-
PDF
- Split View
-
Views
-
Cite
Cite
Alison J Rattray, Carolyn B McGill, Brenda K Shafer, Jeffrey N Strathern, Fidelity of Mitotic Double-Strand-Break Repair in Saccharomyces cerevisiae: A Role for SAE2/COM1, Genetics, Volume 158, Issue 1, 1 May 2001, Pages 109–122, https://doi.org/10.1093/genetics/158.1.109
Close - Share Icon Share
Abstract
Errors associated with the repair of DNA double-strand breaks (DSBs) include point mutations caused by misincorporation during repair DNA synthesis or novel junctions made by nonhomologous end joining (NHEJ). We previously demonstrated that DNA synthesis is ∼100-fold more error prone when associated with DSB repair. Here we describe a genetic screen for mutants that affect the fidelity of DSB repair. The substrate consists of inverted repeats of the trp1 and CAN1 genes. Recombinational repair of a site-specific DSB within the repeat yields TRP1 recombinants. Errors in the repair process can be detected by the production of canavanine-resistant (can1) mutants among the TRP1 recombinants. In wild-type cells the recombinational repair process is efficient and fairly accurate. Errors resulting in can1 mutations occur in <1% of the TRP1 recombinants and most appear to be point mutations. We isolated several mutant strains with altered fidelity of recombination. Here we characterize one of these mutants that revealed an ∼10-fold elevation in the frequency of can1 mutants among TRP1 recombinants. The gene was cloned by complementation of a coincident sporulation defect and proved to be an allele of SAE2/COM1. Physical analysis of the can1 mutants from sae2/com1 strains revealed that many were a novel class of chromosome rearrangement that could reflect break-induced replication (BIR) and NHEJ. Strains with either the mre11s-H125N or rad50s-K81I alleles had phenotypes in this assay that are similar to that of the sae2/com1Δ strain. Our data suggest that Sae2p/Com1p plays a role in ensuring that both ends of a DSB participate in a recombination event, thus avoiding BIR, possibly by regulating the nuclease activity of the Mre11p/Rad50p/Xrs2p complex.
MUTATIONS result from the balance between the formation of premutagenic DNA lesions and the ability of the cell to recognize and repair these lesions (see Friedberg et al. 1995; Wood 1996 for reviews). Although DNA repair presumably exists to avoid mutations, some repair mechanisms may also introduce mutations, perhaps in the process of attempting to repair potentially lethal DNA damage. DNA double-strand breaks (DSBs) are catastrophic lesions for a chromosome, and if left unrepaired, can result in lethality (Resnick and Martin 1976; Chlebowicz and Jachymczyk 1979; Malone and Esposito 1980). However, DSBs are normally repaired very efficiently by one of three known mechanisms (for a review see Paques and Haber 1999). Precise religation of the broken ends is by definition nonmutagenic and may be a common DSB repair pathway, but the inability to distinguish between substrate and products has made the overall contribution of this mechanism difficult to ascertain. Nonhomologous end joining (NHEJ) between sequences with little or no identity is by definition mutagenic. NHEJ is predominant when cell survival depends upon joining DNA sequences that do not share homology and is the preferred pathway of DSB repair in organisms that contain a large proportion of noncoding repetitive DNA (Roth and Wilson 1985). In these organisms, such as mammalian cells, random broken ends are less likely to fall within an open reading frame (ORF), and thus ligation between nonhomologous broken ends is unlikely to cause phenotypic mutation. In addition, repair by homologous recombination between highly repetitive DNA sequences could lead to deleterious gross chromosomal rearrangements such as translocations or deletions. Homologous recombination is considered to be an error-free pathway of repair where a sister chromatid, homologous chromosome, or other homologous sequence is used as a template for DNA synthesis to restore sequences bridging the break. Both sides of the broken chromosome are thought to participate in the recombination reaction, resulting in Holliday junctions that can then be resolved as either a crossover or a noncrossover, depending upon the strands cleaved (Resnick 1976; Strathern et al. 1982; Szostak et al. 1983). Homologous recombination is the preferred mode of DSB repair in organisms like Saccharomyces cerevisiae, in which most of the genome is comprised of unique sequence.
We previously monitored the fidelity of recombination between homologous chromosomes in a diploid using a substrate that allowed us to initiate high levels of recombination at a specific site with the HO endonuclease. The efficiency of recombination was monitored by measuring the frequency of His+ prototrophs that resulted from recombination between his3 heteroalleles on one side of the DSB. The fidelity of recombination was monitored by measuring the frequency of reversion of identical trp1 alleles on the other side of the break. Reversion of trp1 was found to be 10- to 1000-fold higher (depending upon the allele used) among the recombinants than in cultures that had not been induced to express the HO endonuclease (Strathern et al. 1995; McGill et al. 1998). Because this assay was dependent upon reversion of point mutations, it focused on misin-corporation errors. Recombination errors that resulted in gene rearrangements were undetectable.
To identify factors that affect the fidelity of DSB repair, we developed a genetic screen for monitoring the frequency of mutations associated with DSB repair in haploid cells. A site-specific DSB introduced within one copy of an intrachromosomal inverted repeat was repaired by utilizing homology of the uncut inverted-repeat sequence as a template for repair synthesis (Figure 1). Recombination was monitored by the formation of TRP1 prototrophs between two truncated but overlapping alleles of trp1. Additional homology was provided on the other side of the DSB by sequences of the CAN1 gene, which encodes the arginine permease and renders cells sensitive to the toxic arginine analog canavanine (Grenson et al. 1966). Mutations generated during repair of the DSB utilizing the homologous CAN1 sequences could result in can1 mutants and were measured by determining the frequency of canavanine-resistant mutants associated with a TRP1 recombinant (Figure 1). Because this assay is based on forward mutation selection, it allows for monitoring mutations that are due to DNA synthetic errors as well as mutations that arise by improper recombinational repair, resulting in chromosomal rearrangements.
In this article we present a characterization of the inverted-repeat substrate from a wild-type strain, where we studied the repair of induced DSB events. We found that the majority of DSBs appeared to be repaired via homologous recombination, resulting in gene conversions either with or without an associated crossover. Consistent with our previous data, we also found that the frequency of mutations associated with DSB repair was much higher than the spontaneous mutation frequency. The structure of most of the recombination-associated mutations suggests that they are point mutations. We also describe a mutant strain that we isolated from a screen for mutants with altered fidelity. This particular mutant, which was determined to be an allele of SAE2/COM1, affects the integrity of the recombination event itself, resulting in a unique class of gene rearrangement events.
Yeast strains
| Name . | Relevant genotype or description . |
|---|---|
| GRY1509 | MATa-inc::URA3 ade2-101 his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 |
| GRY1559 | MATα-inc::URA3 cyh2 his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 tyr7-1 |
| GRY1593 | GRY1509 + MATa-inc::mush18/21a |
| GRY1595 | GRY1559 + MATα-inc:: mush18/21 |
| GRY1644 | MATa-inc::mush18/21 ade2-101 can1Δ::hisG his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 |
| GRY1650 | GRY1644 + pGALHO |
| GRY1647 | MATα-inc::mush18/21 can1Δ::hisG his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 tyr7-1 |
| GRY1654 | GRY1647 + pGALHO |
| yAR523 | GRY1654 sae2Δ::KanMX |
| yAR574 | GRY1647 sae2Δ::KanMX |
| yAR577 | GRY1647 + pCHOL |
| yAR579 | yAR574 + pCHOL |
| yAR581 | GRY1647 rad50s::URA3 |
| yAR583 | yAR581 + pCHOL |
| yAR626 | GRY1647 mre11-H125N |
| yAR627 | yAR626 + pCHOL |
| Name . | Relevant genotype or description . |
|---|---|
| GRY1509 | MATa-inc::URA3 ade2-101 his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 |
| GRY1559 | MATα-inc::URA3 cyh2 his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 tyr7-1 |
| GRY1593 | GRY1509 + MATa-inc::mush18/21a |
| GRY1595 | GRY1559 + MATα-inc:: mush18/21 |
| GRY1644 | MATa-inc::mush18/21 ade2-101 can1Δ::hisG his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 |
| GRY1650 | GRY1644 + pGALHO |
| GRY1647 | MATα-inc::mush18/21 can1Δ::hisG his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 tyr7-1 |
| GRY1654 | GRY1647 + pGALHO |
| yAR523 | GRY1654 sae2Δ::KanMX |
| yAR574 | GRY1647 sae2Δ::KanMX |
| yAR577 | GRY1647 + pCHOL |
| yAR579 | yAR574 + pCHOL |
| yAR581 | GRY1647 rad50s::URA3 |
| yAR583 | yAR581 + pCHOL |
| yAR626 | GRY1647 mre11-H125N |
| yAR627 | yAR626 + pCHOL |
mush18/21 refers to the inverted-repeat construct that is inserted near the MAT locus (see materials and methods).
