-
PDF
- Split View
-
Views
-
Cite
Cite
Yinhua Zhang, Lisa L Maduzia, Mutations in Caenorhabditis elegans eIF2β Permit Translation Initiation From Non-AUG Start Codons, Genetics, Volume 185, Issue 1, 1 May 2010, Pages 141–152, https://doi.org/10.1534/genetics.110.115485
Close - Share Icon Share
Abstract
Recognition of the AUG start codon on mRNAs during translation initiation in eukaryotes occurs in a preinitiation complex that includes small ribosomal subunits and multiple translation initiation factors. The complexity of this process and the lack of appropriate tools have prevented its genetic study in multicellular organisms. Here we describe a genetic system in the nematode Caenorhabditis elegans to study how the AUG start codon is selected. We have generated a sensitive reporter assay that allows for the isolation of mutants with reduced fidelity to recognize the AUG start codon. Two mutants were identified to have dominant missense mutations in iftb-1, which encodes the β-subunit of eIF2 (eIF2β). Both mutations occur in a conserved region located outside of the C2–C2 zinc finger domain where yeast SUI3 mutations are localized in Saccharomyces cerevisiae eIF2β. C. elegans iftb-1, as well as mutant eIF2βs carrying the equivalent SUI3 mutations, are able to initiate translation at non-AUG codons that retain two potential base-pairing interactions with the anticodon of the initiator methionyl tRNA. These analyses further support the critical role of eIF2β in start codon selection, and two functional domains within eIF2β are likely involved, one defined by our C. elegans mutants and the other by the yeast SUI3 mutants.
RECOGNITION of the start codon AUG during translation initiation is critical for decoding the genetic information carried on mRNAs. In prokaryotes, the Shine–Dalgarno sequence located immediately 5′ to the AUG on the mRNA is complementary to a region on the 16S rRNA of the ribosome. This base-pairing interaction positions the mRNA so that the initiator tRNA finds the AUG efficiently. In contrast, such a base-pairing interaction between the mRNA and the ribosome is not found in eukaryotes. Instead, translation initiation appears to rely on the base pairing of the AUG codon with the anticodon as proposed in a ribosomal scanning model (Kozak 1989). In this model, a preinitiation complex (PIC), consisting of the small ribosomal subunit (40S), multiple translation initiation factors (eIFs), and an initiator methionyl tRNA (Met-tRNAi), moves from 5′ to 3′ along the mRNA to examine each nucleotide triplet for a start codon. The first AUG triplet is recognized as the start site for initiation. Perfect base pairing between the AUG and the anticodon signals to the rest of the initiation complex to shift to the elongation phase. Although many molecular components required for AUG selection have been identified (Pestova et al. 2007), it still remains unclear how the base-pairing interactions are recognized during ribosomal scanning.
Saccharomyces cerevisiae mutants that have reduced fidelity in start codon selection provide evidence supporting the involvement of key molecular components in AUG start codon recognition. In a genetic screen, suppressor of initiation codon (sui) mutants were isolated on the basis of their ability to express a modified His4 gene with an AUU at the native start site (Donahue et al. 1988). Subsequent analyses of these mutants revealed that they were able to initiate translation from a downstream in-frame UUG codon (the third codon of the His4 coding region). These mutations were found in five genes (SUI1-5) and multiple alleles of each gene were isolated. Three of the five genes correspond to the three subunits of the heterotrimeric GTPase eIF2: SUI2 is the α-subunit (Cigan et al. 1989), SUI3 is the β-subunit (Donahue et al. 1988), and SUI4 is the γ-subunit (Huang et al. 1997). The other two genes, SUI1 and SUI5, correspond to eIF1 (Yoon and Donahue 1992) and eIF5 (Huang et al. 1997), respectively. Increased translation initiation at the UUG codon by these mutants indicates that they are unfaithful in selecting the start codon AUG. Such genetic defects demonstrate that these initiation factors play critical roles in start codon recognition in wild-type cells.
The analyses of these yeast mutants, together with biochemical and structural studies, established that an important mechanism in start codon recognition involves the coupling of GTPase hydrolysis with the presence of the AUG codon (Hinnebusch et al. 2007; Pestova et al. 2007). The GTPase activity resides in the γ-subunit of eIF2 and it is tightly regulated by the α- and β-subunits as well as several other initiation factors such as eIF1 and eIF5. During AUG scanning, GTP is partially hydrolyzed into GDP·Pi by eIF2 and the presence of AUG triggers the release of Pi, resulting in start codon recognition (Algire et al. 2005). A SUI4 mutation in the GTP-binding G-domain of eIF2γ increases dissociation between eIF2 and Met-tRNAi in the absence of GTP hydrolysis and leads to abnormal initiation at a UUG codon (Huang et al. 1997). SUI3 mutations in eIF2β elevate the intrinsic rate of GTP hydrolysis of eIF2 and this leads to aberrant initiation at the UUG codon (Huang et al. 1997). eIF1 promotes scanning at non-AUG codons and thus prevents initiation at incorrect sites. Some SUI1 mutations in eIF1 appear to impair this function (Cheung et al. 2007). eIF5 is a typical GTPase-activating protein that stimulates eIF2 GTPase activity (Das et al. 2001; Paulin et al. 2001). A SUI5 mutation in eIF5 shows increased stimulation of GTP hydrolysis and again leads to translation initiation at non-AUG codons (Huang et al. 1997). Thus, the accuracy of AUG recognition relies on the precise control of GTP hydrolysis in response to base pairing with the anticodon, and the regulation requires at least eIF1, eIF2, and eIF5. Relaxed control of GTPase activity due to mutations in eIF2 or its regulators leads to the loss of initiation fidelity and improper initiation at non-AUG start codons.
