca-1 strains
| Time . | 74-OR23-14 . | ORS6a . | WC11 . | WC13 . | WC15 . |
|---|---|---|---|---|---|
| 47 | 0.12 ± 0.06a | 0.22 ± 0.05 | 0.051 ± 0.009 | 0.10 ± 0.03 | 0.11 ± 0.04 |
| 66 | 3.8 ± 1.0 | 3.1 ± 0.6 | 1.7 ± 0.02 | 1.4 ± 0.1 | 1.9 ± 0.9 |
| 96 | 9.5 ± 3.7 | 8.7 ± 1.0 | 7.2 ± 2.0 | 7.1 ± 1.4 | 8.3 ± 0.6 |
| Time . | 74-OR23-14 . | ORS6a . | WC11 . | WC13 . | WC15 . |
|---|---|---|---|---|---|
| 47 | 0.12 ± 0.06a | 0.22 ± 0.05 | 0.051 ± 0.009 | 0.10 ± 0.03 | 0.11 ± 0.04 |
| 66 | 3.8 ± 1.0 | 3.1 ± 0.6 | 1.7 ± 0.02 | 1.4 ± 0.1 | 1.9 ± 0.9 |
| 96 | 9.5 ± 3.7 | 8.7 ± 1.0 | 7.2 ± 2.0 | 7.1 ± 1.4 | 8.3 ± 0.6 |
Total yeild of conidia (× 10−7) from flasks of Vogel's minimal medium containing 1.5% sucrose were inoculated and grown at 25° for the time (hr) indicated prior to harvesting.
Standard deviation of triplicate samples.
fld mutants are initially aconidial, but following prolonged incubation they do produce reduced numbers of macroconidia (not shown). Approximately 50% of the progeny were initially aconidial as expected for fld mutants and half of these were hygromycin resistant, indicating that they carried the Δrca-1 construct. We were unable to distinguish any phenotypic differences in the extent or timing of macroconidiation in fld and Δrca-1; fld strains.
We also tested the possibility that the rca-1 deletion could affect the expression of genes known to be activated during macroconidiation. As shown in Figure 5, we examined the timing and level of expression of eas, con-6, con-8, and con-10 in wild type and in the Δrca-1 mutant. In every case, transcript levels and the timing of their expression were unaltered in the Δrca-1 mutant strain.
We next examined the effect of deleting rca-1 on formation of the second N. crassa asexual spore type, the microconidium, and found that the Δrca-1 strain was capable of producing microconidia. However, because of the difficulty in obtaining synchronous production of large quantities of microconidia we could not distinguish whether or not the rca-1 deletion had any subtle effects on the timing or extent of microconidiation. We therefore crossed Δrca-1 into the pe, fl (peach, fluffy) genetic background. pe, fl strains do not produce macroconidia and the pe mutant allele enhances microconidiation. Of 50 progeny examined, we observed 26 fl strains and 19 of these produced abundant microconidia, consistent with the known linkage of fl and pe. We found 9/19 pe, fl progeny to be hygromycin resistant and therefore carry Δrca-1. Five pe, fl strains (DE41–DE45) and 5 pe, fl; Δrca-1 strains (DE46–DE50) were examined for microconidiation and microconidia production. No visual difference in the timing of microconidiophore production was observed. After 7 days of growth on synthetic cross agar in 7-cm-diameter petri plates, microconidia were harvested for quantitation. Yields ranged from 6.4 × 108 microconidia/plate to 3.2 × 109 microconidia/plate with no clear correlation of yield to genotype. The combined rca-1+ isolates averaged 1.0 × 109 microconidia/plate and Δrca-1 strains averaged 1.7 × 109 microconidia/plate.
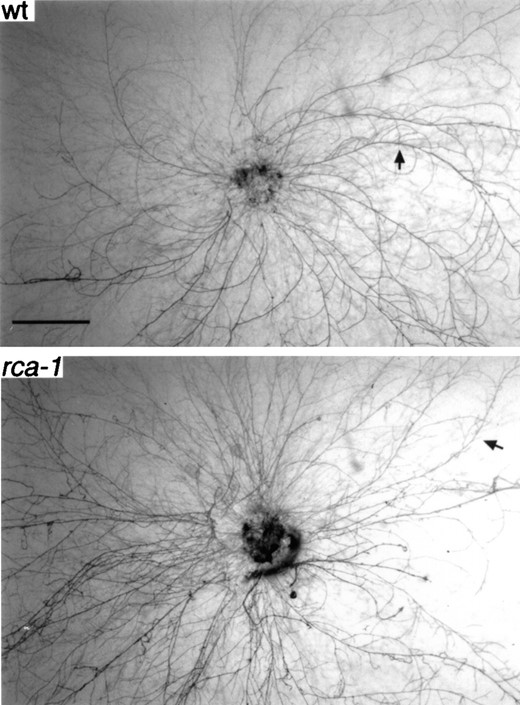
Δrca-1 mutants are altered in spiral growth: During our characterization of the Δrca-1 mutants we noticed that they did not have the pronounced clockwise spiral growth pattern of typical N. crassa colonies during the first 24–36 hr of growth (Figure 6). In fact, the mutants appeared in many cases to display counterclockwise spiral growth (Figure 6). The spiral growth phenotype segregated completely with the hygromycin resistance marker at the rca-1 locus. This phenotype was easily scored among the pe, fl progeny described above and several pe+, fl+; Δrca-1 strains tested. Although an A. nidulans strain (A17) was previously reported to have clockwise spiral growth (Trinci et al. 1979), we were unable to detect significant and consistent spiral growth of the A. nidulans FGSC 26 wild-type strain. No difference in the growth of hyphae following germination of conidia was observed during comparison of the wild-type and flbD mutant strains.
