-
PDF
- Split View
-
Views
-
Cite
Cite
Jean-Pascal Capp, Noise-Driven Heterogeneity in the Rate of Genetic-Variant Generation as a Basis for Evolvability, Genetics, Volume 185, Issue 2, 1 June 2010, Pages 395–404, https://doi.org/10.1534/genetics.110.118190
Close - Share Icon Share
Abstract
Molecular biologists have long searched for molecular mechanisms responsible for tuning the rate of genetic-variant generation (RGVG) in fluctuating environments. In spite of several bacterial examples, no regulated variation in the RGVG has been identified in eukaryotic systems. Based notably on the example of industrial and pathogenic yeasts, this article proposes a nonregulated molecular evolutionary mechanism for the appearance of the transient increase of the RGVG in eukaryotic cell populations facing challenging environments. The stochastic nature of gene expression allows a model in which the RGVG in the population can be rapidly tuned as a result of a simple Darwinian process acting on noise-driven heterogeneity in the RGVG from cell to cell. The high flexibility conferred through this model could resolve paradoxical situations, especially concerning the mutator phenotype in cancer cells.
Anecdotal, Historical and Critical Commentaries on Genetics
LIVING organisms evolve to survive in environments fluctuating in terms of competitors, nutritional resources, and physical or chemical stresses. This necessity to diversify is variable depending on environmental constraints, but always exists. What is the driving force for this diversification? In the neo-Darwinian perspective, genetic variants appear stochastically and the environment selects the fittest ones. But is environmental pressure able to increase the rate of appearance of genetic variants? In the past 35 years, these fundamental issues have often been questioned by looking for regulated stress-inducible molecular mechanisms that could have been selected for their ability to produce genetic diversity.
Miroslav Radman introduced, in the 1970s, the SOS hypothesis in which mutation was no longer seen simply as a rare stochastic chemical accident but as an active inducible cellular process (Radman 1975). Then he extensively described and analyzed these ideas about “adaptative evolution” in which inducible variations of the rate of genetic-variant generation (RGVG) can lead to accelerated mutations under stress in bacteria (Radmanet al. 1999). In 1988, John Cairns discussed “experiments suggesting that cells may have mechanisms for choosing which mutations will occur,” experiments that were done on bacterial populations under stress (Cairnset al. 1988). This phenomenon of nonrandom mutation, initially called “directed mutation,” provoked vigorous discussion among biologists and philosophers of science (Lenski and Mittler 1993). This Lamarckian hypothesis was rejected in favor of more conventional Darwinian explanations, but it stimulated the debate about adaptative mutagenesis (Lenski and Sniegowski 1995).
Studies on bacteria have revealed mechanisms of regulated increase in the RGVG that are entirely consistent with the modern Darwinian concept of adaptation by natural selection on randomly occurring variation (Foster 2007). Nevertheless, equivalents in eukaryotic systems have not been observed. As variations in the RGVG are also observed in these cells, an alternative model is needed for the appearance of genetic plasticity in fluctuating environments and for the modulation of evolvability.
Evolvability relates to the intrinsic capacity of organisms to evolve (Sniegowski and Murphy 2006). It reflects the extent of genetic variability in populations. In asexual species, the mutation rate is the main parameter affecting evolvability. Mutation rates can easily evolve in asexual conditions because an allele that increases the mutation rate (mutator allele) is not eliminated from the population by recombination even if it generates many deleterious mutations (Hurst 2009). Bacterial mutator alleles provide good examples of systems in which the ability to evolve is selected because of its immediate beneficial consequences (Sniegowskiet al. 1997). Such systems are unlikely to evolve in sexual conditions because meiotic recombination rapidly breaks down the linkage between the mutator allele and its beneficial consequences (Pigliucci 2008).
Mitotic recombination might itself be mutagenic owing to errors introduced during double-strand break repair (Lercher and Hurst 2002). Indeed, the rate of recombination and the rate of substitutions are positively correlated (Lercher and Hurst 2002). Recombination can also lead to gross chromosomal rearrangements (Lambertet al. 2005). Thus mutation and recombination rates involve different kinds of genetic alterations, with mutation conferring gene-level changes, and recombination, in contrast, generating genome-level alterations (Heng 2009). The consequences of their effect on the genome are radically different, but the selective process acting on the genetic diversity that they generate is the same. Variations of mutation and recombination rates can be selected for the positive or negative impact of the genetic alterations that they produce. Therefore, the RGVG is globally defined here as the sum of mutation and recombination rates.
After reviewing experimental evidences of variations of the RGVG in stressful environments and their molecular origins, this article proposes that noise-driven heterogeneity in the RGVG could provide the framework for a Darwinian process that facilitates rapid tuning of the RGVG in eukaryotic cell populations facing challenging environments. This article focuses mainly on asexual conditions with examples among bacteria under stress, industrial and pathogenic yeasts, and cancer cells, but I describe here a general evolutionary mechanism to account for variations of the RGVG. Meiotic recombination can also be considered as affected by random fluctuations in DNA recombination genes, and meiotic recombination rates might therefore be heterogeneous among sexual cells. Thus, rise and fall of meiotic recombination rates are possible by this mechanism although this will depend on the environmental constraints and the selective value of a more or less shuffled genome.
RAPID ACQUISITION OF GENOMIC MODIFICATIONS IN STRESSFUL ENVIRONMENTS IS WIDESPREAD, BUT WHAT ARE THE UNDERLYING MECHANISMS?