Specific mutations in the components of the initiation complex obtained by yeast genetic screens have helped not only to pinpoint their particular roles, but also to identify important amino acids and define functional domains. To date, such genetic systems have been available only in yeast and have not yet been explored in multicellular organisms. We have developed a sensitive and efficient genetic screen in the nematode Caenorhabditis elegans to study the fidelity of start codon recognition. The screen is based on a GFP reporter with an altered translation start site. An advantage of this system is that mutants do not require the reporter product for survival, as was the case for His4 selection in yeast. Due to its high sensitivity, this screen allowed the isolation of mutants with subtle effects on translation initiation. Here we report the identification and characterization of two C. elegans eIF2β mutants, iftb-1(nb101) and iftb-1(nb131). Our analyses of these mutants and the C. elegans equivalents of the yeast SUI3 mutations further support its involvement in start codon selection and indicate that eIF2β appears to play a critical role in recognizing base pairing between the codon and the anticodon.
MATERIALS AND METHODS
Construction of reporter:
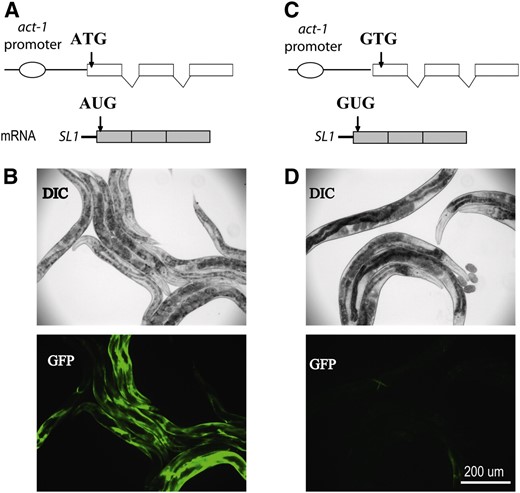
The act-1 (T04C12.6) promoter region (2929 bp) was PCR amplified from genomic DNA (forward primer, actgGTCGACgatttattagattaggaggaac; reverse primer, actgGGTACCatgtacctgaaaaattaaattg; inserted SalI and KpnI restriction enzymes sites used for cloning are indicated in uppercase). This fragment was inserted into the C. elegans GFP expression vector pPD95.75 before the GFP coding sequence to generate the plasmid pIP52 (Figure 1A). The translation initiation site for GFP in this vector is preceded with bases GGTAGAAAAA that contain the C. elegans consensus sequence (AAAA) found before the translation initiation site of endogenous mRNAs (Blumenthal and Steward 1997). Next, pIP56 was made by introducing two mutations into the reporter. The first mutation altered the GFP translation initiation codon ATG to GTG (Figure 1C), while the second mutation inserted the sequence AATCAAAAT before GTGTTATATT, generating an AUG codon (underlined) in the outron region (Krause and Hirsh 1987). During pre-mRNA maturation, the outron region including the insertion is replaced by a 22-base SL1 leader sequence through a process called RNA trans-splicing (Blumenthal 2005). Both mutations were engineered with USER enzymes (New England Biolabs, Ipswich, MA) (Bitinaite et al. 2007), using a 672-bp SacI-XhoI fragment that included both mutation sites. These constructs were injected at 30 μg/ml with 48 μg/ml of pRF4 as a cotransformation marker to generate transgenic worms containing extrachromosomal arrays. RT–PCR analyses of the GFP mRNA derived from pIP52 and pIP56 confirmed the expected patterns of splicing and the insertion-containing outron is efficiently removed by SL1 trans-splicing.
Integration of transgene:
A novel method utilizing methyl methanesulfonate (MMS) [Sigma (St. Louis) M-4016] instead of irradiation was performed to integrate the pIP56 reporter extrachromosomal arrays. Three microliters of MMS was added to mixed-stage worms in 2 ml of M9 buffer and slowly shaken at room temperature for 30 min. After washing three times with M9 buffer, worms were transferred onto large (9 cm) NGM plates previously seeded with Escherichia coli OP50 and allowed to recover for 2–3 hr. Next, 15 transgenic roller worms at the late L4 larval or young adult stages were transferred to each of 20 small (6 cm) NGM plates. These plates were incubated at 20° for 10–14 days. During this period, the frequency of the integrated transgene is enriched relative to that of the extrachromosomal arrays. Up to 20 roller worms from each starved plate were singled out to examine whether or not they carried an integrated transgene on the basis of 100% transmission of the roller phenotype. After screening 400 rollers, six integration lines were obtained. Integrated strains were out crossed twice to remove unwanted mutations. Two strains (nbIs2 and nbIs4) were used as reporter strains in mutant screens and the integration sites of the transgenes were mapped to chromosomes IV and I, respectively.
Mutant screens:
Two separate screens were performed to obtain iftb-1(nb101) and iftb-1(nb131) mutants. The nb101 mutant was isolated from the nbIs2 reporter strain using the mutagen ethyl methanesulfonate (EMS) (Wood 1988). Briefly, worms were treated with 0.5% EMS for 4 hr and then five hermaphrodites at the late L4 larval or young adult stages were placed onto each of 40 large NGM plates. In the F2 generation, worms that showed visible GFP expression under a fluorescence dissecting microscope (Leica MZFLIII) were singled out as candidate mutant strains. The nb131 allele was obtained from the nbIs4 reporter strain following mutagenesis with N-ethyl-N-nitrosourea (ENU) (De Stasio and Dorman 2001). In this screen, P0 worms synchronized at L4 larval and young adult stages were treated with 1 mm ENU for 4 hr. F1 eggs (∼75,000) were transferred onto 20 large NGM plates and allowed to hatch and grow up. Once gravid, F1 adult worms from each plate were bleached separately and F2 eggs were transferred onto new plates. These F2 worms were screened for GFP-expressing mutants upon reaching the young adult stage.