Yeast strains
| Name . | Relevant genotype or description . |
|---|---|
| GRY1509 | MATa-inc::URA3 ade2-101 his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 |
| GRY1559 | MATα-inc::URA3 cyh2 his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 tyr7-1 |
| GRY1593 | GRY1509 + MATa-inc::mush18/21a |
| GRY1595 | GRY1559 + MATα-inc:: mush18/21 |
| GRY1644 | MATa-inc::mush18/21 ade2-101 can1Δ::hisG his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 |
| GRY1650 | GRY1644 + pGALHO |
| GRY1647 | MATα-inc::mush18/21 can1Δ::hisG his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 tyr7-1 |
| GRY1654 | GRY1647 + pGALHO |
| yAR523 | GRY1654 sae2Δ::KanMX |
| yAR574 | GRY1647 sae2Δ::KanMX |
| yAR577 | GRY1647 + pCHOL |
| yAR579 | yAR574 + pCHOL |
| yAR581 | GRY1647 rad50s::URA3 |
| yAR583 | yAR581 + pCHOL |
| yAR626 | GRY1647 mre11-H125N |
| yAR627 | yAR626 + pCHOL |
| Name . | Relevant genotype or description . |
|---|---|
| GRY1509 | MATa-inc::URA3 ade2-101 his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 |
| GRY1559 | MATα-inc::URA3 cyh2 his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 tyr7-1 |
| GRY1593 | GRY1509 + MATa-inc::mush18/21a |
| GRY1595 | GRY1559 + MATα-inc:: mush18/21 |
| GRY1644 | MATa-inc::mush18/21 ade2-101 can1Δ::hisG his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 |
| GRY1650 | GRY1644 + pGALHO |
| GRY1647 | MATα-inc::mush18/21 can1Δ::hisG his3-Δ200 leu2-Δ1 lys2Δ::hisG trp1Δ::hisG ura3-52 tyr7-1 |
| GRY1654 | GRY1647 + pGALHO |
| yAR523 | GRY1654 sae2Δ::KanMX |
| yAR574 | GRY1647 sae2Δ::KanMX |
| yAR577 | GRY1647 + pCHOL |
| yAR579 | yAR574 + pCHOL |
| yAR581 | GRY1647 rad50s::URA3 |
| yAR583 | yAR581 + pCHOL |
| yAR626 | GRY1647 mre11-H125N |
| yAR627 | yAR626 + pCHOL |
mush18/21 refers to the inverted-repeat construct that is inserted near the MAT locus (see materials and methods).
MATERIALS AND METHODS
Media and general methods: Media were made as described by Sherman et al. (1986). Yeast cells were transformed by either the spheroplast (Hinnen et al. 1978) or the LiAc (Ito et al. 1983) transformation protocol.
Plasmids: The HO endonuclease was expressed from plasmids pGalHO (Kolodkin et al. 1986) or pCHOL (a gift from D. Nissley), which consists of the HindIII to EcoRV fragment from pGalHO cloned into YCplac33 (Gietz and Sugino 1988). pMUSHΔ17 was used for making a replacement of the CAN1 ORF with can1-Δ17. The can1-Δ17 allele has a deletion/gene replacement beginning 140 bp 5′ of the CAN1 ORF and terminating 196 bp 3′ of the CAN1 ORF. The deletion was made by the method of Alani et al. (1987), which results in a substitution of the deleted sequence by the S. typhimurium hisG sequence. pMJ536 (a gift from M. Lichten) was used for deletion/replacement of the SAE2 ORF with sae2Δ::KanMX (Borde et al. 2000). pNKY349 (a gift from N. Kleckner) was used for introduction of the rad50s-K81I, aided by a tightly linked URA3 marker (Alani et al. 1990).
Strains: The strains used in this study and their genotypes are listed in Table 1. The construction of strains GRY1509 and GRY1559 was described previously (McGill et al. 1993). Construction of the inverted-repeat substrate mush18/21 is described below. Strain yAR626 (mre11-H125N) was constructed by crossing GRY1647 with LSY781 (Moreau et al. 1999) and selecting for spores with the desired genotype as noted in Table 1 for yAR626. These spores were then backcrossed twice to strain GRY1644 to produce a congenic derivative with the genotype listed in Table 1 for strain yAR626. The presence of the mre11-H125N allele was confirmed by PCR and restriction digest analysis.
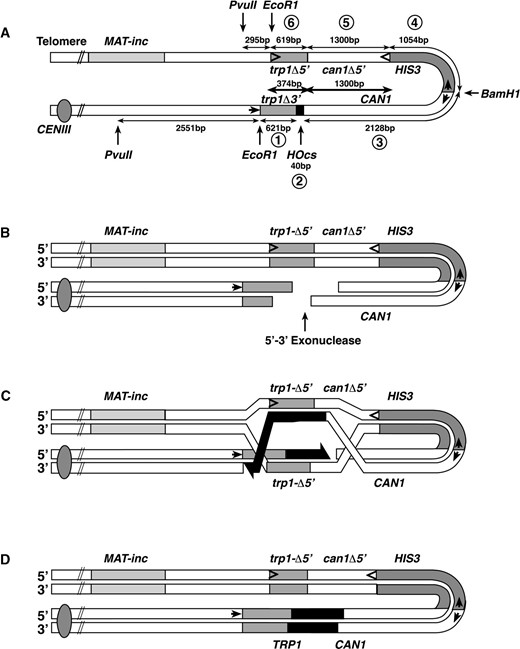
Construction of the inverted-repeat substrate: The inverted repeat shown in Figure 1 is a 5.8-kb insertion at the native EcoRI site, just centromere proximal to MAT. The normal MAT locus was mutated to be uncleavable by HO (MAT-inc, Table 1; Figure 1). The insertion is made up of the following six segments (Figure 1): (1) trp1-3′Δ is a 621-bp EcoRI-HindIII fragment of TRP1 that includes 103 bp of the promoter and the first 518 bp of the 672-bp TRP1 ORF; (2) HOcs (cleavage site) is a 40-bp oligonucleotide containing a 30-bp recognition site for the HO-endonuclease in inverted orientation relative to MAT; (3) CAN1 is a 2128-bp fragment carrying the entire 1770-bp CAN1 ORF including 154 bp of upstream DNA sequence with the CAN1 promoter and 204 bp of downstream sequence; (4) HIS3 is a 1054-bp fragment carrying the entire 657-bp HIS3 ORF including 200 bp of upstream sequence carrying the promoter and 197 bp of downstream sequence; (5) can1-5′Δ is a 1300-bp EcoRI-BglII fragment of can1 carrying the last 1096 bp of the 1770-bp CAN1 ORF and 204 bp of downstream sequence; and (6) trp1-5′Δ is a 619-bp fragment of TRP1 including the last 528 bp of the 672-bp ORF and 91 bp of downstream sequence. The inverted repeat was inserted into chromosome III by a two-step process: First, segments 4 (carrying a his3-190 mutation), 5, and 6 were inserted as a substitution for URA3 in the MAT-inc strains GRY1509 and GRY1559 by selection for Ura- (FOAr; Boeke et al. 1987). Then, segments 1-3 and a HIS3 version of segment 4 were inserted by selection for His+. The entire inverted repeat is designated mush18/21 (Table 1). The resulting strains were then deleted for the CAN1 ORF by transformation with a SacI-XhoI fragment from pMUSHΔ17 containing the can1-Δ17 allele (see above) and selecting for Ura+. The URA3 gene was recombined out of the canavanine-resistant transformants by selecting for 5-fluoroorotic acid resistance (Alani et al. 1987; Boeke et al. 1987), resulting in strains GRY1644 and GRY1647, respectively.
Mutagenesis: Log-phase cells from strains GRY1650 and GRY1654 grown on SC -Ura media to select for the pGALHO plasmid were plated on solid YEPD agar in dilutions varying from 102 to 106 cells per plate and were irradiated together in a Stratalinker (Stratagene, La Jolla, CA) at several doses between 0 and 200 J/m2. Independent colonies from plates showing 7-30% survival after UV treatment were then patched onto SC -Ura and monitored for DSB-induced recombination and mutation by replica-plating. First, the SC -Ura plates were replica-plated to SC -Trp, SC -Arg +Can (SC +Can), and SC -Arg -Trp +Can (SC -Trp +Can) to determine the spontaneous levels of recombination, mutation, and mutation associated with recombination, respectively, and to SC -Ura + galactose (SC -Ura +Gal) to induce expression of the HO endonuclease. After ∼24 hr, the SC -Ura +Gal plates were replica-plated to SC -Trp, SC +Can, and SC -Trp +Can to determine the DSB-induced levels of recombination, mutation, or mutation associated with recombination, respectively. Patches showing a DSB-dependent phenotype different from the wild type were colony purified and several colonies were retested by the patch assay. The remaining candidates were then scored for growth differences on YEPD at 20°, 30°, and 37° for sensitivity to 3% formamide, 3% ethanol, 0.02% methyl methanesulfonate (MMS), or irradiation with 150 J/m2 UV. Mutant cells were crossed to a wild-type strain of the opposite mating type (GRY1650 or GRY1654) to determine if the mutation was dominant or recessive, due to a single locus, and to generate mutant spores with opposite mating types. Mutant spores of opposite mating types resulting from the crosses were then crossed to each other to determine if they displayed any homozygous diploid phenotypes, including the ability to sporulate.