Stress-associated mutagenesis in bacteria:
Bacteria with elevated mutation rates are frequently found among natural isolates of Escherichia coli (Bjedovet al. 2003) and other bacteria (Denamur and Matic 2006). These populations contain strains having high mutation rates with frequencies ranging from 0.1 to >60%. Natural environments seem to positively select for high mutation rates despite the potential deleterious variants among the generated mutations. The conclusion of much work on bacteria is that mutator strains are indirectly selected along with the favorable mutations that they generate, which counterbalance deleterious mutations that are also produced. [This is “second-order” selection (Tenaillonet al. 2001)]. Such a result is now associated with the idea that if mutation rates increase when mutations could be advantageous, they return to normal levels when adaptation is achieved. Indeed, mutator phenotypes would be counterselected in adapted populations because of the deleterious mutations that they generate.
Evolution of bacterial populations probably happens through alternating periods of high and low mutation rates. But the increase in the mutation rate greatly varies among natural isolates. For example, starvation-induced mutagenesis among 787 E. coli natural isolates showed that a 7-fold increase in mutagenesis was observed on average, with 20% of strains showing an increase of >100-fold and 1.5% showing an increase of >1000-fold. Strong and weak mutators can be distinguished and exhibit different properties in the face of stressful environments (Bjedovet al. 2003).
The example of antibiotic resistance has been extensively examined to evaluate how selection modulates mutation rates. In vitro and in vivo studies have shown that antibiotic treatment contributes to the selection of mutators (Maoet al. 1997; Giraudet al. 2002) because it generates antibiotic-resistance-conferring mutations at a higher rate. Nevertheless, no correlation between multiple antibiotic resistance and high mutation rates has been found in E. coli natural isolates. Among these isolates, weak mutators exhibit the highest frequency of antibiotic resistance, while strains harboring high mutation rates have significantly lower antibiotic resistance (Denamuret al. 2005). This result provides evidence that selective conditions probably favor weak mutator strains because they persist for much longer than strong mutators and have more chance to accumulate multiple resistance (Denamur and Matic 2006). At the molecular level, these different populations of mutators correspond to different molecular mechanisms.
Regulated changes in DNA repair and maintenance genes expression level in bacteria:
Mutator phenotypes result from structural or expression changes in genes coding for DNA repair enzymes and for proteins that assure the accuracy of DNA replication (Denamur and Matic 2006). Strong mutators in bacteria usually result from a defective mismatch repair (MMR) system due to the inactivation of mutS or mutL genes. In E. coli and other bacteria, the MMR complex recognizes base mismatches in the DNA and excises the newly synthesized DNA strand; the old strand then serves as a template for new synthesis. Inactivation of MutS or MutL provides a 102-fold increased rate of transition and a 103-fold increased rate of frameshift mutations. In addition, mutS or mutL knockout mutants have a strong hyper-recombination effect, resulting in a 101- to 103-fold increase in the rate of chromosomal rearrangements (Denamur and Matic 2006). This strong constitutive increase of the RGVG is found only in a very small proportion of natural isolates exhibiting an increase in mutant frequency (1%). Nevertheless, >80% of these natural isolates showed stress-inducible mutagenesis after 7 days in an aging colony. This suggests that inducible mutators are widespread and that they make a greater contribution to natural variation within a population than constitutive mutators do.
Several global stress responses providing stress-induced mutagenesis have been identified in E. coli (Foster 2007). They include the SOS response, the general stress response, and the heat-shock response. These pathways enhance spontaneous mutation rates during stress mainly by two mechanisms. The first one is the downregulation of enzymes responsible for MMR, notably MutS and MutH. The other one is the overexpression of DNA polymerases that have the ability to replicate damaged DNA, especially Pol IV and Pol V. These polymerases temporarily take the place of the replicative polymerase (Pol III) and synthesize past blocking lesions in the DNA template. Pol IV and V belong to the widely distributed Y-family of specialized polymerases. This family is characterized not only by the ability to replicate damaged DNA, but also by the lack of processivity and low fidelity when copying an undamaged template (Foster 2007). When induced via global stress responses, both Pol IV and Pol V increase the spontaneous mutation rate even in the absence of DNA damage.
Even though it is still unknown whether these inductions have been directly selected due to the adaptative advantage that they confer in stressful environments, or whether they are indirect consequences of selection for other mechanisms in global stress responses, they provide a remarkable potential for fine tuning of the RGVG. Here bacteria show us that variations in DNA repair and maintenance gene (DRMG) expression are much more efficient in tuning the RGVG than constitutive mutations in these genes.
Recently, sublethal levels of antibiotics have been shown to lead to multidrug resistance via radical-induced mutagenesis in bacteria (Kohanskiet al. 2010). As levels of reactive oxygen species significantly vary from cell to cell (Kohanskiet al. 2007), it has been suggested that heterogeneity in the RGVG might exist among the population (Kaufmann and Hung 2010). In the final part of this article, I will present an alternative and complementary hypothesis for the appearance of heterogeneity in the RGVG.
Acquisition of genomic rearrangements in industrial yeasts by exposure to environmental stress:
Most studies of yeast evolvability and RGVG are focused on industrial or pathogenic strains. Indeed, both situations provide fluctuating environments where yeasts have to cope with permanently varying growth conditions.
Industrial strains are mainly polypoid. They include wine strains, brewing strains, and ethanol-producing strains. Under fermentation conditions, they all are exposed to numerous environmental stresses that affect their viability and favor the emergence of genetic variants that are better adapted to new environmental conditions. These stresses include high osmotic pressure, high alcohol concentrations, anaerobiosis, or temperature fluctuations.