Genetic analyses:
The chromosomal linkage of the mutant gene was assessed relative to the location of the transgene to exclude potential GFP reporter reversion mutants. F1 double heterozygous males (e.g., m/+, nbIs2/+) were used to mate with wild-type N2 hermaphrodites. In the next generation, eight cross-progeny hermaphrodites (F2) were singled out and examined to determine whether they segregate GFP-expressing mutants. If a mutation is linked to the transgene, all of these F2 worms will segregate GFP-expressing mutants. If unlinked, approximately half of them will segregate GFP mutants. These analyses revealed that the iftb-1(nb101) mutation was unlinked to the reporter nbIs2, while iftb-1(nb131) was linked to nbIs4.
Chromosomal deficiency and duplication analyses with the iftb-1(nb101) allele were performed as follows. The strain IP447: unc-13(e51) iftb-1(nb101))/nDf24; nbIs2 was constructed to obtain deficiency-containing animals. Non-Unc-13 worms were identified as nb101/nDf24 since the deficiency does not cover the unc-13 loci. The genotype of iftb-1 was also confirmed by sequencing PCR fragments amplified from these non-Unc-13 worms. The strain IP445: unc-13(e51) iftb-1(nb101); nDp4; nbIs2 was constructed to generate duplication-carrying worms that were non-Unc-13 since the duplicated chromosomal region in nDp4 includes unc-13. The presence of a wild-type copy of iftb-1 was also confirmed by DNA sequencing.
Genetic mapping and gene identification:
Using SNP mapping techniques with the Hawaiian strain (CB4856) as described previously (Davis et al. 2005), iftb-1(nb101) was shown to be linked to chromosome I. Several three-factor or multifactor crosses further localized the mutation to a narrow genomic region. With strains carrying double markers dpy-5 sem-4 or lin-11 unc-75, the mutation was placed between sem-4 and lin-11. With markers unc-13 lin-11 or unc-13 lin-10, the mutation was placed between unc-13 and lin-10 with the following results: unc-13 (5/14) iftb-1(nb101) (9/14) lin-11 and unc-13(6/6) [iftb-1(nb101) lin-10]. Further SNP mapping with the CB4856 strain using the triple mutant dpy-5 iftb-1(nb101) lin-11 generated 96 Lin-11 nonDpy-5 recombinants. Analyses of SNP patterns among these recombinants placed iftb-1(nb101) in a genomic region limited by SNP markers SNP_H05L14[3] and uCE1-955.
To map iftb-1(nb131), a chromosome containing unc-35(e259) dpy-5(e61) iftb-1(nb131) nbIs4 was constructed for SNP mapping with the CB4856 strain. Forty-eight Dpy non-iftb-1(nb131) recombinants were obtained. All recombination events were found to have occurred to the right of SNP_H05L14[3]. One recombination event occurred between uCE1-948 and SNP_H05L14[3] and two others occurred between SNP_D2005[1] and uCE1-944 . These results placed iftb-1(nb131) very close to or to the right of SNP_H05L14[3].
To identify the gene mutated in iftb-1(nb101), we sequenced candidate genes located between SNP_H05L14[3] and uCE1-955. The coding regions of 12 predicted genes (F18C12.4, K04G2.10, K04G2.7, K04G2.11, K04G2.4, K04G2.1, C54G4.9, C54G4.7, C54G4.6, C54G4.1, C54G4.2, and C54G4.3) were PCR amplified from the iftb-1(nb101) homozygous strain, sequenced, and compared to the reference sequences in Wormbase. Only K04G2.1 was found to contain a single-base change.
A transgenic phenocopy method was used to confirm the gene identified. A genomic fragment containing the coding region of K04G2.1 (970 bp) and its upstream (3076 bp) and downstream (396 bp) sequences was PCR amplified from wild-type or iftb-1(nb101) mutant animals using Phusion DNA polymerase (New England Biolabs). Two cosmids [K04G2 and C54G4, gifts from A. Coulson, Medical Research Council (MRC)] that contain the wild-type copy of K04G2.1 were also tested. The cosmid DNA (5 μg/ml) or PCR product (1 μg/ml) was co-injected with pIP56 and pRF4 into wild-type worms, and extrachromosomal arrays were subsequently introduced into the iftb-1(nb101) background.
Suppression of unc-62(t2012) by iftb-1(nb101):
A strain with the genotype +/iftb-1(nb101); nbIs2; +/eT1; unc-62(t2012) dpy-11(e224)/eT1 was constructed to obtain homozygous unc-62(t2012) dpy-11(e224) animals. Of 62 Dpy worms singled, 19 of them gave viable progeny as a result of suppression and the remainder gave only dead eggs. The progeny of 12 worms were randomly picked for genotyping by DNA sequencing. All 12 were found to be +/iftb-1(nb101); unc-62(t2012). Three of these viable Dpy strains were then singled a second time and those animals that grew to adults were sequenced again to confirm they were all of the genotype +/iftb-1(nb101); unc-62(t2012). An identical genetic strain was constructed using unc-62(ct344) and analyzed for suppression. Progeny from unc-62(ct344) mutants gave similar lethality whether or not iftb-1(nb101) was present.
Determination of GFP expression:
To estimate GFP protein expressed in mutant strains, worm lysate was prepared by boiling 100 animals in 15 μl of 1× SDS sample buffer. Western Blotting was performed using anti-GFP monoclonal antibody (Roche 11 814 460 001) (dilution 1:1000). After detecting with a secondary antibody labeled with infrared fluorescent dye (LiCor 926-32220), the signal was imaged and analyzed using a LiCor/Odyssey scanner.
Visual scoring of GFP levels was performed under a Leica MZFLIII with the objective at 5× zoom. Fluorescent worms were mounted on a 2% agarose pad for digital imaging on a Zeiss Axiovert 200M with a 10× objective, using a 16-bit camera with AxioVision 4.7 software. Identical settings were used and images were taken within several days for each set of samples. A histogram of gray values was used to calculate relative GFP signal levels following methods for quantitative fluorescence microscopy (Waters 2009). The background signal for each image was determined using a region that did not contain worms and its average gray value per pixel and associated standard deviation were calculated using the Zeiss software. After subtracting background, fluorescent signals from pixels with gray values higher than the background mean by three standard deviations (thus excluding >99% of background signals) were summed as a total fluorescent signal from GFP. To compensate for variations in worm number between images, which ranged from 5 to 10 worms, a mean gray value per pixel was calculated as the relative GFP signal level. The mean gray value represents the average brightness of GFP signal-containing pixels and is relatively independent of worm number variation.