Cloning of m342: A homozygous diploid strain of m342 lacking the pGalHO plasmid was transformed with aliquots from several pools of a genomic YCp50 library (Rose et al. 1987), and transformants were selected on SC -Ura. Patches of transformed cells grown on SC -Ura were replica-plated to sporulation media for 3 days. The sporulation plates were then replica-plated to unsupplemented 2% agar plates, incubated overnight, followed by replica-plating to SC -Ura plates. We found that the overnight incubation on the unsupplemented agar plates greatly enhanced the killing of unsporulated cells. From ∼1500 library transformants, we isolated a single patch that survived the replica-plating regimen and that was also noted to have asci upon examination of cells from the sporulation plate. The plasmid was rescued into Escherichia coli cells and retransformed into the homozygous diploid cells to confirm complementation of the sporulation defect. Sequence analysis of the plasmid identified a 7.8-kb fragment of chromosome VII containing the full-length ORFs of genes YGL174-YGL179 and only short 3′-terminal coding regions for the KEM1/SEP1 and APG1 ORFs. Further subcloning and complementation indicated that plasmids harboring the full-length SAE2/COM1 ORF alone (YGL175C) were sufficient for full complementation of the sporulation, MMS sensitivity, and mutator phenotypes (data not shown).
Measurements of recombination frequencies: Recombination frequencies were measured by streaking the appropriate strain on minimal media agar plates to select for the HO plasmid (SC -Ura for pGalHO or SC -Leu for pCHOL). Between 3 and 11 single colonies were inoculated into selective liquid media (SC -Ura or SC -Leu) and grown to a density of ∼1-8 × 107 cells/ml. The cells were pelleted, washed twice with sterile water, and the appropriate dilutions were plated to determine preinduction titers. An aliquot of each culture was transferred into liquid selective galactose media to induce expression of the HO endonuclease and then grown with aeration at 30° for ∼16 hr. Cells were pelleted, washed with sterile water, and appropriate dilutions were plated to determine HO-induced titers. Total cell titers were determined by plating appropriate dilutions on YEPD and either SC -Ura or SC -Leu. Spontaneous and HO-induced events were determined by plating on SC -Trp, SC +Can, and SC -Trp +Can. The frequency was determined by the method of the median (Lea and Coulson 1949), and the average of at least three independent fluctuation tests is shown in Table 2 (± SD). A minimum of 17 independent colonies was examined for each strain.
Confirmation of disomy: Strains that were candidates for chromosome III disomy were crossed to strain GRY1509, which has a URA3 inserted at the same position as the recombination substrate. Hence, URA3 and the HIS3-marked recombination substrate should act as alleles and be coupled to MATa and MATα, respectively. While the parent strain yAR577 crossed to GRY1509 segregated two His+ Ura- MATα and two His- Ura+ MATa spores in each tetrad, segregants from the putative disomes gave an excess of His+ spores, including spores that were His+ Ura+ and nonmaters.
Southern blot analysis: Independent recombinants were colony-purified from the appropriate selective media from cells either before (spontaneous) or after (HO-induced) galactose induction. DNA was prepared by the glass bead disruption method (Hoffman and Winston 1987). Aliquots were digested with the appropriate restriction endonucleases according to manufacturer’s instructions (New England Biolabs, Beverly, MA) and electrophoresed in agarose. The DNA was then transferred to a Hybond N+ nylon membrane (Amersham, Piscataway, NJ) and hybridized under standard conditions (Ausubel et al. 1994) using the appropriate 32P-labeled fragment as a probe (Rediprime II; Amersham). After washing, the radioactivity was visualized by either autoradiography or phosphorimager analysis.
RESULTS
Inverted-repeat substrate for monitoring the fidelity of DSB repair in haploid cells: The detailed construction of the inverted-repeat substrate is described in materials and methods. The chromosomally integrated construct is shown in Figure 1A. The normal MAT HO endonuclease recognition site was mutated to be noncleavable by HO (MAT-inc) in all strains used (Table 1). These strains are also deleted for the chromosomal alleles of can1, his3, leu2, and trp1; have a ura3-52 disruption; and harbor the inverted-repeat substrate (mush18/21) on chromosome III, adjacent to the MAT-inc locus (Figure 1A). The substrate consists of a 3′-end truncation of TRP1 (trp1-3′Δ) adjacent to a recognition sequence for the HO endonuclease (HOcs), which is the only cleavable HO recognition site in the genome. Adjacent to the HOcs is a full-length wild-type CAN1 gene that encodes arginine permease and confers sensitivity to the cell-toxic arginine analog canavanine. The opposite side of the inverted repeat has a 5′ truncation of TRP1 (trp1-5′Δ) resulting in 374 bp of homology between the trp1 alleles, as well as a 5′ truncation of the CAN1 gene (can1-5′Δ), which shares 1.3 kb of homology with the full-length CAN1 insert. A wild-type copy of the HIS3 gene separates the inverted repeats. The HO endonuclease was expressed from a centromere-containing plasmid (pGalHO or pCHOL, Table 1) under control of the GAL1 promoter. Removal of glucose from the growth media and addition of galactose results in transcription and translation of the HO-endonuclease protein, which then cleaves at the HO recognition sequence within the inverted repeat, producing a DSB with 3′ overhangs (Figure 1B; Nickoloff et al. 1986). This DSB can be repaired by homologous recombination using the trp1 and can1 sequences from the other unbroken repeat (Figure 1C). This results in a loss of the HO-endonuclease recognition sequence and reconstitution of a full-length wild-type TRP1 gene (Figure 1D), which can be assayed by monitoring recombinants on media lacking tryptophan. Mutations made during DSB repair that inactivate the wild-type CAN1 gene become resistant to canavanine and can be monitored as cells that can grow in the presence of this drug.
Frequency of recombination and mutation in wild-type, sae2Δ, mre11s-H125N, and rad50s-K81I strains
| Relevant genotype (strain)a . | Spontaneous frequency × 106 (±SD)b . | HO-induced frequency × 106 (±SD) . | ||||
|---|---|---|---|---|---|---|
| Trp+ . | Canr . | Trp+ Canr . | Trp+ . | Canr . | Trp+ Canr . | |
| Wild type (yAR577) | 4.0 | 1.1 | <0.012 | 24,000 | 150 | 3.7 |
| (1.4)b | (0.86) | (0.0045) | (14,000) | (33) | (2.4) | |
| sae2Δ (yAR579) | 5.3 | 3.3 | <0.015 | 18,000 | 410 | 22 |
| (4.1) | (2.7) | (0.0017) | (7,900) | (87) | (8.3) | |
| mre11s-H125N (yAR627) | 5.6 | 2.0 | 0.0087 | 25,000 | 280 | 26 |
| (3.4) | (0.97) | (0.0038) | (17,000) | (57) | (11) | |
| rad50s-KI81 (yAR583) | 2.6 | 2.0 | <0.011 | 31,000 | 460 | 20 |
| (1.4) | (1.0) | (0.0052) | (7,400) | (120) | (3.7) | |
| Relevant genotype (strain)a . | Spontaneous frequency × 106 (±SD)b . | HO-induced frequency × 106 (±SD) . | ||||
|---|---|---|---|---|---|---|
| Trp+ . | Canr . | Trp+ Canr . | Trp+ . | Canr . | Trp+ Canr . | |
| Wild type (yAR577) | 4.0 | 1.1 | <0.012 | 24,000 | 150 | 3.7 |
| (1.4)b | (0.86) | (0.0045) | (14,000) | (33) | (2.4) | |
| sae2Δ (yAR579) | 5.3 | 3.3 | <0.015 | 18,000 | 410 | 22 |
| (4.1) | (2.7) | (0.0017) | (7,900) | (87) | (8.3) | |
| mre11s-H125N (yAR627) | 5.6 | 2.0 | 0.0087 | 25,000 | 280 | 26 |
| (3.4) | (0.97) | (0.0038) | (17,000) | (57) | (11) | |
| rad50s-KI81 (yAR583) | 2.6 | 2.0 | <0.011 | 31,000 | 460 | 20 |
| (1.4) | (1.0) | (0.0052) | (7,400) | (120) | (3.7) | |
Frequencies are the average of at least three independent fluctuation tests as described in materials and methods.
Complete genotypes are listed in Table 1.
Numbers in parentheses indicate standard deviation of the average median frequency from the fluctuation tests.