Interestingly, stress-tolerant yeast strains used to produce lager beer have been obtained during a single 8-day round of fermentation (Jameset al. 2008). The cells exposed to stress conditions, such as fermentation in high-specific-gravity wort (the liquid extracted from the mashing process that contains the sugars to be fermented by the brewing yeast to produce alcohol) or growth at higher-than-normal temperatures, undergo gross chromosomal rearrangements, small deletions, and regional amplifications whereas “standard” fermentation conditions do not generate significant changes (Jameset al. 2008). This result shows that the lager yeast genome displays high plasticity, specifically under stressful fermentation conditions demonstrating that acquisition of chromosome rearrangements in allopolyploid yeast genomes can be influenced by exposure to environmental stress (Querol and Bond 2009).
Other examples of chromosomal rearrangements occurring in industrial yeast strains during fermentation have been observed. Sparkling-wine Saccharomyces cerevisiae strains display natural variability of their karyotypes, and, interestingly, the rate of chromosomal changes may be influenced by the medium in which the cells grow (Nadalet al. 1999). The cause–effect relationship is unknown, but an environmental influence on the RGVG clearly seems to be involved in this difference in the frequency of chromosomal changes.
Starvation is another environmental constraint that has been associated with the appearance of genomic rearrangements. The month-long starvation of a champagne vineyard yeast in chemostat led to an exceptional increase in the frequency of genomic rearrangements in replicate cultures derived from a common ancestor (Coyle and Kroll 2008). Fine-scale mutation frequency was also increased severalfold. These results showed that starvation affects the rate at which genomic rearrangements and point mutations accumulate in yeast population.
Interestingly, high genetic instability and loss of heterozygosity (LOH) in natural wine yeast strains during laboratory propagation under nonselective conditions have also been observed, but not in the common laboratory strains of S. cerevisiae (Ambronaet al. 2005; Ambrona and Ramirez 2007). In contrast to laboratory strains, wine yeast strains do not maintain genetically homogeneous populations because of this propensity to undergo genetic modifications.
Rapid evolution of pathogenic yeasts during passage through host or exposure to drugs:
Candida albicans constitutes the leading cause of fungal disease in humans and provides a model for the study of opportunistic pathogens notably because it harbors a high degree of genetic variability among isolates (Legrandet al. 2004). Clinical studies reveal the accumulation of variations in host-associated populations, but rates of mitotic recombination at specific genome regions have been evaluated primarily from in vitro studies (Lephartet al. 2005). High chromosomal instability in C. albicans has already been observed during growth in rich medium (Rustchenko 2007), but LOH is also widespread in natural isolates (Diogoet al. 2009). Nevertheless, no estimation of the rate of LOH occurring during in vivo propagation was available until recently.
An important study now highlights the influence of in vivo propagation in a susceptible host (mouse) on the RGVG by comparing results to those obtained for the same strain propagated in liquid cultures (Forcheet al. 2009). In particular, the rates and types of short- and long-range LOH were compared between in vivo and in vitro propagation. The rate of LOH at GAL1 is 1.7 × 10−4 (±2.12 × 10−4 SD) events/generation across 13 in vivo propagating populations and 6.0 × 10−6 (±2.0 × 10−6 SD) events/generation for the 20 in vitro cultures. Thus, the rate of recombination at GAL1 is ∼28-fold greater during in vivo growth than during in vitro growth. Here C. albicans populations generate considerably more genetic variants during stressful growth in a living host than in the relatively nonstressful environment of in vitro culture.
Moreover, naturally heterozygous diploid C. albicans cells also undergo LOH and/or aneuploidy at high frequencies when exposed to antifungal drugs. Azole resistance is acquired during drug exposure in patients (Marret al. 1998) and in vitro (Cowenet al. 2000). It has been associated with aneuploidy in general and with a specific segmental aneuploidy, consisting of an isochromosome composed of the two left arms of chromosome 5 (Selmeckiet al. 2006). The number of extra copies of this isochromosome is correlated with the resistance level. Here again high environmental pressure is associated with the acquisition of adaptative genomic modifications. Similar mechanisms have been recently observed in Candida glabrata (Polakovaet al. 2009).
WHAT ARE THE DETERMINANTS OF STRESS-ASSOCIATED EVOLVABILITY IN YEAST?
Industrial and pathogenic yeasts are mainly aneuploid or polyploid (or both) (Querol and Bond 2009) and notably undergo many LOH events, suggesting that the maintenance of an unbalanced chromosome set is advantageous. These complex genomes allow loss, duplication, inversion, or translocation of genetic material that forms at a faster rate than other types of mutations (Hastingset al. 2009). These genetic modifications occur mainly by two double-strand breaks (DSB) repair pathways: homologous recombination (HR), and nonhomologous end joining (NHEJ).