Expression of non-AUG reporters in iftb-1(nb101):
GFP reporters containing additional non-AUG codons were constructed using pIP56 described above. Desired mutations were introduced by site-directed mutagenesis using Phusion DNA polymerase. These constructs were injected at 15 μg/ml along with pRF4 into wild-type worms. Extrachromosomal arrays were then crossed into the genotype dpy-5 iftb-1(nb101)/+ + to examine the effect of heterozygous iftb-1(nb101) on the expression of these non-AUG reporters. Some reporters were also introduced into iftb-1 homozygotes with the genotype dpy-5 iftb-1(nb101) +/+ iftb-1(nb101) unc-13.
Expression of non-AUG reporters in transgenic worms carrying SUI3 equivalent mutations:
The effects of SUI3 equivalent mutations were assayed in transgenic worms. To make eIF2β mutations, K04G2.1 was first cloned into pNEB206A using the USER cloning strategy (New England Biolabs). Mutations for iftb-1(nb131) and four SUI3 alleles (worm mutation/yeast mutation: K205T/R248T, L209P/L254P, S219Y/S264Y, and V223G/V268G) were generated by site-directed mutagenesis. Each construct was injected at 2 μg/ml along with non-AUG reporter constructs. The frequencies of extrachromosomal array formation from F1 transgenic worms were similar: 6.9% for wild type, 7.6% for iftb-1(nb101), 3.0% for iftb-1(nb131), 6.2% for K205T, 8.5% for L209T, 7.1% for S219Y, and 7.0% for V223G, suggesting that these mutations did not have an apparent toxic effect on the formation of arrays. The GFP expression level was scored relative to the GFP expressed from worms carrying the iftb-1(nb101) transgene and a GUG-containing reporter.
RESULTS
A reporter system to screen for mutants that permit the use of non-AUG start codons:
We generated a sensitive reporter system using the promoter of the highly expressed C. elegans act-1 gene that encodes a muscle actin (Willis et al. 2006). A transcriptional GFP reporter containing the act-1 promoter produces abundant GFP expression in the body wall muscles at all developmental stages from late embryogenesis to adult (Figure 1, A and B). When the translation start codon ATG of GFP was mutated to GTG, the expression of GFP was almost completely eliminated (Figure 1, C and D). This GTG-containing reporter was integrated into the genome (nbIs2 or nbIs4) and then used to screen for mutants that expressed GFP. It is likely that these GFP-expressing strains are able to initiate translation from non-AUG codons. Two mutant alleles described here, iftb-1(nb101) and iftb-1(nb131), were obtained from separate mutagenesis screens (see materials and methods).
Construction of a GFP reporter for screening translation initiation mutants. (A) Transcriptional reporter of act-1∷GFP containing the native ATG translation start codon of the GFP gene. (B) GFP expression from act-1∷GFP reporter in wild-type worms. (C) Non-AUG GFP reporter carrying a mutation that alters the ATG translation start codon to GTG. (D) GFP expression from GTG–GFP reporter in wild-type worms. Reporter expressions shown in both B and D were obtained from extrachromosomal arrays.
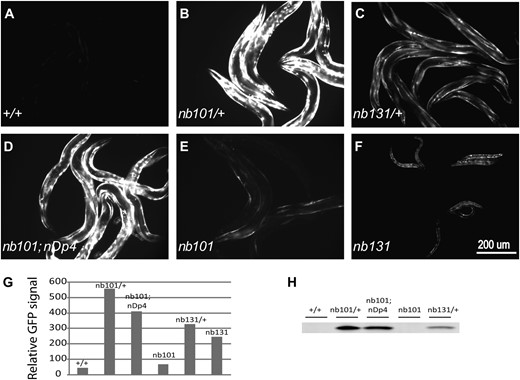
During outcrossing and genetic mapping, both iftb-1 alleles were found to be dominant in regard to the expression of the GTG-containing GFP reporter. To test the dominant nature of iftb-1(nb101), iftb-1(nb101)/+; nbIs2/+ males were crossed with dpy-11; nbIs2 hermaphrodites. Among the cross progeny, 31 of 127 worms showed GFP expression. All of these GFP-expressing worms segregated both iftb-1(nb101) and wild-type worms and all were found to be homozygous for the reporter nbIs2. These genetic segregations indicated that iftb-1(nb101) is dominant and two copies of the transgene nbIs2 are required for the GFP phenotype. Consistent with this dosage effect of the reporter gene, dpy-5 iftb-1(nb101)/+; nbIs2/+ heterozygous worms showed little GFP. A similar dominant property and reporter dosage effect was observed with iftb-1(nb131). Worms with genotype iftb-1(nb131) nbIs4/+ nbIs4 showed GFP expression while iftb-1(nb131) nbIs4/++ worms did not.
For both alleles, a high level of GFP expression was observed in heterozygous animals (Table 1, lines 1–3; Figure 2, A–C, G, and H) or in homozygous worms also carrying a wild-type gene on a chromosomal duplication (Table 1, line 4; Figure 2, D, G, and H). iftb-1(nb101) and iftb-1(nb131) homozygous worms showed different GFP expression and growth phenotypes. iftb-1(nb101) homozygotes or iftb-1(nb101) overdeficiency worms are viable but express GFP at very low levels (Table 1, lines 5 and 6; Figure 2, E, G, and H). After removing the reporter transgene, we examined the fertility and growth characteristics of iftb-1(nb101) homozygous animals. The brood size of these mutants was ∼52% [146/278, average progeny per hermaphrodite, iftb-1(nb101)/wild type], 86% (189/218), and 66% (251/377) of wild type at 15°, 20°, and 25°, respectively. The decreased brood size appeared to be largely attributed to a reduced fertility since only a slight increase in embryonic lethality (∼3%) was observed at any incubation temperature. These mutant worms also showed a slow growth defect. Most mutant embryos only developed to the L4 larval stage by the time their wild-type counterparts reached adulthood at 15° or 25°. In contrast, iftb-1(nb131) homozygotes showed GFP expression at levels similar to the heterozygote and arrested at the L1 larval stage (Table 1, lines 3 and 7; Figure 2, C, F, and G).