Frequency of recombination and mutation in wild-type, sae2Δ, mre11s-H125N, and rad50s-K81I strains
| Relevant genotype (strain)a . | Spontaneous frequency × 106 (±SD)b . | HO-induced frequency × 106 (±SD) . | ||||
|---|---|---|---|---|---|---|
| Trp+ . | Canr . | Trp+ Canr . | Trp+ . | Canr . | Trp+ Canr . | |
| Wild type (yAR577) | 4.0 | 1.1 | <0.012 | 24,000 | 150 | 3.7 |
| (1.4)b | (0.86) | (0.0045) | (14,000) | (33) | (2.4) | |
| sae2Δ (yAR579) | 5.3 | 3.3 | <0.015 | 18,000 | 410 | 22 |
| (4.1) | (2.7) | (0.0017) | (7,900) | (87) | (8.3) | |
| mre11s-H125N (yAR627) | 5.6 | 2.0 | 0.0087 | 25,000 | 280 | 26 |
| (3.4) | (0.97) | (0.0038) | (17,000) | (57) | (11) | |
| rad50s-KI81 (yAR583) | 2.6 | 2.0 | <0.011 | 31,000 | 460 | 20 |
| (1.4) | (1.0) | (0.0052) | (7,400) | (120) | (3.7) | |
| Relevant genotype (strain)a . | Spontaneous frequency × 106 (±SD)b . | HO-induced frequency × 106 (±SD) . | ||||
|---|---|---|---|---|---|---|
| Trp+ . | Canr . | Trp+ Canr . | Trp+ . | Canr . | Trp+ Canr . | |
| Wild type (yAR577) | 4.0 | 1.1 | <0.012 | 24,000 | 150 | 3.7 |
| (1.4)b | (0.86) | (0.0045) | (14,000) | (33) | (2.4) | |
| sae2Δ (yAR579) | 5.3 | 3.3 | <0.015 | 18,000 | 410 | 22 |
| (4.1) | (2.7) | (0.0017) | (7,900) | (87) | (8.3) | |
| mre11s-H125N (yAR627) | 5.6 | 2.0 | 0.0087 | 25,000 | 280 | 26 |
| (3.4) | (0.97) | (0.0038) | (17,000) | (57) | (11) | |
| rad50s-KI81 (yAR583) | 2.6 | 2.0 | <0.011 | 31,000 | 460 | 20 |
| (1.4) | (1.0) | (0.0052) | (7,400) | (120) | (3.7) | |
Frequencies are the average of at least three independent fluctuation tests as described in materials and methods.
Complete genotypes are listed in Table 1.
Numbers in parentheses indicate standard deviation of the average median frequency from the fluctuation tests.
Patches of wild-type haploid cells before and after induction of the HO endonuclease are shown in Figure 2 (wild type), clearly demonstrating the efficient production of TRP1 recombinants and the elevated number of can1 mutants after induction of the HO endonuclease. The average of three independent liquid fluctuation tests is shown in Table 2 (wild type). The spontaneous recombination frequency to TRP1 is low (∼4 × 10-6). The spontaneous mutation frequency to can1 is also low (∼1 × 10-6) and is similar to the reported frequency of spontaneous can1 mutations at the native CAN1 locus (Whelan et al. 1979). TRP1 recombinants having an associated can1 mutation are rare (<1.2 × 10-8). Transient expression of the HO endonuclease increases the frequency of TRP1 recombinants by 6000-fold (compare TRP1 spontaneous with TRP1 induced, Table 2, wild type). TRP1 recombinants arise by homologous recombination, and therefore the majority of the HO-induced TRP1 events are expected to be phenotypically His+ Cans. When independent TRP1 recombinants were patched and replica-plated to determine their Can and His phenotypes, we found that 99.6% of the recombinants were in fact CAN1 HIS3 (Table 3, wild type). Therefore, most TRP1 recombinants were apparently repaired by homologous recombination in an error-free fashion. The introduction of a DSB by the HO endonuclease increased the frequency of can1 mutations in wild-type strains by ∼100-fold (wild-type Cans, Table 2). However, when independent can1 events were analyzed by patching and replica plating, we found that the majority (95.4%) appeared to be deletions of the substrate due to loss of the HIS3 gene (Trp- His- and Trp+ His-, Table 3). Subsequent Southern blot analysis of can1 events indicated that the majority had lost sequences that could hybridize with a HIS3 probe and had altered restriction patterns in the region adjacent to the substrate (data not shown). HO induction resulted in a >300-fold increase in TRP1 recombinants that had an associated can1 mutation (wild-type TRP1 can1, Table 2). Although the frequency of TRP1 can1 events appears to be multiplicative (wild type, Table 2), suggesting independent origins for TRP1 recombinants and can1 mutations, this in fact is not the case. Because the majority of can1 events were deleted for the substrate (see above), these events could not contribute to the pool of TRP1 events. If we consider only the proportion of can1 mutants that retain the substrate, then we find that the association between TRP1 recombinants and can1 mutants is at least 15-fold higher than expected.
—Inverted-repeat assay for recombinational fidelity. (A) Relative location, fragment sizes, and alleles used to construct the inverted-repeat substrate. Circled numbers refer to the fragments and double-headed arrows refer to fragment sizes used in the construction as described in materials and methods. Heavier double-headed arrows in the center refer to the lengths of overlapping homology. Small internal arrows indicate the location of the promoters and the direction of transcription, and internal arrowheads indicate the gene orientation on fragments lacking promoters (only shown on one strand). Sites marked EcoRI indicate the location at which the inverted repeat was inserted near the MAT-inc locus. PvuII and BamH1 indicate the location of the restriction sites used for Southern blot analysis in Figure 3. The centromere is indicated by the filled circle. (B) Expression of the HO-endonuclease from a galactose-regulatable promoter results in a site-specific DSB that is presumably resected to produce long 3′ tails from the site of the break. (C) Invasion by the broken ends of the DNA followed by DNA synthesis (black lines) across the site of the break results in a recombination intermediate. (D) Resolution of the intermediate shown in C results in a TRP1 recombinant that can either be a noncrossover (shown) or a crossover (not shown), in which case the intervening sequences including HIS3 would be inverted. Errors associated with the DNA repair synthesis may result in mutations in the CAN1 gene.
We consistently found a greater percentage of the TRP1 events to have an associated can1 mutation in the data obtained by testing patched TRP1 recombinants (Table 3) than by directly plating for TRP1 can1 events (Table 2). We believe that this difference reflects a plating artifact, possibly due to a requirement for a period of outgrowth necessary to remove the arginine permease from the membrane. Also, TRP1 cells harboring the substrate grow slowly on media lacking tryptophan, and it is possible that the high density of cells plated on SC -Trp +Can media further slows the growth of the TRP1 cells. The patching data is likely to be a more accurate monitor of the real proportion of TRP1 can1 events, but fewer cells can be scored in this way. Both approaches demonstrate that the production of can1 mutations is associated with the formation of TRP1 recombinants.
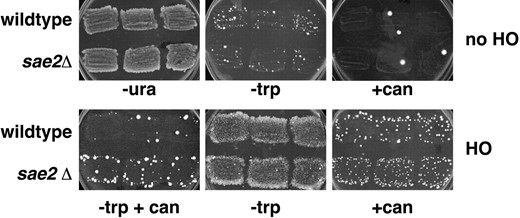
—Phenotype of wild-type cells and sae2Δ mutant cells in inverted-repeat assay. Three individual patches of each strain GRY1654 (wild type) and yAR523 (sae2Δ) grown on SC -Ura + glucose were replica-plated to SC -Trp and to SC +Can to determine spontaneous recombinants and mutants, respectively (no HO), and to SC -Ura + galactose (not shown) to induce expression of the HO endonuclease. After 24 hr, the galactose plates were replica-plated to SC -Trp +Can, SC -Trp, and SC +Can to determine the induced levels of mutations associated with recombination, induced recombinants, and induced mutations, respectively. Note the increased papillae in the SC -Trp +Can patches for sae2Δ mutants after induction with galactose.
Identification of mutants with altered fidelity of DSB repair: Patches of mutagenized yeast cells were scored by replica-plating both before or after galactose induction as described in materials and methods. Cells from mutagenized patches showing altered levels of TRP1 can1 events after HO induction were colony-purified and several colonies of each candidate were retested by the same patching and replica-plating sequence. Six candidates showed an altered frequency of mutations associated with recombination after the secondary screen, and they were saved for further characterization.
Several different phenotypes were scored for each candidate, as described in materials and methods. A summary of the relevant phenotypes is shown in Table 4.