Indeed, karyotype rearrangements in a wine yeast strain occur by rad52-dependent and rad52-independent mechanisms (Carroet al. 2003). RAD52 mediates an early step in the HR pathway and is consequently required for HR-mediated chromosomal rearrangements. In this study, the authors showed that deletion of rad52 partially stabilized the karyotype by suppressing recombination at telomeric and subtelomeric regions and by reducing the rate of changes in chromosomal size by 30% (Carroet al. 2003). Interestingly, fermentation of lager yeasts in high-gravity wort also results in a greater degree of chromosomal instability and/or gene amplification, particularly in telomeric regions (Jameset al. 2008). Carroet al. (2003) also showed that many of the karyotypic variations are due to nonhomologous, rad52-independent recombination. Recently, another report highlighted that the occurrence of deletions is favored in S. cerevisiae when RAD52-dependent homologous recombination is inactivated (Fritschet al. 2009). The Ku-independent NHEJ mechanism contributes to the occurrence of deletions and duplications. Finally, some genomic elements favor error-prone events by HR and NHEJ. For example, recombination between retrotransposons is a source of chromosome rearrangements in yeast (Mieczkowskiet al. 2006) and cancer cells (Konkel and Batzer 2010). It mediated multiple translocations in a wine strain of S. cerevisiae (Rachidiet al. 1999) and rearrangements in the experimental evolution of S. cerevisiae (Dunhamet al. 2002).
While numerous examples of regulated stress-induced mutagenesis are found in bacteria, no such mechanism has been described in eukaryotic cells. In these cells, is the appearance of chromosomal modifications associated with a regulated increase of the RGVG? Or is it the result of selection in a stressful environment without an inducible mechanism that promotes the rapid acquisition of genetic modifications? Several results indicate that the exposure of yeasts to stressful environments is associated with variations of the RGVG. These examples of micro-organisms acquiring genomic modifications in stressful environments with, when examined, an increase of the RGVG, raise the question of the behavior of higher eukaryotic cells in similar situations. The best situation for studying the acquisition of genetic modifications in a highly selective environment is probably that of carcinogenesis.
Genetic plasticity in cancer cells:
Cancer cells exhibit a high genome plasticity that allows the rapid acquisition of supplementary genetic alterations to cope with biological, physical, or chemical “barriers” in the organism. However, the origins of this ability to rapidly acquire genetic alterations are subject to debate. Peter Nowell's concept of intrinsic genetic instability (the ability for cancer cells to accumulate genetic modifications at an higher rate than normal cells) (Nowell 1976) has been supported by, for example, Lawrence Loeb. He proposed that an elevated mutation rate is present in all tumors because the rate of spontaneous mutagenesis in somatic cells is insufficient to account for the multiple mutations observed (Loeb 1991). Bert Vogelstein subsequently introduced the concept of “just right” instability enabling passage through selection barriers without genetic drift caused by too high genetic instability (Cahillet al. 1999).
Nevertheless, other researchers, using a panel of arguments, ruled out the requirement of genetic instability for tumor development, notably the limited range of mutations in DRMGs and the fact that not all cancers are chromosomally unstable (Bodmeret al. 2008). Using a mathematical model for colon cancer, Luebeck and Moolgavkar (2002) showed that the “mutator phenotype” is not an explanation for the origin of common cancers. Other researchers have demonstrated that the mutation frequency in colon cancer cells is actually the same as in the normal epithelium (Wanget al. 2002). A mutator phenotype therefore may not be necessary to explain the existing data on the number of mutations detected in tumors (Tomlinsonet al. 2002). From that perspective, an increased proliferation rate associated with normal RGVG is sufficient to account for the genetic alterations found in cancers.
Finally, an alternative explanation could come from the fact that significant changes in the RGVG could occur during cancer progression (Frank and Nowak 2004). These changes could produce “bursts” of new genetic alterations when the environmental pressure is high and a slowing down of the RGVG when the environment is more benign, that is, when high genetic instability becomes more deleterious than beneficial.
Variations of the RGVG in cancer cells as a consequence of expression variations:
Well-known forms of inherited susceptibility to cancer are associated with germline mutations in DRMG. For example, heritable colorectal cancers are due to germline mutations in hMLH1 or hMSH2 genes controlling the repair of mismatched DNA lesions. However, mutations of DRMGs rarely, if ever, occur in sporadic cancers. hMLH1 or hMSH2 genes' epigenetic silencing is associated with loss of MMR function in 10–20% of sporadic colorectal cancers, but no somatic mutation has been described (Futrealet al. 2004). The situation is identical for excision repair pathways genes (Bodmeret al. 2008). Somatic mutations are described only in recombinational repair (HR and NHEJ).
On the basis of these observations, we have already argued that the limited range of DRMG mutations found in tumors indicates that genetic alterations are rather acquired through deregulation of DNA repair and maintenance pathways (DRMPs) associated with the aberrant expression of genes involved in these pathways (Capp 2005). Indeed, DRMPs are often strongly deregulated in cancers, and tumor cells can exhibit a highly altered diversity of a DRMG expression profile when compared to normal cells (Castroet al. 2007). Many studies show that deregulation of DRMGs in cancer cells accelerates the acquisition of genetic modifications and enhances the tumorigenic potential.
Nevertheless, the same questions as before (for yeast) can be raised: are these deregulations of DRMG expression due to regulated inducible mechanisms, or do they result from a second-order selection? One can imagine that these expression variations appear without any regulated mechanism and that they are subjected to a Darwinian process favoring the most variable DRMGs and the most impaired pathways because they provide adaptative genetic modifications under high environmental pressure. Work on the stochastic nature of gene expression may provide insights into such adaptive behaviors.