DISCUSSION
Extensive genetic analysis combined with gene characterization has identified several genes in A. nidulans that control conidiation (Adams 1995). We sought homologs of these genes in N. crassa to examine the possibility that these distantly related ascomycetous fungi use common regulatory mechanisms to control asexual sporulation. The only A. nidulans gene for which we successfully identified a related N. crassa gene was flbD. The role of flbD in A. nidulans conidiation has been established by the observations that flbD mutants are delayed in conidiation and that forced overexpression of flbD induces development at inappropriate times (Wieser et al. 1994; Wieser and Adams 1995). flbD mutations have no effect on the mycelial growth of A. nidulans, suggesting that flbD functions in a conidiation-specific manner. Remarkably, the N. crassa rca-1 gene appears to be functionally equivalent to flbD in A. nidulans in that rca-1 restored conidiation to the flbD mutant strain and forced expression of rca-1-induced A. nidulans development in submerged culture.
In N. crassa, there was no discernible effect of the rca-1 deletion on growth rate, fertility, and microconidiation. Under conditions of synchronous induction of conidiation we did not observe a delay in conidiation-specific gene expression or morphogenesis. There was an overall
Spiral growth in wild-type and rca-1 mutants. Strains were grown on synthetic cross medium containing 1% glucose and inoculated with small blocks of agar. Growth was for 16 hr. Arrows indicate a primary hypha growing along the surface that displays clockwise (wt) or counterclockwise (rca-1) spiral growth. Bar, 1 mm.
tendency with cultures grown in flasks for Δrca-1 strains to produce somewhat fewer conidia than wild-type strains. However, because conidial yields were somewhat variable it was difficult to detect a statistically significant difference between conidial yields. A much more extensive analysis of conidia production by several Δrca-1 and wild-type strains will be needed to determine whether Δrca-1 mutants have a slight reduction in conidial yield. At this time we cannot exclude the possibility that rca-1 has a subtle role in N. crassa macroconidiation but it is clearly not as important to this phase of the life cycle as flbD is to conidiation by A. nidulans. The lack of any major phenotypic consequence of deleting rca-1 might be explained if N. crassa possesses a rca-1 homolog that can compensate for the loss of rca-1. However, we have been unable to detect a second copy of the gene by probing with rca-1 in Southern blots or by PCR amplification of genomic DNA isolated from the Δrca-1 mutant using the degenerate oligonucleotide primers used to initially amplify rca-1 from wild type.
Epistasis analysis of A. nidulans flbD with other developmental mutants suggests that flbD participates in one of two independent pathways that are both needed to efficiently activate brlA expression and development. flbE, flbD, and flbB form one pathway, and flbC represents an independent pathway for brlA activation (Wieser et al. 1994). It is possible that a similar set of pathways exist in N. crassa but that they have greater redundancy so that loss of the flbD homolog, rca-1, is largely compensated for by the activity of a flbC homolog. Alternatively, rca-1 has no specific role in controlling development in N. crassa, but during the evolution of the regulatory circuit governing conidiation, A. nidulans recruited this Myb-like transcription factor to regulate conidiation-specific events.