Phenotypic distribution of events
| . | . | Phenotype (%) . | |||
|---|---|---|---|---|---|
| Strain . | Trp+ . | His+ Cans . | His+ Canr . | His- . | . |
| Wild type | 1494 (99.6) | 6 (0.4) | 0 (<0.06) | ||
| sae2Δ | 458 (87.5) | 61 (11.7) | 4 (0.8) | ||
| Canr | Trp- His+ | Trp- His- | Trp+ His+ | Trp+ His- | |
| Wild type | 23 (3.1) | 711 (94.7) | 11 (1.5) | 5 (0.7) | |
| sae2Δ | 21 (2.8) | 681 (90.8) | 26 (3.5) | 22 (2.9) | |
| . | . | Phenotype (%) . | |||
|---|---|---|---|---|---|
| Strain . | Trp+ . | His+ Cans . | His+ Canr . | His- . | . |
| Wild type | 1494 (99.6) | 6 (0.4) | 0 (<0.06) | ||
| sae2Δ | 458 (87.5) | 61 (11.7) | 4 (0.8) | ||
| Canr | Trp- His+ | Trp- His- | Trp+ His+ | Trp+ His- | |
| Wild type | 23 (3.1) | 711 (94.7) | 11 (1.5) | 5 (0.7) | |
| sae2Δ | 21 (2.8) | 681 (90.8) | 26 (3.5) | 22 (2.9) | |
Cells were patched on the media originally selected (SC -Trp or SC + Can) and were subsequently replicaplated to SC -His, SC +Can, and SC -Trp.
Phenotypic distribution of events
| . | . | Phenotype (%) . | |||
|---|---|---|---|---|---|
| Strain . | Trp+ . | His+ Cans . | His+ Canr . | His- . | . |
| Wild type | 1494 (99.6) | 6 (0.4) | 0 (<0.06) | ||
| sae2Δ | 458 (87.5) | 61 (11.7) | 4 (0.8) | ||
| Canr | Trp- His+ | Trp- His- | Trp+ His+ | Trp+ His- | |
| Wild type | 23 (3.1) | 711 (94.7) | 11 (1.5) | 5 (0.7) | |
| sae2Δ | 21 (2.8) | 681 (90.8) | 26 (3.5) | 22 (2.9) | |
| . | . | Phenotype (%) . | |||
|---|---|---|---|---|---|
| Strain . | Trp+ . | His+ Cans . | His+ Canr . | His- . | . |
| Wild type | 1494 (99.6) | 6 (0.4) | 0 (<0.06) | ||
| sae2Δ | 458 (87.5) | 61 (11.7) | 4 (0.8) | ||
| Canr | Trp- His+ | Trp- His- | Trp+ His+ | Trp+ His- | |
| Wild type | 23 (3.1) | 711 (94.7) | 11 (1.5) | 5 (0.7) | |
| sae2Δ | 21 (2.8) | 681 (90.8) | 26 (3.5) | 22 (2.9) | |
Cells were patched on the media originally selected (SC -Trp or SC + Can) and were subsequently replicaplated to SC -His, SC +Can, and SC -Trp.
Mutant m362 was found to be dominant and the mutation was substrate linked. This mutant is probably a mutation in the truncated repeat of can1 (can1-5′Δ, Figure 1), resulting in a very high transfer of can1 to the wild-type allele upon recombination induction.
Mutants m342 and m431 were sensitive to MMS and were also sporulation defective. These mutants were crossed to strains disrupted for rad51, rad52, rad55, or rad57. All four disruption strains complemented m342, suggesting that the mutation is not in any of these genes. Further characterization of this mutant is described below. Mutant m431 was not complemented by a rad57 mutant strain, was partially complemented by a rad55 mutant strain, and was fully complemented by strains with null mutations in rad51 and rad52. Restoration of sporulation, MMS resistance, and loss of both the spontaneous and HO-induced mutator phenotypes was obtained for mutant m431 upon transformation with a plasmid bearing a wild-type copy of the RAD57 gene. Further characterization of m431 will be described elsewhere along with a characterization of strains with null mutations in several members of the RAD52 epistasis group (A. J. Rattray and J. N. Strathern, unpublished data).
Two additional mutants, m499 and m500, had very high levels of spontaneous mutation to can1. The mutants are not in the same gene, as they are recessive, complement one another, and heterozygous diploids produce wild-type spores, suggesting that they are not linked. Mutant m403 appeared to be slightly temperature sensitive for growth, but it proved too leaky to allow cloning of the wild-type gene by complementation. No additional phenotypes were detected for mutants m403, m499, and m500, and they have not been further characterized at this time.
Mutant m342 is allelic to SAE2/COM1: Homozygous diploid m342 strains were used for cloning of the wild-type allele by complementation of the sporulation defect as described in materials and methods. We isolated a single complementing plasmid, and further subcloning and complementation indicated that a plasmid containing only the full-length SAE2/COM1 ORF (YGL175C) was sufficient for full complementation of the sporulation, MMS sensitivity, and DSB-induced mutator phenotypes (data not shown). We also found that a strain disrupted for the SAE2 ORF (sae2Δ::KanMX) was unable to complement m342, and that a sae2Δ strain had a DSB-induced mutator phenotype identical to the original m342 allele (data not shown). All subsequent experiments were performed using the sae2Δ allele rather than the original point mutation.
Mutants and phenotypes
| . | Temp. sensitivity . | MMS sensitivity . | Sporulation . | Spontaneous mutator . | DSB-induced mutator . | Other . |
|---|---|---|---|---|---|---|
| Wild type | No | No | Yes | No | No | |
| m362 | No | No | Yes | NAa | NA | Substrate linked |
| m431 | No | Very | No | Yes | Yes | Recombination deficient |
| m342 | No | Slightly | No | No | Yes | |
| m499 | No | No | Yes | Yes | Yes | |
| m500 | No | No | Yes | Yes | Yes | |
| m403 | Slightly | No | Yes | No | Yes |
| . | Temp. sensitivity . | MMS sensitivity . | Sporulation . | Spontaneous mutator . | DSB-induced mutator . | Other . |
|---|---|---|---|---|---|---|
| Wild type | No | No | Yes | No | No | |
| m362 | No | No | Yes | NAa | NA | Substrate linked |
| m431 | No | Very | No | Yes | Yes | Recombination deficient |
| m342 | No | Slightly | No | No | Yes | |
| m499 | No | No | Yes | Yes | Yes | |
| m500 | No | No | Yes | Yes | Yes | |
| m403 | Slightly | No | Yes | No | Yes |
Phenotypes were determined as described in materials and methods.
NA, not applicable, see text.
Mutants and phenotypes
| . | Temp. sensitivity . | MMS sensitivity . | Sporulation . | Spontaneous mutator . | DSB-induced mutator . | Other . |
|---|---|---|---|---|---|---|
| Wild type | No | No | Yes | No | No | |
| m362 | No | No | Yes | NAa | NA | Substrate linked |
| m431 | No | Very | No | Yes | Yes | Recombination deficient |
| m342 | No | Slightly | No | No | Yes | |
| m499 | No | No | Yes | Yes | Yes | |
| m500 | No | No | Yes | Yes | Yes | |
| m403 | Slightly | No | Yes | No | Yes |
| . | Temp. sensitivity . | MMS sensitivity . | Sporulation . | Spontaneous mutator . | DSB-induced mutator . | Other . |
|---|---|---|---|---|---|---|
| Wild type | No | No | Yes | No | No | |
| m362 | No | No | Yes | NAa | NA | Substrate linked |
| m431 | No | Very | No | Yes | Yes | Recombination deficient |
| m342 | No | Slightly | No | No | Yes | |
| m499 | No | No | Yes | Yes | Yes | |
| m500 | No | No | Yes | Yes | Yes | |
| m403 | Slightly | No | Yes | No | Yes |
Phenotypes were determined as described in materials and methods.
NA, not applicable, see text.
Characterization of the DSB-induced mutator phenotype in sae2Δ strains: Figure 2 shows a comparison of patches from wild-type and sae2Δ mutant strains before and after HO induction. In particular, note the higher level of TRP1 can1 colonies in the sae2Δ mutant after HO induction, reflecting increased mutations associated with recombination. The average (± SD) of three independent fluctuation tests (each consisting of between 3 and 11 colonies) to measure recombination and mutation frequencies in the inverted-repeat substrate is shown in Table 2. As was found for the wild-type cells, induction of the HO endonuclease in sae2Δ cells stimulates recombination to TRP1 by three to four orders of magnitude. There was no significant difference in the spontaneous or induced recombination rate to TRP1 between SAE2 and sae2Δ strains, indicating that SAE2 is not important for recombination per se. Induction of HO increased mutation to can1 by ∼100-fold. Although there was a slight increase in the spontaneous and induced can1 frequencies in the sae2Δ strains as compared to wild-type strains, these are not statistically significant by chi-square analysis (P > 0.1). Spontaneous TRP1 can1 events are rare, but we did detect an ∼1500-fold increase in these events after HO induction to a level 6-fold above that seen in wild-type cells. The difference between wild-type and sae2Δ strains is readily apparent in the phenotypic analysis of TRP1 recombinants (Table 3). We find that 11.7% of the DSB-induced TRP1 recombinants were also canavanine resistant in the sae2Δ strain as compared to 0.4% in the wild-type strain. As noted above for the wild-type strain, we found that the data obtained by plating (Table 2) underestimated the proportion of TRP1 recombinants having an associated can1 mutation as determined by phenotypic analysis (Table 3). In any event, both analyses found that TRP1 can1 events were increased in sae2Δ strains, and that the plating data indicated a similar difference between the two strains. We found that the proportion of events that had deleted the substrate (His- Canr) was slightly decreased in the sae2Δ strain relative to the wild-type strain (93.7 vs. 95.4%, Table 3); however, most can1 events were still associated with a deletion of the substrate, indicating that the level of TRP1 can1 events was ∼50-fold greater than would be expected for independent association of these events.