STOCHASTIC GENE EXPRESSION AS A SOURCE OF PHENOTYPIC HETEROGENEITY FAVORABLE TO POPULATION SURVIVAL
Gene expression variability affects population dynamics and survival:
Development of single-cell analysis by flux cytometry and construction of synthetic gene networks have recently led to the discovery of large fluctuations in gene expression levels among individual cells in isogenic populations (Elowitzet al. 2002; Blakeet al. 2003). Noise in gene expression is the stochastic variation in the expression level of a gene under constant environmental conditions (Raser and O'Shea 2005). The parameter called “noise” used to quantify the heterogeneity of the population is the variance across the population divided by the mean (Kaernet al. 2005). Downstream effects of noise can have profound phenotypic consequences, drastically affecting the stability of gene expression (Blakeet al. 2003). Variability could be an advantage in that it would allow heterogeneous phenotypes even in clonal populations, enabling a population of organisms to contain subpopulations with different behaviors and favoring the emergence of adapted cells when the environment is fluctuating and when stress appears (Fraser and Kaern 2009).
As noise seems to be advantageous in regard to environmental fluctuations, are genes related to stress responses noisier than housekeeping genes? In 2006, a strategy that used a library of GFP-tagged yeast strains allowed rapid and precise monitoring of protein levels at single-cell resolution by high-throughput flow cytometry. Thus Newmanet al. (2006) showed that dramatic protein-specific differences in noise are strongly correlated with modes of transcription and the protein's function. Proteins responding to environmental changes are noisy whereas those involved in the mechanism of protein synthesis are quiet. Noise levels seem to have been selected to reflect the costs and potential benefits of this variation. In the same way, noise is minimized for essential and complex-forming proteins, and TATA box-containing genes are noisy and associated with stress responses (Fraseret al. 2004; Newmanet al. 2006).
Stochastic gene expression creates nongenetic heterogeneity that favors the appearance of resistant subpopulations (Blakeet al. 2006) or confers better fitness in the face of fluctuating environments (Acaret al. 2008). The hypothesis of stochastic gene expression as a major source of phenotypic diversification is reinforced by the fact that promoter elements conferring high expression variability (TATA boxes) are generally associated with genes implicated in stress response (Basehoaret al. 2004; Huisinga and Pugh 2004).
These data are in accordance with recent work showing how selection influences phenotypic fluctuations in evolutionary experiments. Indeed, an important study on E. coli provides evidence that mutants with a large degree of phenotypic fluctuation emerged under strong selection pressure (Itoet al. 2009). Fluctuations in fluorescence were attributable to the variance in mRNA abundance. Here, an increase in phenotypic fluctuation through noise in gene expression is clearly a relevant evolutionary strategy.
How stochastic gene expression and RGVG might be linked?
In addition to phenotypic changes acquired by genetic modifications, an increase in phenotypic fluctuation acts as an evolutionary strategy to produce extreme phenotypes under severe selective environments. But considering these sources of phenotypic diversification as independent could be wrong. Like any other phenotype, the maintenance of genome integrity is under the influence of the genes expressed with stochastic fluctuations. The RGVG could be variable as a consequence of stochastic fluctuations in DRMG expression from cell to cell.
No study has explored the variability of DRMG expression at the single-cell level and its potential consequences on the RGVG. Nevertheless, DRMG expression levels are known to influence the RGVG. For example, in E. coli, the frequency and structure of recombinant products are influenced by the level of MutL protein in the population (Elezet al. 2007). MutL is involved in MMR and also influences recombination events. Elezet al. (2007) have shown that the deletion-generating recombination is inversely related to the amount of cellular MutL. Various expression levels of this DRMG generate differences in the genomic instability level of the population. This is a provocative demonstration of the importance of a DRMG expression level for the RGVG.
In the yeast S. cerevisiae, reduced levels of the replicative α- or δ DNA polymerases (Lemoineet al. 2005, 2008) result in greatly elevated frequencies of chromosome translocations and chromosome loss. This instability was proposed to occur through the spontaneous appearance of DSBs at fragile sites by stalling replication forks. The results obtained with changes in Polα and -δ expression implicate a finely tuned balance of DRMG expression in genome stability. Consequently, cell-to-cell variation of these proteins may be potential sources of chromosome aberrations (Resnick 2005).
Interestingly, despite their numerous genetic alterations, cancer cells rarely harbor mutations or rearrangements in DRMGs. Genes involved in nucleotide-excision repair (NER) exemplify this phenomenon (Castroet al. 2007). NER malfunctioning is due to expression fluctuations of its components and could account for random point mutations scattered throughout the cancer cell genome. Again, the origin and the dynamics of this expression variability are interesting with regard to the fitness of cells harboring these destabilized DRMPs. Why does selection among cancer cell populations seem to favor genetic instability obtained by expression fluctuations rather than by structural modifications in DRMGs? One causal explanation could be the stochastic nature of gene expression. This article explores the concept of noise-driven heterogeneity in the RGVG as a basis for genetic plasticity in stressful environments and provides a model for the rapid tuning of the RGVG in fluctuating environments.
A MODEL FOR TUNABILITY OF THE RGVG IN STRESSFUL AND FLUCTUATING ENVIRONMENTS
High variability in DMRG expression might be advantageous in fluctuating environments:
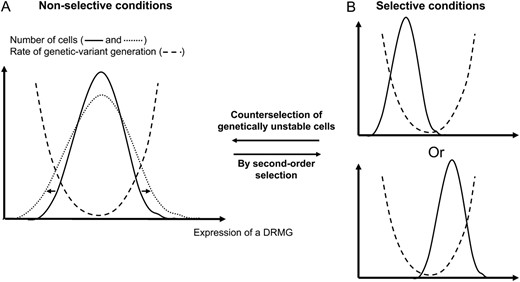
DRMG expression levels strongly influence efficiency and fidelity of DRMPs. Following variability of DRMG expression, the DRMPs may display variable efficiency and fidelity from cell to cell. Consequently, the appearance of genetic alterations could also be variable among the population as consequence of the stochastic appearance of DRMP malfunctioning (Figure 1). Genetic modifications could be facilitated and the RGVG could increase in “extreme” subpopulations where a DRMG is under- or overexpressed compared to the bulk of the population.