Characterization of mutant phenotypes
Line . | Genotype . | GFP level . |
|---|---|---|
| 1 | nbIs2 (or nbIs4) | — |
| 2 | iftb-1(nb101)/+; nbIs2 | +++ |
| 3 | iftb-1(nb131) nbIs4/+nbIs4 | +++ |
| 4 | iftb-1(nb101); nDP4; nbIs2 | +++ |
| 5 | iftb-1(nb101); nbIs2 | +/− |
| 6 | iftb-1(nb101)/nDf24; nbIs2 | +/− |
| 7 | iftb-1(nb131) nbIs4 | +++ |
Line . | Genotype . | GFP level . |
|---|---|---|
| 1 | nbIs2 (or nbIs4) | — |
| 2 | iftb-1(nb101)/+; nbIs2 | +++ |
| 3 | iftb-1(nb131) nbIs4/+nbIs4 | +++ |
| 4 | iftb-1(nb101); nDP4; nbIs2 | +++ |
| 5 | iftb-1(nb101); nbIs2 | +/− |
| 6 | iftb-1(nb101)/nDf24; nbIs2 | +/− |
| 7 | iftb-1(nb131) nbIs4 | +++ |
Characterization of mutant phenotypes
Line . | Genotype . | GFP level . |
|---|---|---|
| 1 | nbIs2 (or nbIs4) | — |
| 2 | iftb-1(nb101)/+; nbIs2 | +++ |
| 3 | iftb-1(nb131) nbIs4/+nbIs4 | +++ |
| 4 | iftb-1(nb101); nDP4; nbIs2 | +++ |
| 5 | iftb-1(nb101); nbIs2 | +/− |
| 6 | iftb-1(nb101)/nDf24; nbIs2 | +/− |
| 7 | iftb-1(nb131) nbIs4 | +++ |
Line . | Genotype . | GFP level . |
|---|---|---|
| 1 | nbIs2 (or nbIs4) | — |
| 2 | iftb-1(nb101)/+; nbIs2 | +++ |
| 3 | iftb-1(nb131) nbIs4/+nbIs4 | +++ |
| 4 | iftb-1(nb101); nDP4; nbIs2 | +++ |
| 5 | iftb-1(nb101); nbIs2 | +/− |
| 6 | iftb-1(nb101)/nDf24; nbIs2 | +/− |
| 7 | iftb-1(nb131) nbIs4 | +++ |
Increased GFP expression from a non-AUG reporter in iftb-1 mutants. (A) Wild-type worms. (B) iftb-1(nb101)/+. (C) iftb-1(nb131)/+. (D) iftb-1(nb101);nDp4. (E) iftb-1(nb101). (F) iftb-1(nb131). All panels contain adult worms except F, which contains developmentally arrested L1 larvae. (G) Relative GFP signal levels of images A–F determined by digital image analysis. (H) Amount of GFP protein detected by Western blot, using 100 adult worms per sample.
Genetic suppression of an initiator codon mutation in unc-62(t2012) by iftb-1(nb101):
The expression of the GTG-containing reporter in iftb-1(nb101) animals suggests that these worms might initiate translation from a non-AUG codon. To confirm this possibility, genetic suppression experiments were performed to determine whether iftb-1(nb101) could also initiate translation from another non-AUG codon within an endogenous gene. The mutation responsible for the unc-62(t2012) phenotype is a nucleotide change within the initiator AUG that results in the non-AUG start codon AUA (Van Auken et al. 2002). unc-62(t2012) worms are maternal-effect lethal such that they grow up normally but their progeny arrest as either unhatched embryos or early larvae. This lethal phenotype therefore provided a sensitive test to examine the effects of iftb-1(nb101) on this mutant. We find that in the presence of one copy of the iftb-1(nb101) mutation but not two, unc-62(t2012) worms produce viable progeny that develop into reproductive adults (see materials and methods for details). This suppression is specific to the unc-62(t2012) allele since iftb-1(nb101) heterozygotes or homozygotes do not improve the lethality of unc-62(ct344) mutant animals, which contain a 7.2-kb deletion in the promoter region. Suppression of the maternal-effect lethal phenotype of unc-62(t2012) by iftb-1(nb101) indicates that essential UNC-62 protein was synthesized from mRNAs containing the non-AUG start codon AUA. This supports the notion that iftb-1(nb101) allows translation to initiate at non-AUG codons on mRNAs derived either from endogenous or from reporter genes.