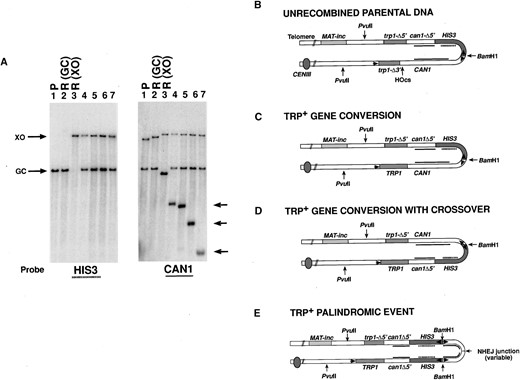
SAE2 is required to avoid a unique class of DSB-induced gene rearrangements: The structure of the products of spontaneous and HO-induced TRP1 CAN1 and TRP1 can1 recombinants from both wild-type and sae2Δ strains were analyzed by Southern blots. Figure 3A shows an example of DNAs digested with BamHI and PvuII from an unrecombined (Trp-) parental cell (P), a TRP1 gene conversion event (GC), and a TRP1 gene conversion associated with a crossover (XO; Figure 3A, lanes 1-3, respectively). The expected products of these digests are shown in Figure 3, B-D, respectively. In all three cases, digestion with BamHI and PvuII is expected to hybridize with a single fragment when probed with a HIS3 32P-labeled fragment. The DNA fragments migrate with the expected size of 3.28 kb for the unrecombined parent and for a noncrossover gene conversion event and with a size of 5.87 kb for a crossover-associated event (Figure 3A). The blots were stripped and reprobed with a 32P-labeled CAN1 DNA fragment. As expected (see Figure 3, B-D), the CAN1 probe hybridizes with two fragments for each type of event: 3.28- and 5.45-kb fragments for the unrecombined parental DNA, 3.28- and 5.64-kb fragments for DNA from a TRP1 gene conversion event, and 3.05- and 5.87-kb fragments in DNA from a TRP1 event with an associated crossover.
—Southern blot analysis of Trp+ recombinants. (A) Southern blot hybridization of DNAs from a sae2Δ strain (yAR579) digested with PvuII and BamHI. Expected fragments and location of the probes utilized are shown in B-D. Lane 1, unrecombined Trp- parental DNA (P in B); lane 2, Trp+ Cans recombinant with a simple gene conversion event (R-GC, in C); lane 3, Trp+ Cans recombinant with a gene conversion associated with a crossover (R-XO, in D). Lanes 4-7 are four independent Trp+ Canr recombinants showing palindromic gene rearrangements (E). The blot was first hybridized with a 32P-labeled probe of the HIS3 ORF as shown on the left. The probe was washed off and the blot was subsequently hybridized with a 32P-labeled HindIII fragment from the CAN1 gene. Arrows indicate variably sized novel CAN1 hybridizing bands in the palindromic rearrangements. (B) Structure of unrecombined parental DNA. Sites for cleavage by PvuII and BamHI are indicated. Locations of probes used in A are indicated by the internal lines. Internal arrows indicate the location of the promoters and the direction of transcription. (C) Structure of a Trp+ gene conversion event unassociated with a crossover. (D) Structure of a Trp+ gene conversion event associated with a crossover inverting HIS3 relative to the centromere (solid circle). (E) An example of the deduced structure of the palindromic events shown in lanes 4-7 of A.
A summary of the Southern blot analysis for the wild-type strain is shown in Table 5 (wild type). We found that 18/18 spontaneous TRP1 events were all gene conversions without an associated crossover. In the analysis of HO-induced cultures shown in Table 5 (wild type), we found that 23/45 TRP1 CAN1 events appeared to be gene conversions without crossover while 21 had an associated crossover. Some of the TRP1 recombinants from HO-induced cultures had restriction patterns that were consistent with having two copies of chromosome III, where at least one copy had an apparent crossover event. Disomy for chromosome III was confirmed by classical genetics (see materials and methods). We interpret these disomes as being G2 events that failed to segregate properly. Only 1/45 TRP1 CAN1 events analyzed had an unpredicted chromosomal rearrangement, suggesting that recombinational repair was efficient and fairly accurate. As noted above, 0.4% of the HO-induced TRP1 recombinants in wild-type cells had associated can1 mutations. Southern blot analysis of these events indicated that the majority of the mutations appear to be point mutations made during normal recombination, resulting in simple gene conversions either without (31/44) or with (10/44) an associated crossover. Only 3/44 had aberrant rearrangements of can1.
Distribution of events by physical analysis
| Genotypea . | Phenotype of selected event . | Gene conversion . | Gene conversion with crossover . | Palindromic event . | Other rearrangement . |
|---|---|---|---|---|---|
| Wild type | Spontaneous Trp+ | 18 | |||
| HO-induced Trp+ Cans | 23 | 21b | 1 | ||
| HO-induced Trp+ Canr | 31 | 10 | 1 | 2 | |
| sae2Δ | Spontaneous Trp+ | 18 | 1 | 1 | |
| HO-induced Trp+ Cans | 23 | 6b | 1 | ||
| HO-induced Trp+ Canr | 21 | 5 | 27 | 3 | |
| mre11s-H125N | HO-induced Trp+ Canr | 14 | 4 | 16 | 2 |
| rad50s-K81I | HO-induced Trp+ Canr | 15 | 5 | 14 | 2 |
| Genotypea . | Phenotype of selected event . | Gene conversion . | Gene conversion with crossover . | Palindromic event . | Other rearrangement . |
|---|---|---|---|---|---|
| Wild type | Spontaneous Trp+ | 18 | |||
| HO-induced Trp+ Cans | 23 | 21b | 1 | ||
| HO-induced Trp+ Canr | 31 | 10 | 1 | 2 | |
| sae2Δ | Spontaneous Trp+ | 18 | 1 | 1 | |
| HO-induced Trp+ Cans | 23 | 6b | 1 | ||
| HO-induced Trp+ Canr | 21 | 5 | 27 | 3 | |
| mre11s-H125N | HO-induced Trp+ Canr | 14 | 4 | 16 | 2 |
| rad50s-K81I | HO-induced Trp+ Canr | 15 | 5 | 14 | 2 |
See Table 1 for full genotypes. Wild type, GRY1654 and yAR577; sae2Δ, yAR523 and yAR579; rad50s-K81I, yAR583; mre11s-H125N, yAR627.
Includes events disomic for chromosome III (see text).
Distribution of events by physical analysis
| Genotypea . | Phenotype of selected event . | Gene conversion . | Gene conversion with crossover . | Palindromic event . | Other rearrangement . |
|---|---|---|---|---|---|
| Wild type | Spontaneous Trp+ | 18 | |||
| HO-induced Trp+ Cans | 23 | 21b | 1 | ||
| HO-induced Trp+ Canr | 31 | 10 | 1 | 2 | |
| sae2Δ | Spontaneous Trp+ | 18 | 1 | 1 | |
| HO-induced Trp+ Cans | 23 | 6b | 1 | ||
| HO-induced Trp+ Canr | 21 | 5 | 27 | 3 | |
| mre11s-H125N | HO-induced Trp+ Canr | 14 | 4 | 16 | 2 |
| rad50s-K81I | HO-induced Trp+ Canr | 15 | 5 | 14 | 2 |
| Genotypea . | Phenotype of selected event . | Gene conversion . | Gene conversion with crossover . | Palindromic event . | Other rearrangement . |
|---|---|---|---|---|---|
| Wild type | Spontaneous Trp+ | 18 | |||
| HO-induced Trp+ Cans | 23 | 21b | 1 | ||
| HO-induced Trp+ Canr | 31 | 10 | 1 | 2 | |
| sae2Δ | Spontaneous Trp+ | 18 | 1 | 1 | |
| HO-induced Trp+ Cans | 23 | 6b | 1 | ||
| HO-induced Trp+ Canr | 21 | 5 | 27 | 3 | |
| mre11s-H125N | HO-induced Trp+ Canr | 14 | 4 | 16 | 2 |
| rad50s-K81I | HO-induced Trp+ Canr | 15 | 5 | 14 | 2 |
See Table 1 for full genotypes. Wild type, GRY1654 and yAR577; sae2Δ, yAR523 and yAR579; rad50s-K81I, yAR583; mre11s-H125N, yAR627.
Includes events disomic for chromosome III (see text).
The summary of the Southern blot analysis for sae2Δ strains is shown in Table 5. We found that 18/20 spontaneous TRP1 recombinants were gene conversion events unassociated with a crossover (sae2Δ, Table 5), 1/20 was a gene conversion associated with a crossover, and 1/20 was a gene rearrangement (see below). We found that most HO-induced TRP1 CAN1 events were due to gene conversions without a crossover (23/30), and six were gene conversions associated with a crossover. We again found some events to be disomic for chromosome III. Only one event appeared to have an aberrant rearrangement (see below).