Expected link between heterogeneity in DRMG expression and the RGVG. (A) The stochastic nature of gene expression generates a heterogeneity in the expression of DRMGs (solid curve). As good functioning of DRMPs is highly dependent on the DRMG expression level, this heterogeneity is expected to generate heterogeneity in RGVG from cell to cell (dashed curve). Even if the bulk of the population is genetically stable in nonselective conditions (with mean expression corresponding to the lower value of RGVG) because genetic modifications are deleterious, extreme subpopulations with a high or low expression level of one particular DRMG might accumulate genetic modifications at higher rate. (B) When the population is in selective conditions, this heterogeneity could have profound phenotypic consequences because DRMP malfunctioning in extreme subpopulations could become advantageous. High or low DRMG expression level could be selected for along with the favourable mutations that they generate. When adaptation is achieved and if the environment is constant, genetically unstable cells are counterselected and mean DRMG expression comes back to its optimal level in nonselective conditions. When exposed to permanently fluctuating environments, evolution (arrows) toward a higher noise level in DRMG expression (dotted curve in A) could be advantageous due to the broader range of RGVG and the higher number of cells with high RGVG that can rapidly generate adaptative genetic modifications (A). Then tuning of the RGVG could be more rapid and efficient. Moreover, extreme subpopulations have a higher RGVG in the case of noisier DRMG expression than for a less noisy expression, enabling higher genetic instability in highly selective conditions. But, in nonselective conditions, this noisier DRMG expression is deleterious because it generates more genetic modifications.
This deleterious situation in nonselective conditions is expected to have profound implications when stress appears. Indeed, subpopulations with high or low DMRG expression and a higher RGVG would favor the appearance of potentially adaptative genetic modifications. In this case, these under- or overexpression properties could be selected for through second-order selection because of these adaptative modifications. The consequence would be a global increase of the RGVG and an increase or decrease of DRMG expression (Figure 1).
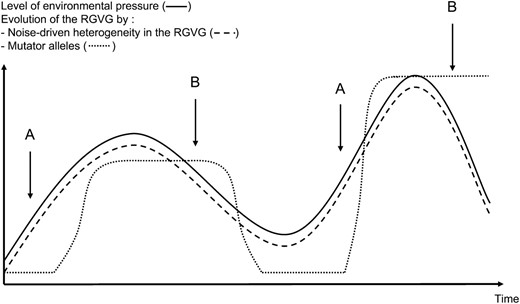
This Darwinian strategy allows fine tuning of the RGVG in fluctuating environments because noise-driven variations of the RGVG from cell to cell could enable rapid and permanent selection of cells harboring the level of instability just right for a given environment (Figure 2). In contrast, a structural modification of a DRMG generating a “mutator allele” provides an abrupt jump from a precise RGVG to another without intermediate levels (Figure 2). Tuning through noise-driven heterogeneity in the RGVG avoids these severe variations and allows for a perfect fit between RGVG and environmental pressure. This is especially true when this environmental pressure lowers because counterselection will rapidly allow decrease of the RGVG and a return to the optimal DRMG expression level in nonselective conditions (Figure 1) whereas mutator alleles would continue to produce deleterious genetic variability (Figure 2). Their counterselection would certainly be slower because a genetically caused increase of the RGVG in these cells is not reversible (Figure 2). Bacterial regulated pathways that tune DRMG expression levels probably reflect the evolutionary advantage of expression variations compared to mutator alleles.
Comparison between noise-driven evolution of the RGVG and evolution of the RGVG through acquisition of mutator alleles in fluctuating environments. When environmental pressure fluctuates, heterogeneity in the RGVG among the population allows permanent selection of cells harboring the “just right” RGVG in the noise-driven hypothesis. In this case, evolution of the RGVG (dashed curve) perfectly fits with evolution of environmental pressure (solid curve). On the contrary, evolution of the RGVG in the mutator alleles model proceeds by abrupt jumps of the RGVG in the population from low to high values (dotted curve). The RGVG is never perfectly adapted to the level of environmental pressure. Before the appearance of a mutator allele (A), the RGVG remains low even if adaptative genetic modifications are already needed. If the mutator allele dominates in the population (B), it will produce high genetic variability longer than an increase in DRMG expression when environmental pressure lowers. Indeed, its genetic basis implies that counterselection is longer than for an epigenetic event.
Adaptation through genetic modifications conferring high variability in DRMG expression:
When the environment is highly variable, the rapid tuning of the genetic variability level is expected to be advantageous for a population. In our model, this tuning would be easier with a higher noise level in DRMG expression. Indeed, a broader range of DRMG expression levels could optimize the emergence of cells harboring the “just right” level of genetic variability (Figure 1). Noisier DRMG expression would facilitate variations of the RGVG because at any time more cells would have, by chance, the level of instability required to adapt to a given stress level. The same evolutionary scenario probably governs the selection of high noise in the expression of genes related to stress response and other environmental cues.