Mutations in a novel domain of eIF2β lead to non-AUG translation:
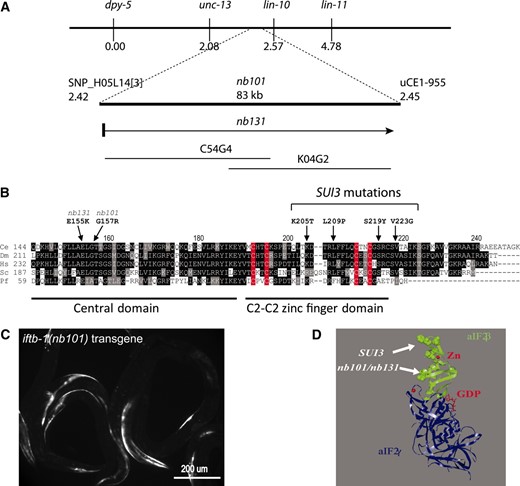
To determine the identity of genes mutated in iftb-1(nb101) and iftb-1(nb131), we performed standard three-factor and SNP mapping. We placed iftb-1(nb101) in an 83-kb genomic region between two SNPs, SNP_H05L14[3] and uCE1-955, which is covered by two overlapping cosmids (C54G4 and K04G2) (Figure 3A). After sequencing the coding regions of 12 predicted genes located in this region, a single missense mutation was found in the predicted coding region of K04G2.1: a G469A base change that results in a G157R amino acid substitution in the protein sequence (Figure 3B). In separate SNP mappings, iftb-1(nb131) was placed very close and to the right of SNP_H05L14[3]. Due to the close proximity and similar GFP expression phenotype to that of iftb-1(nb101), the genomic DNA of K04G2.1 was then amplified and sequenced from L1-arrested iftb-1(nb131) homozygotes and adult heterozygotes. A single-base change, G463A, was identified that leads to an E155K amino acid substitution that is located only two residues away from the iftb-1(nb101) mutation (Figure 3B).
eIF2β mutations are responsible for GFP expression from the GTG-containing reporter. (A) Genetic mapping of mutants. (B) Locations of amino acid changes found in iftb-1(nb101) and iftb-1(nb131). Four mutations equivalent to yeast SUI3 alleles are also shown and labeled according to the C. elegans sequence. Part of the central region and the C-terminal C2–C2 domain of eIF2βs from C. elegans (Ce), Drosophila melanogaster (Dm), Homo sapiens (Hs), Saccharomyces cerevisiae (Sc), and the archaea Pyrococcus furiosus (Pf) aIF2β were aligned to compare identical (shaded black) and similar (shaded gray) residues. Cysteine residues that form the zinc finger domain are highlighted in red. (C) GFP expression from a GTG–GFP reporter carried on extrachromosomal arrays that also coexpress the iftb-1(nb101) mutant eIF2β. (D) Positions of eIF2β mutations mapped onto the P. furiosus aIF2 structure (2DCU) consisting of the β- (green) and γ-subunits (blue). Residues of aIF2β corresponding to iftb-1 and SUI3 mutations are highlighted as solid atoms. The amino acids that form the C2–C2 zinc finger are shown as ball and stick atoms surrounding the Zn ion.
To confirm gene identity, we took advantage of the fact that both iftb-1(nb101) and iftb-1(nb131) are dominant and tested for their ability to confer reporter GFP expression when carried on a transgene. The K04G2.1 coding region along with its associated promoter and downstream region was amplified either from iftb-1(nb101) or from wild-type worms. Plasmid carrying the iftb-1(nb131) mutation was generated by site-directed mutagenesis. These DNA fragments, as well as cosmids containing the wild type gene, were individually co-injected with the GTG-containing reporter construct into wild-type worms. GFP expression was observed in worms carrying the iftb-1(nb101)- or iftb-1(nb131)-containing transgene, but not in worms carrying the wild-type transgene either on cosmids or as an amplified genomic fragment (Figure 3C and data not shown). In reciprocal experiments, transgene-containing wild-type or mutant K04G2.1 were introduced into the iftb-1(nb101) worms. GFP expression was observed in these iftb-1(nb101) mutant worms carrying the wild-type transgenes but not in worms carrying the iftb-1(nb101) transgene. Thus, these results demonstrate that the mutant phenotypes can be assayed even when the gene is expressed from a transgene, further reflecting the dominant nature of these iftb-1 alleles. We conclude that these missense mutations in K04G2.1 are responsible for the GFP phenotype.
K04G2.1 is the only predicted eIF2β in the C. elegans genome (Rhoads et al. 2006). The predicted gene structure was confirmed by sequencing RT–PCR products. This gene encodes a 250-amino-acid protein, which is relatively small in size among eukaryotic eIF2βs (i.e., human eIF2β is 343 amino acids in length). Like typical eIF2βs, it has a diverged N-terminal domain and highly conserved central and C-terminal C2–C2 zinc finger domains (Figure 3B; supporting information, Figure S1). A prominent feature of the N-terminal domain is that it contains stretches of lysine (Lys) residues. Interestingly, only two stretches of Lys residues could be identified in C. elegans while three Lys stretches are present in vertebrates, arthropods, and yeast. The presence of only two stretches of Lys residues is a common characteristic among eIF2βs found in other nematode and plant species (Figure S1). The G157R mutation in iftb-1(nb101) and the E155K mutation in iftb-1(nb131) are located within the central domain. Both amino acids are conserved in all eukaryotic eIF2βs. In addition, they are also conserved in aIF2β from Archaea, which have a translation system similar to eukaryotes. Pyrococcus furiosus aIF2β has an identical residue at the corresponding position of E155 and has a conserved residue corresponding to G157 (Figure 3B). In comparison, SUI3 mutations of yeast eIF2β are localized within the vicinity of the C2–C2 zinc finger domain. When modeled onto an archaeal IF2β crystal structure, these two residues that are mutated in the iftb-1 strains are located in a region adjacent to the zinc finger domain where the SUI3 mutations reside (Figure 3D). Thus, the iftb-1(nb101) and iftb-1(nb131) mutations together define a new functional region within eIF2β.
eIF2β containing the iftb-1(nb101) mutation allows translation at a subset of non-AUG start codons:
The restoration of GFP expression from our GTG-containing reporter is unlikely due to translation initiation at a cryptic start site within the GFP coding frame. There is no upstream in-frame AUG codon within the mature mRNA and the next downstream in-frame AUG codon is located at amino acid 95. If used as the start codon, this AUG codon would lead to a truncated and nonfluorescent GFP fragment, since evidence suggests that even the first 8 amino acids are essential for GFP fluorescence (Dopf and Horiagon 1996; Li et al. 1997). By Western blot analysis, the size of the GFP produced in these mutants was found to be identical to that of GFP expressed from the unmodified ATG reporter (data not shown). This suggests that translation likely initiates at or near the GUG codon. To further address whether this codon is utilized for translation initiation, we examined the expression of GFP reporters that contain other non-AUG codons at this position. If translation initiates from this site, one would expect different levels of GFP expression due to the variable interactions of these non-AUG codons with the translation initiation complex. However, if translation were to initiate at a position different from this codon location, then it would be expected that GFP expression would be independent of these base changes.