It is important to recognize that flbD and rca-1 mRNAs are each present in both mycelial and developing cultures (Wieser and Adams 1995), although (ironically) rca-1 accumulates to higher levels during the course of macroconidiation. Given that flbD is required specifically during sporulation, this result has led to the proposal (Wieser and Adams 1995) that as with myb-related genes in other organisms, an important aspect of flbD regulation is probably post-transcriptional (Luscher and Eisenman 1990; Myrset et al. 1993). In this sense, it is particularly intriguing that the similarity between RCA-1 and FlbD is limited primarily to the putative DNA-binding domain at the N termini of both proteins. The amino acid residues implicated in specific interactions of c-Myb with DNA are conserved in most Myb-like proteins (Ogata et al. 1992; Myrset et al. 1993; Ogata et al. 1994), including rca-1 and flbD, making it likely that FlbD and RCA-1 bind to similar sequences that resemble the AACNG c-Myb binding site. If the site for post-translational control of FlbD/RCA-1 is within the DNA-binding domain, one possible mechanism for regulating their activity in controlling sporulation is through changes in the environment. Aspergillus does not normally conidiate unless hyphae are exposed to an air interface (Adams 1995). It has been shown that one mechanism for controlling c-Myb binding involves a redox-regulated conformational change within the DNA-binding domain (Myrset et al. 1993; Ogata et al. 1994). This redox-sensing mechanism could provide a means of changing the activity of both FlbD and RCA-1 as hyphae are exposed to air. However, it is important to recognize that the DNA-binding activity of c-Myb is inactivated by oxidative environments (Myrset et al. 1993; Ogata et al. 1994), and the condition under which FlbD activity is needed for development is presumably oxidative. Alternatively, regulation of FlbD/RCA-1 activity could be through as-yet-uncharacterized residues in the C terminus, or posttranslational modification of FlbD may not be necessary for function. In any case, it is likely that we can learn more about the role of FlbD and its regulation by investigating the ability of RCA-1 to activate A. nidulans sporulation. FlbD and RCA-1 are most similar to the Myb-like proteins in plants that are commonly found to be involved in tissue-specific gene expression and morphogenesis. For example, maize C1 is required for production of anthocyanin pigment in certain tissues (Paz-Ares et al. 1987) and Arabidopsis GL1 is involved in leaf trichome differentiation (Oppenheimer et al. 1991). It appears that similar roles in tissue-specific gene expression and development occur in fungi given the phenotypes of the flbD and rca-1 mutants.
The only readily detectable phenotype in N. crassa rca-1 mutants is an alteration in spiral growth. Spiral growth has been observed in the colonies of many fungi (Madelin et al. 1978). In both N. crassa and A. nidulans clockwise spiral growth has been noted for young colonies (Trinci et al. 1979). It has been postulated that the hyphal tip rotates about its axis in a clockwise direction with respect to the subapical regions of the hypha (Madelin et al. 1978). Because the subapical regions are fixed to the substrate surface, the rotation of the tip causes the hyphae to curve as they grow across the substrate. This phenomenon could be subject to many environmental factors including nutrients, pH, and signals from other nearby hyphae. rca-1 mutants may be perturbed in some subtle process that influences events at the hyphal tip. Interestingly, another regulatory gene of N. crassa, rco-1, has reversed spiral growth (Yamashiro et al. 1996). RCO-1 has sequence similarity to Tup1p, a protein involved in repression of many genes in Saccharomyces cerevisiae (Yamashiro et al. 1996). Tup1p is involved in cell type-specific expression in S. cerevisiae and this also appears to be a role for RCO-1 (Yamashiro et al. 1996). One role for rco-1 may be to influence a pathway involving rca-1 that affects hyphal tip growth. Further examination of rca-1, rco-1 double mutants will be needed to explore this possibility. We could not detect any difference between A. nidulans flbD− and wild-type strains that would suggest that FlbD influences orientation of hyphal tip growth in A. nidulans.
Acknowledgement
This work was supported by Public Heath Service Grants GM-45252 to T.H.A. and R29GM47977 to D.J.E. J.W. was supported in part by a National Science Foundation training grant DGE-9354891 to the Program for the Biology of Filamentous Fungi.
Footnotes
Communicating editor: J. J. Loros
LITERATURE CITED




![Expression of rca-1 in A. nidulans using the alcA promoter. A. nidulans strains TTA11 (panel A; wild type), TJW29.1 [panel B; alcA(p)::flbD], and TWC41 [panel C; alcA(p)::rca-1] were grown in liquid medium containing glucose for 14 hr and then shifted to liquid minimal medium with threonine and allowed to grow. Photographs were taken14 hr after the shift.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/genetics/148/3/10.1093_genetics_148.3.1031/20/m_gen7051.f4.jpeg?Expires=1716366218&Signature=k-yCkmL3mem4GYGF8nnLO971qzjCkN~tvcn22Lf0zpBPGt9akPuQHbFN6jZptzag-XP-oap5sGcb1pbN1LkiNRoCKXpjImfDV1EZ88bIa3LfBhfCAPHXgGhKY47yludQyUbdkvr4LZFS4OYWHZXBIEa~3MPlfhRLSQHHWB951mQ1FUs4Zd4d0o4wM89atJgQCef8fof1Ogguf-JMis4c5C5uoNG7QXhpgGSCtu6hGIhwHwE9ooLO7zQGgdo0cfRa2o6LQ35-T~0Z8Fe79jlgRHHxlTBE6RS5NC2G2i3rCYkH~yYGLRH-aua~YlGxda8yjJbcSPlMc7-6NU-2KJ2Z0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)