The rise or fall of noise level in DRMG expression is possible through genetic modifications of their promoters. Some promoter elements have been shown to modify expression noise; e.g., TATA boxes increase noise level (Blakeet al. 2006). Some mutations in these elements have been used to modulate the level of expression noise with profound phenotypic consequences (Blakeet al. 2006; Murphyet al. 2007, 2010). Gross chromosomal rearrangements are also possibly involved in changes of expression noise because chromosomal positioning affects both expression and noise levels in S. cerevisiae (Becskeiet al. 2005). The same mechanism should apply to the DRMGs when the environment is variable. Promoter modifications or new chromosomal positions modulating noise level could be selected for, depending on the frequency of environmental variations, because of the easier tuning of the RGVG and the fitness advantage that they can confer.
A plausible and explanatory model from bacteria to yeasts to cancer cells:
Industrial or pathogenic yeast populations are exposed to stressful and fluctuating environments and can probably take advantage of a high tunability of the RGVG. High noise in expression of genes involved in DSB repair, for example, could confer a broad range of RGVG and favor emergence of subpopulations with higher genetic variability in times of stress, thanks to a second-order selection process. One hypothesis could be that these strains have evolved to exhibit higher noise in DRMG expression than laboratory strains do because they are exposed to more variable growth conditions. Higher heterogeneity in DRMG expression in industrial and pathogenic yeasts could explain their ability to rapidly acquire genetic modifications in stressful environments and their genomic instability in nonselective conditions.
I (Capp 2005) and now others (Brocket al. 2009) have proposed that noise-driven nongenetic heterogeneity among cancer cells could be a driving force in cancer evolution. This assumption predicts that high noise in the expression of genes involved in cancer progression could be beneficial in overcoming physical or chemical barriers in the organism. From the same perspective, high noise in DRMG expression could be an evolutionary strategy allowing rapid tuning of the RGVG in cancer, with bursts of genetic instability when environmental pressure is high and rapid counterselection for more stable cells when genetic instability is no longer needed to proliferate. This heterogeneity could be acquired by promoter modifications or genomic rearrangements as proposed for yeasts, but also by the global increase of noise in gene expression generated by the loss of micro-environmental control of cellular phenotypes (Capp 2005). Apparent paradoxes concerning the mutator phenotype in cancer cells are readily resolved by this model because the rapid rise and fall of the RGVG in cancer cells (Frank and Nowak 2004) would be expected and the mutator phenotype is probably transient during carcinogenesis.
Finally, even if bacteria have developed regulated pathways modulating the RGVG, the same mechanism could be at work in circumstances that do not induce global stress responses. Moreover, this selective process could be at the origin of bacterial stress-induced mutagenesis because genes differently expressed in a time of stress because of this Darwinian process could become a target for gene regulation by transcriptional factors that were present only at that time.
Conclusion:
Considering the RGVG as identical in all cells among a population may well be incorrect. The RGVG, like all other phenotypes, depends on the expression of proteins that are subjected to random fluctuations. But the RGVG constitutes one particular case because the phenotype itself allows phenotypic variations. This is why it is important to consider its evolvability in the light of stochasticity in gene expression. One value of RGVG can be selected for via second-order selection through the benefits conferred by the adaptative mutations that it generates. This model allows a permanent adjustment of the RGVG because cells that are not harboring the optimum value of the RGVG are spontaneously put at a disadvantage and replaced by cells expressing the DRMG at levels conferring the highest fitness. If the environment is permanently changing, cells expressing the DRMG with higher noise levels will be favored due to a better tunability of the RGVG. Noise in DRMG expression not only provides variations of the RGVG for second-order selection, but also aids the emergence of genomes harboring higher noise in DRMG expression or in expression of other genes. Then noisier gene expression confers higher tunability of the RGVG and higher evolvability in changing environments. This interplay might appear complex but provides a more complete interpretative framework for the results presented here. It also provides testable hypotheses.
Searching for heterogeneity and fluctuations of DRMG expression during carcinogenesis through live-cell imaging could give a first indication of the validity of this hypothesis. An important study has recently used transgenic rat models with fluorescent reporter and real-time confocal imaging of fluorescent pituitary tissue to analyze the spatiotemporal patterns of gene expression in relation to tissue structure (Harperet al. 2010). This study showed a long-term coordination of pituitary cell behavior when the tissue structure appears whereas dispersion of cells in a primary culture provides high variability and independence of promoter activity. This is the first evidence of gene expression coordination in living cells in intact tissue from transgenic rats. This study also opens the opportunity to study the nature of transcriptional patterning during hyperplasia and adenoma formation and the possible loss of coordination of cell behavior. By fusing a DRMG to a fluorescent reporter, it seems feasible to conduct such a study to show in vivo if carcinogenesis is associated with enhanced heterogeneity and fluctuations in DRMG expression. Dispersion of cells would then enable the immediate sorting of subpopulations harboring different levels of DRGM expression and the study of their DNA repair or maintenance abilities (depending on the fused gene), their RGVG, and their genetic content. The repetition of these experiments at different stages of the cancer process would help to test the hypothesis developed in this article.
In silico experiments using multi-agent simulation would also help to test this model. By modulating environmental constraints on the agents, such simulations might provide indications on variations of the RGVG strictly driven by heterogeneity in DRMG expression.
Finally, the links between selection and genetic variability that have been highlighted elsewhere (Rando and Verstrepen 2007) become even closer with this model. Far from being inconsistent with neo-Darwinian theory, this model updates it by considering the stochastic nature of gene expression itself as a driving force in evolvability.
Acknowledgements
I thank members of my lab, particularly Jean-Marie François, Gustavo De Billerbeck, Jean-Luc Parrou, and Thomas Walther for helpful discussions; Christine Rettew (Drexel University, Philadelphia) for copyediting the manuscript; and several colleagues for their critical reading. I am also very grateful to the referees and the editor of this work for their useful remarks and suggestions.