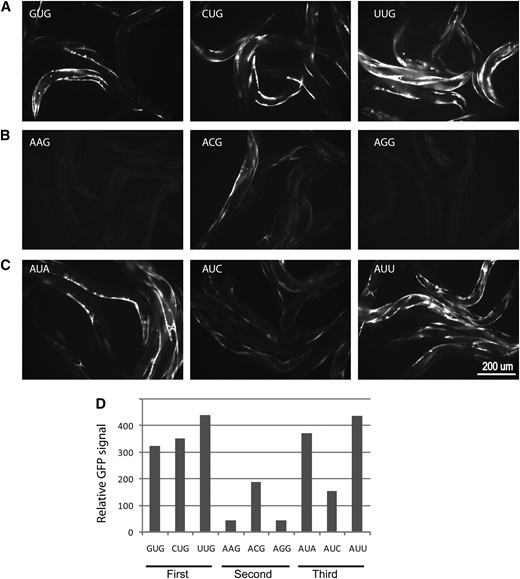
We assayed reporters containing all possible single-base changes relative to AUG as well as several 2- or 3-base change combinations. Most of these reporters showed little GFP expression in wild-type worms with the exception of weak signals produced from the CUG and AUU reporters upon visual inspection using a dissecting microscope (Table 2), indicating that these non-AUG codons are not efficiently used for translation initiation. When these reporters were introduced into the iftb-1(nb101) heterozygous background, they gave variable GFP expression that correlates with the position of the base change. Reporters with one base change at the first or the third base of the AUG codon showed a significant increase in GFP expression (Table 2, lines 2–4 and 8–10; Figure 4). The GFP expression levels among these reporters were mostly similar. Reporters that had changes at the second base position gave no apparent increase in GFP expression levels with the exception of the ACG reporter that showed an increased level of expression over background (Table 2, lines 5–7; Figure 4). In contrast, no expression was observed from reporters that contained various 2- or 3-base change combinations (Table 2, lines 11–15). The expression of these non-AUG reporters was also assayed in iftb-1(nb101) homozygotes. Most reporters that expressed GFP in the iftb-1(nb101) heterozygotes also showed increased expression in the homozygotes although to a lesser extent, with the exception of the CUG- and AUA-containing reporters where GFP signal was undetectable (Table 2). In summary, only a subset of non-AUG reporters is expressed in iftb-1(nb101), particularly those with a single-base change either at the first or at the third base position of the AUG start codon. These data support that translation of GFP in our mutants is initiated from the same position as the native AUG start site, even when it consists of a non-AUG codon.
Expression of GFP from non-AUG reporters in iftb-1(nb101) mutants
Line . | Reporter . | wt . | iftb-1(nb101)/+ . | iftb-1(nb101) . |
|---|---|---|---|---|
| 1 | AUG | +++++ | +++++ | +++++ |
| 2 | GUG | − | +++ | NA |
| 3 | CUG | + | +++ | − |
| 4 | UUG | − | +++ | ++ |
| 5 | AAG | − | − | − |
| 6 | ACG | − | ++ | + |
| 7 | AGG | − | − | NA |
| 8 | AUA | − | +++ | − |
| 9 | AUC | − | ++ | +/− |
| 10 | AUU | + | +++ | ++ |
| 11 | CCG | − | − | NA |
| 12 | GUC | − | − | − |
| 13 | UUC | − | − | − |
| 14 | GGC | − | − | NA |
| 15 | UAC | − | − | NA |
Line . | Reporter . | wt . | iftb-1(nb101)/+ . | iftb-1(nb101) . |
|---|---|---|---|---|
| 1 | AUG | +++++ | +++++ | +++++ |
| 2 | GUG | − | +++ | NA |
| 3 | CUG | + | +++ | − |
| 4 | UUG | − | +++ | ++ |
| 5 | AAG | − | − | − |
| 6 | ACG | − | ++ | + |
| 7 | AGG | − | − | NA |
| 8 | AUA | − | +++ | − |
| 9 | AUC | − | ++ | +/− |
| 10 | AUU | + | +++ | ++ |
| 11 | CCG | − | − | NA |
| 12 | GUC | − | − | − |
| 13 | UUC | − | − | − |
| 14 | GGC | − | − | NA |
| 15 | UAC | − | − | NA |
The GFP expression intensity was scored under a dissecting microscope as follows: −, no expression; +, weak expression; ++, moderate expression; +++, high expression. The GFP expression from reporters carrying the native AUG start codon (line 1) is considerably higher (+++++) than that from any other non-AUG reporters. NA, strain not available for scoring. wt, wild type.