From the Southern blot analysis of TRP1 can1 events from sae2Δ strains (Table 5) we found that 21/56 were noncrossover gene conversion events and 5/56 were gene conversions associated with a crossover. Remarkably, we found that 30/56 events had an associated gene rearrangement, and that at least 27 of these rearrangements appeared to have a related structure. Four independent representative examples are shown in Figure 3A, lanes 4-7. We found that these events had two bands that hybridized with the HIS3 probe, indicating a duplication of HIS3 sequences. These bands migrated with the 3.28-kb fragment expected for a simple gene conversion event and with the 5.87-kb fragment expected for a crossover-associated event. After stripping and rehybridization of the same blots with a CAN1 probe, we found that the same two bands hybridized with CAN1 sequences as well as a third band of variable size. Digestion with BamHI alone releases an identically variably sized fragment that hybridizes with CAN1 sequences (data not shown). By digestion with different restriction enzymes we determined that the structure appears to be symmetrical. The resulting structure is shown in Figure 3E, where the loop end consists of can1 sequences of variable size. Furthermore, the aberrant TRP1 CAN1 events in sae2Δ strains (one spontaneous and one induced, see above) had a similar structure. In the discussion we consider possible mechanisms by which this type of structure might be formed.
Several different conclusions can be made from the Southern blot data.
Spontaneous TRP1 events are primarily resolved as simple gene conversions, removing the HO-endonuclease site in both the wild-type and sae2Δ strains.
In both wild-type and sae2Δ strains, induction of the HO endonuclease primarily stimulates recombination to TRP1 by a mechanism that appears to involve homologous recombination, resulting in gene conversions either with or without an associated crossover in an error-free fashion. Some of the crossover events appear to result in disomy for chromosome III.
In a wild-type strain, most (93%) of the induced TRP1 can1 events were gene conversions with (23%) or without (70%) an associated crossover and show no other alteration of the substrate, suggesting that the can1 mutations are point mutations.
In contrast, in a sae2Δ strain 54% of the TRP1 can1 events were rearrangements of the substrate in which sequences of the inverted repeat become duplicated to produce palindromic molecules.
rad50s-K81I and mre11s-H125N mutants have a phenotype indistinguishable from sae2Δ mutants: The SAE2/COM1 gene was identified in two similar meiotic screens for mutants defective after the initiation of SPO11-induced meiotic DSBs, but before resolution of recombination intermediates (McKee and Kleckner 1997; Prinz et al. 1997). The meiotic phenotype of sae2Δ mutants is very similar to that of the separation of function or “s” alleles of either RAD50 or MRE11. In rad50s, mre11s, and sae2Δ strains meiotic DSBs are formed, but remain unresected, resulting in poor spore formation and spore viability (Alani et al. 1990; Nairz and Klein 1997). Neither a rad50s-K81I nor a mre11s-H125N mutant was found to have a significant mitotic defect (Alani et al. 1990; Nairz and Klein 1997; Moreau et al. 1999). Because of the similarity of the meiotic phenotype of sae2Δ to rad50s and mre11s mutants, we examined whether either of these mutants were similar to sae2Δ in giving an elevated level of DSB-induced rearrangements in our inverted-repeat assay. The construction of these strains is described in materials and methods and their genotypes are listed in Table 1. We found that neither a rad50s-K81I nor a mre11s-H125N mutant strain affected the overall rate of TRP1 formation as compared to a wild-type strain (Table 2). However, as in a sae2Δ strain, induction of the HO endonuclease in either strain resulted in an ∼10-fold increase in TRP1 can1 events as compared to the wild-type strain (Table 2). We examined 36 independently induced TRP1 can1 events from both a rad50s-K81I and a mre11s-H125N strain by Southern blot analyses, and the data are summarized in Table 5. As with sae2Δ mutants, we found that about one-half of the events were associated with a palindromic type of rearrangement as depicted in Figure 3E. The indistinguishable phenotypes of rad50s-K81I, mre11s-H125N, and sae2D strains in our assay suggests that all three proteins have a similar function during mitotic DSB repair.
DISCUSSION
A single unrepaired DNA DSB is lethal to cells, but DSBs are normally efficiently repaired. For example, during meiosis, the repair of >100 breaks is required for proper chromosome segregation in meiosis I. The mutation rate during meiosis is higher than the spontaneous mitotic rate, and many of the mutations appear to be associated with nearby crossover events (Magni and von Borstel 1962; Magni 1964; Esposito and Bruschi 1993). We previously found that DSB-induced recombination between heteroalleles on homologous chromosomes in diploid yeast cells resulted in an ∼100-fold decrease in the fidelity of DNA synthesis as compared to normal S-phase DNA synthesis (Strathern et al. 1995; Holbeck and Strathern 1997; McGill et al. 1998). Because those experiments depended on the reversion of point mutations, they specifically addressed the fidelity of the DNA synthesis associated with repair. Here, we have extended these observations by examining DSB repair in haploid cells, where recombination could be monitored by the formation of TRP1 cells between trp1 heteroalleles. The presence of an associated forward mutation reporter (CAN1) allowed us to monitor the fidelity of the DNA repair synthesis as well as the integrity of the recombination event itself. We found that in wild-type cells most DSB-induced events were faithfully repaired via standard recombination mechanisms to yield gene conversions either with or without an associated crossover. Many of the products that were scored as mutants, i.e., can1, appeared to be due to gross chromosomal rearrangements in which the entire substrate was deleted (and were therefore His- and Trp-, Table 3) and were presumably repaired via an NHEJ pathway. However, if we examined only those mutations that arose during recombinational repair by virtue of their having become TRP1, we found that 0.4% of all TRP1 events in wild-type cells had an associated mutation in CAN1 (Table 3). Physical analyses of TRP1 can1 events indicated that most of these also arose by normal recombinational repair mechanisms, since 93% were gene conversions either with or without an associated crossover and had no other detectable alteration (Table 5). We conclude that these are likely to be point mutations generated during DNA repair synthesis and reconfirm our previous observation that the fidelity of DNA synthesis associated with DSB repair is lower than the fidelity of S-phase DNA synthesis.
The ability to monitor mutations associated with DSB repair in haploid cells allowed us to use this assay in a screen to identify recessive mutants with altered fidelity of DSB repair. From 4000 independent patches of UV-mutagenized cells we identified 6 that had altered levels of DSB-induced mutations associated with TRP1 recombination. Three of the mutants, m403, m499, and m500, have not been further characterized. One mutant, m362, was both dominant and substrate linked and is probably a point mutation in the can1-5′Δ allele of the inverted-repeat substrate, resulting in very high transfer of the mutation upon induction of recombination. Another mutant, m431, proved to be an allele of the RAD57 gene. The characterization of m362 and m431 will be described in more detail elsewhere (A. J. Rattray and J. N. Strathern, unpublished data). Here we have focused on the characterization of one mutant, m342, which showed an ∼10-fold increase in DSB-induced TRP1 can1 events as compared to the wild-type strain, and was found to be an allele of SAE2/COM1. SAE2/COM1 encodes a 345-amino-acid protein with no known homologs. It was originally identified in two similar screens (McKee and Kleckner 1997; Prinz et al. 1997) for meiotic mutants that would result in lethality if meiotic DSBs were made, but which could survive in the absence of DSBs by virtue of a meiosis I bypass due to an additional mutation in spo13. Additional characterization demonstrated that sae2 mutants are proficient for the formation of meiotic DSBs, but that the Spo11 protein remains bound to the 5′ strand of the break (Keeney and Kleckner 1995). The DSBs are not resected or rejoined, and as a consequence the cells die rapidly on sporulation media. No mitotic defect was noted for null alleles of sae2, other than a slight sensitivity to ionizing radiation and/or MMS. All of the noted phenotypes were indistinguishable from the non-null “separation of function” alleles of MRE11 and RAD50 (Alani et al. 1990; Nairz and Klein 1997; Tsubouchi and Ogawa 1998; Moreau et al. 1999), which are discussed further below.