References
Acar, M., J. T. Mettetal and A. van Oudenaarden,
Ambrona, J., and M. Ramirez,
Ambrona, J., A. Vinagre and M. Ramirez,
Basehoar, A. D., S. J. Zanton and B. F. Pugh,
Becskei, A., B. B. Kaufmann and A. van Oudenaarden,
Bjedov, I., O. Tenaillon, B. Gerard, V. Souza, E. Denamur et al.,
Blake, W. J., K. A. M, C. R. Cantor and J. J. Collins,
Blake, W. J., G. Balazsi, M. A. Kohanski, F. J. Isaacs, K. F. Murphy et al.,
Bodmer, W., J. H. Bielas and R. A. Beckman,
Brock, A., H. Chang and S. Huang,
Cahill, D. P., K. W. Kinzler, B. Vogelstein and C. Lengauer,
Cairns, J., J. Overbaugh and S. Miller,
Capp, J.-P.,
Carro, D., E. Bartra and B. Pina,
Castro, M. A., J. C. Mombach, R. M. de Almeida and J. C. Moreira,
Cowen, L. E., D. Sanglard, D. Calabrese, C. Sirjusingh, J. B. Anderson et al.,
Coyle, S., and E. Kroll,
Denamur, E., and I. Matic,
Denamur, E., O. Tenaillon, C. Deschamps, D. Skurnik, E. Ronco et al.,
Diogo, D., C. Bouchier, C. d'Enfert and M. E. Bougnoux,
Dunham, M. J., H. Badrane, T. Ferea, J. Adams, P. O. Brown et al.,
Elez, M., M. Radman and I. Matic,
Elowitz, M. B., A. J. Levine, E. D. Siggia and P. S. Swain,
Forche, A., P. T. Magee, A. Selmecki, J. Berman and G. May,
Foster, P. L.,
Frank, S. A., and M. A. Nowak,
Fraser, D., and M. Kaern,
Fraser, H. B., A. E. Hirsh, G. Giaever, J. Kumm and M. B. Eisen,
Fritsch, E. S., J. Schacherer, C. Bleykasten-Grosshans, J. L. Souciet, S. Potier et al.,
Futreal, P. A., L. Coin, M. Marshall, T. Down, T. Hubbard et al.,
Giraud, A., I. Matic, M. Radman, M. Fons and F. Taddei,
Harper, C. V., K. Featherstone, S. Semprini, S. Friedrichsen, J. McNeilly et al.,
Hastings, P. J., J. R. Lupski, S. M. Rosenberg and G. Ira,
Heng, H. H.,
Huisinga, K. L., and B. F. Pugh,
Hurst, L. D.,
Ito, Y., H. Toyota, K. Kaneko and T. Yomo,
James, T. C., J. Usher, S. Campbell and U. Bond,
Kaern, M., T. C. Elston, W. J. Blake and J. J. Collins,
Kaufmann, B. B., and D. T. Hung,
Kohanski, M. A., D. J. Dwyer, B. Hayete, C. A. Lawrence and J. J. Collins,
Kohanski, M. A., M. A. DePristo and J. J. Collins,
Konkel, M. K., and M. A. Batzer,
Lambert, S., A. Watson, D. M. Sheedy, B. Martin and A. M. Carr,
Legrand, M., P. Lephart, A. Forche, F. M. Mueller, T. Walsh et al.,
Lemoine, F. J., N. P. Degtyareva, K. Lobachev and T. D. Petes,
Lemoine, F. J., N. P. Degtyareva, R. J. Kokoska and T. D. Petes,
Lenski, R. E., and J. E. Mittler,
Lenski, R. E., and P. D. Sniegowski,
Lephart, P. R., H. Chibana and P. T. Magee,
Lercher, M. J., and L. D. Hurst,
Loeb, L. A.,
Luebeck, E. G., and S. H. Moolgavkar,
Mao, E. F., L. Lane, J. Lee and J. H. Miller,
Marr, K. A., C. N. Lyons, T. R. Rustad, R. A. Bowden and T. C. White,
Mieczkowski, P. A., F. J. Lemoine and T. D. Petes,
Murphy, K. F., G. Balazsi and J. J. Collins,
Murphy, K. F., R. M. Adams, X. Wang, G. Balazsi and J. J. Collins,
Nadal, D., D. Carro, J. Fernandez-Larrea and B. Pina,
Newman, J. R., S. Ghaemmaghami, J. Ihmels, D. K. Breslow, M. Noble et al.,
Nowell, P. C.,
Polakova, S., C. Blume, J. A. Zarate, M. Mentel, D. Jorck-Ramberg et al.,
Querol, A., and U. Bond,
Rachidi, N., P. Barre and B. Blondin,
Radman, M.,
Radman, M., I. Matic and F. Taddei,
Rando, O. J., and K. J. Verstrepen,
Raser, J. M., and E. K. O'Shea,
Resnick, M. A.,
Rustchenko, E.,
Selmecki, A., A. Forche and J. Berman,
Sniegowski, P. D., and H. A. Murphy,
Sniegowski, P. D., P. J. Gerrish and R. E. Lenski,
Tenaillon, O., F. Taddei, M. Radmian and I. Matic,
Tomlinson, I., P. Sasieni and W. Bodmer,
Wang, T. L., C. Rago, N. Silliman, J. Ptak, S. Markowitz et al.,