Although Sae2p has not been reported to be a component of the large protein complex (MRX) that includes Mre11p, Rad50p, and Xrs2p, it is tempting to speculate that it is either a part of that complex or that it plays a role in regulating its activity. MRX has a variety of nuclease activities and additional roles in DNA metabolic processes, including ionizing radiation damage repair, telomere maintenance, NHEJ, and the formation and processing of meiotic DSBs (reviewed in Paques and Haber 1999). The nuclease activities of the MRX complex reside in Mre11p, which is homologous to the E.coli SbcD nuclease (Sharples and Leach 1995). The mre11s-H125N allele is due to a single amino acid change in domain IV of the phosphoesterase motif and has been shown to be deficient for all nuclease activities in vitro (Moreau et al. 1999). The mre11-H125N mutation was also found to be synthetically lethal with a mutation in rad27 (Moreau et al. 1999). RAD27 encodes the yeast homolog of the mammalian FEN1 flap endonuclease (Harrington and Lieber 1994; Reagan et al. 1995; Sommers et al. 1995; Tishkoff et al. 1997), which is important for the maturation of Okazaki fragments during DNA replication (Goulian et al. 1990; Turchi et al. 1994). Synthetic lethality suggests that the nuclease activity of Mrellp may be necessary for processing Okazaki fragments in the absence of Rad27p. Rad50p is a member of the SMC protein family. DNA binding is required for protein dimerization, suggesting that Rad50p may be directly involved in bringing broken chromosome ends together (Hopfner et al. 2000). The crystal structure was recently solved for the catalytic domain of Rad50p from Pyrococcus furiosus (Hopfner et al. 2000). The rad50s alleles map to a region on the surface of the dimer that is different from both the catalytic and DNA-binding domains (Hopfner et al. 2000). Interestingly, rad50s and sae2Δ mutants have also been found to be synthetically lethal with rad27 (A. J. Rattray and J. N. Strathern, unpublished observations; A. Nicolas, personal communication).
Thus it appears that sae2Δ, rad50s, and mre11s mutants all have very similar phenotypic spectra for meiotic DSB repair, for synthetic lethality with rad27, and, as shown here, for increased levels of DSB-induced symmetrical gene rearrangements. On the basis of our knowledge about the biochemical nature of the mre11-H125N allele and the strong similarities between the phenotypes of all three mutants, we speculate that these mutants result in a deficient or altered nuclease activity but are wild type for the other functions of the MRX complex.
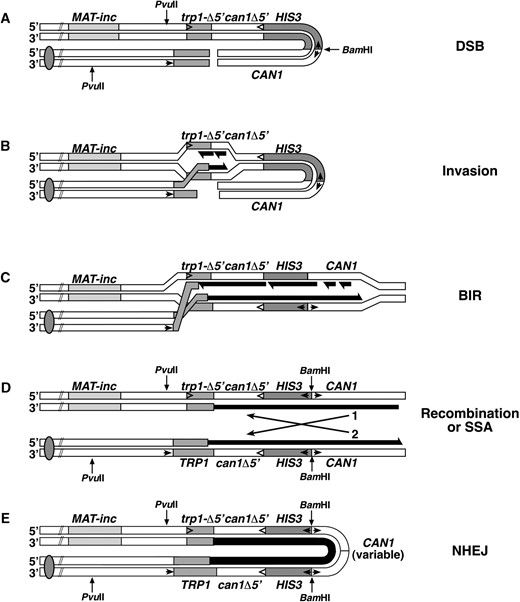
In Figure 4 we present a model for the formation of the rearrangements seen in our study. After the HO-induced DSB is made (Figure 4A), we assume that the initiation of recombination and invasion of the intact homologous duplex by one of the broken DNA strands is normal in sae2Δ, rad50s, and mre11s mutants since the overall frequency of recombination to TRP1 remains at wild-type levels (Figure 4B). We suggest that the loss of nuclease activity of the MRX complex reduces the ability of the second side of the break to participate in the recombination reaction and leads to a one-ended invasion. One-ended invasion may proceed as a full replication fork, with the lagging strand synthesized from the displaced DNA strand, or alternatively, could proceed by a migrating bubble, where the lagging strand uses the newly synthesized strand as a template. This results in de novo DNA synthesis and the formation of a wild-type TRP1 gene (Figure 4B). Replication proceeds to the broken ends of the molecule by BIR (Figure 4C), as has been proposed for repair of DSBs in the absence of large stretches of homology (Malkova et al. 1996; Bosco and Haber 1998). However, BIR would not restore the continuity between the telomere and the centromere of the chromosome. Chromosome continuity could be restored by recombination (or single-strand annealing, SSA) between the CAN1 sequences as shown in Figure 4D or by ligation of the two ends as shown in Figure 4E. Note that recombination (or SSA) between the CAN1 sequences will give products that look like gene conversions either without (Figure 4, D-1) or with (Figure 4, D-2) a crossover. Although both types of events might be expected to occur with equal frequency, the structure of the BIR intermediate may result in a preference for resolution as a noncrossover. Ligation of the ends (Figure 4E) would presumably require functions involved in NHEJ, and we are in the process of testing this hypothesis. Differences in the site of ligation will give the variable extra CAN1-related fragment shown in Figure 3A. This type of pairing (in this case induced by replication) between homologous sequences, followed by ligation of the newly synthesized ends, would result in head-to-head or tail-to-tail symmetrical junctions. Synapsis-mediated head-to-head end joints, resulting in long inverted dimer molecules, are the most common ligation product from in vitro yeast (Symington 1991) and human (Derbyshire et al. 1994) cell extracts. Also, transformation of linear DNA plasmids into yeast cells lacking chromosomal homology to the plasmid result in the formation of inverted dimer plasmid molecules when excess carrier DNA is included in the transformation mixture (Kunes et al. 1985, 1990). Perhaps the excess carrier DNA acts as a sink for nuclease activity, making the cells temporarily nuclease deficient. Events that have a structure consistent with a similar type of symmetrical inverted duplication have also been observed among recombinants from plasmid-borne inverted repeats in wild-type yeast (Embretson and Livingston 1984; Colaiacovo et al. 1999) and in E. coli (Bi and Liu 1996). In the E. coli system, many of the events appear to be derived from replication intermediates, and their formation was shown to be dependent upon SbcCD (Lyu et al. 1999). It would be interesting to determine if a point mutation in a phosphoesterase motif of SbcD (analogous to mre11-H125N) would promote an even higher level of inverted dimer formation.
—Model for the formation of symmetrical amplification events. (A) HO endonuclease cleaves the inverted-repeat substrate to produce a DSB. Arrows and restriction sites are as indicated in Figure 1. (B) One end of the break invades the homologous sequence on the other side of the break. After removal of the nonhomologous sequence, the 3′ hydroxyl from the broken end of trp1-3′Δ can serve as a primer for leading-strand DNA synthesis (black) using trp1-5′Δ as a template to produce a full-length wild-type TRP1 gene. Lagging-strand DNA synthesis may duplicate the displaced strand (shown) or the recently synthesized strand (not shown; see discussion). (C) The inability of the second side of the break to participate in the recombination event results in BIR, which may proceed until it reaches the broken end of the molecule. However, the contiguity between the centromere and telomere is not restored. (D) Chromosome contiguity can be restored by recombination (or SSA) between the duplicated sequences now oriented as direct repeats. This results in products that look like gene conversions either without (1) or with an associated crossover (2). (E) Alternatively, chromosome contiguity could be restored by NHEJ. The position at which the ends become ligated is variable and results in symmetrical molecules with a variably sized loop.
Symmetrically fused sequences are found in a large variety of different organisms (Rayko 1997). They are important for development of many organisms (Kafatos et al. 1985) and are found at the joints from breakage-fusion-bridge events in maize (McClintock 1939), amplified yeast DNA (Moore et al. 2000), and tumorigeneic mammalian cells (Fried et al. 1991; Lengauer et al. 1998). The model that we have proposed in Figure 4 provides a possible mechanism for the initial step in the formation of large intrachromosomal arrays of head-to-head and tail-to-tail concatamers. After a chromosome break, one end of the break could invade DNA on the other side of the break by using a short inverted sequence. DNA synthesis (BIR) would then proceed back toward the break, duplicating the intervening DNA. Synapsis followed by NHEJ would result in a head-to-head inverted-repeat sequence. In a subsequent breakage cycle, a second round of invasion, BIR, and synapsis-mediated NHEJ would further amplify the sequences to produce arrays with the structure tail-head-head-tail-tail-head-head-tail, and each subsequent cycle would further amplify the array. In fact, it has been shown that the introduction of a DSB in a yeast circular plasmid containing inverted duplications located at one end of the molecule and telomere sequences located at the other end of the molecule can result in a linear palindromic minichromosome (Butler et al. 1996). Here we provide support that sequences between inverted repeats can be amplified to produce symmetrical end-to-end joined molecules in the context of a broken chromosome. It will be of interest to determine if a loss of nuclease activity of the MRX complex increases the frequency of gene amplification events, and if tumorigenic cell lines showing high frequencies of gene amplification are altered in their MRX nuclease activity.
Acknowlegement
We thank Nancy Kleckner, Michael Lichten, Dwight Nissley, Mark Rose, and Lorraine Symington for sharing strains and plasmids, and Alain Nicolas for sharing data prior to publication. We also thank current and past members of the Gene Regulation and Chromosome Biology Laboratory for stimulating discussions and helpful insights, and Joan Hopkins for administrative assistance. Research was sponsored by the National Cancer Institute, Department of Health and Human Services, and the ABL-Basic Research Program. The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement from the United States government.
Footnotes
Communicating editor: M. Lichten
LITERATURE CITED
Author notes
These authors contributed equally to this work.