Expression of GFP from non-AUG reporters in iftb-1(nb101) mutants
Line . | Reporter . | wt . | iftb-1(nb101)/+ . | iftb-1(nb101) . |
|---|---|---|---|---|
| 1 | AUG | +++++ | +++++ | +++++ |
| 2 | GUG | − | +++ | NA |
| 3 | CUG | + | +++ | − |
| 4 | UUG | − | +++ | ++ |
| 5 | AAG | − | − | − |
| 6 | ACG | − | ++ | + |
| 7 | AGG | − | − | NA |
| 8 | AUA | − | +++ | − |
| 9 | AUC | − | ++ | +/− |
| 10 | AUU | + | +++ | ++ |
| 11 | CCG | − | − | NA |
| 12 | GUC | − | − | − |
| 13 | UUC | − | − | − |
| 14 | GGC | − | − | NA |
| 15 | UAC | − | − | NA |
Line . | Reporter . | wt . | iftb-1(nb101)/+ . | iftb-1(nb101) . |
|---|---|---|---|---|
| 1 | AUG | +++++ | +++++ | +++++ |
| 2 | GUG | − | +++ | NA |
| 3 | CUG | + | +++ | − |
| 4 | UUG | − | +++ | ++ |
| 5 | AAG | − | − | − |
| 6 | ACG | − | ++ | + |
| 7 | AGG | − | − | NA |
| 8 | AUA | − | +++ | − |
| 9 | AUC | − | ++ | +/− |
| 10 | AUU | + | +++ | ++ |
| 11 | CCG | − | − | NA |
| 12 | GUC | − | − | − |
| 13 | UUC | − | − | − |
| 14 | GGC | − | − | NA |
| 15 | UAC | − | − | NA |
The GFP expression intensity was scored under a dissecting microscope as follows: −, no expression; +, weak expression; ++, moderate expression; +++, high expression. The GFP expression from reporters carrying the native AUG start codon (line 1) is considerably higher (+++++) than that from any other non-AUG reporters. NA, strain not available for scoring. wt, wild type.
Expression of GFP from reporters carrying distinct non-AUG start codons in iftb-1(nb101) heterozygous worms. Fluorescent images obtained from reporters with nucleotide changes at the same base position of the AUG codon are shown in a row. (A) Changes at the first base position (GUG, CUG, and UUG). (B) Changes at the second position (AAG, ACG, and AGG). (C) Changes at the third base position (AUA, AUC, and AUU). (D) Relative GFP signals determined by digital image analysis.
We also assayed whether the iftb-1(nb101) mutant can efficiently recognize the native AUG start site. The expression of GFP from an unmodified AUG reporter was examined in both heterozygous and homozygous mutants. The level of GFP expression was found to be similar among these mutants and comparable to GFP expression levels in wild-type worms (Table 2, line 1). Thus, the iftb-1(nb101) mutation does not significantly affect the ability of eIF2β to recognize native AUG start codons even though it is able to use non-AUG codons to initiate translation. This is consistent with the fact that iftb-1(nb101) homozygous worms are viable with only mild growth defects.
Equivalent SUI3 mutations also allow translation initiation with a subset of non-AUG codons in C. elegans:
In contrast to what we observed with the iftb-1(nb101) mutant described above, yeast eIF2β SUI3 mutants prefer UUG over other non-AUG codons to initiate translation (Huang et al. 1997). It is possible that yeast SUI3 mutations behave differently since they cluster within the zinc finger domain. To address this possibility, we utilized a transgenic assay to study equivalent SUI3 mutations in C. elegans. This assay was derived from our observation that, due to its dominant nature, the iftb-1(nb101) mutant gene was able to confer a phenotype when expressed from a transgene in wild-type worms (Figure 3C). Since SUI3 mutations are dominant in yeast (Castilho-Valavicius et al. 1992), it is possible that similar mutations also behave dominantly in C. elegans. Using this approach, we examined the effects of four SUI3 equivalent mutations (K205T, L209P, S219Y, and V223G) (Figure 3B) on GFP expression from five non-AUG reporters (GUG, UUG, AUU, ACG, and UUC). Wild-type eIF2β, iftb-1(nb101), and iftb-1(nb131) mutants were also included for comparison.
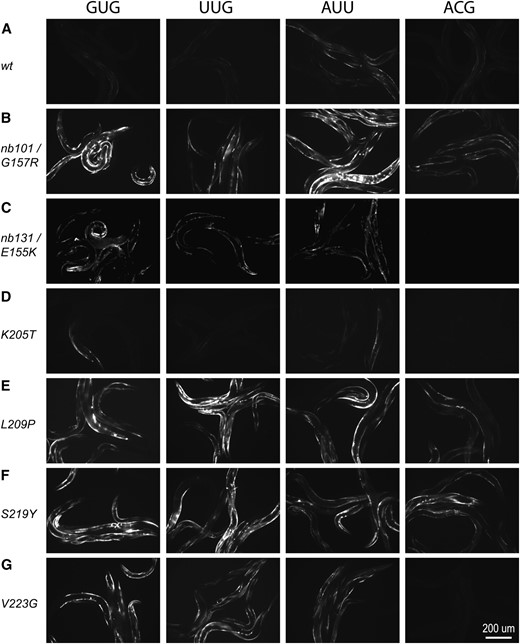
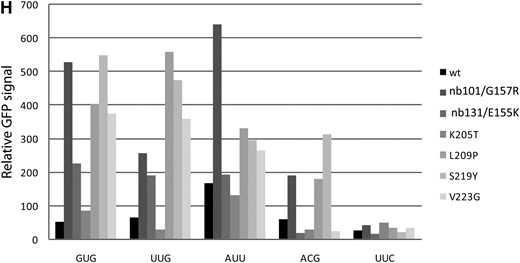
Transgenic worms carrying iftb-1(nb101),iftb-1(nb131), or each of the three SUI3 mutants (L209P, S219Y, and V223G) showed an increase in GFP expression levels from three non-AUG reporters (GUG, UUG, and AUU) (Table 3 and Figure 5). Increased GFP expression is also observed from the ACG reporter with nb101, L209P, and S219Y mutants, but the level of GFP appears to be lower. The increased expression is likely due to the presence of specific eIF2β mutations, since only low levels of GFP expression (most notably in the AUU reporter) were observed when the wild-type gene was present on the transgene (Table 3; Figure 5A). No significant GFP expression was observed with the K205T mutant. The UUC reporter did not show expression in any mutant transgenic worms (Table 3).
Expression of selected non-AUG reporters in worms coexpressing equivalent SUI3 eIF2β mutant genes
Line . | Reporter . | wt . | G157Rnb101 . | E155Knb131 . | K205T . | L209P . | S219Y . | V223G . |
|---|---|---|---|---|---|---|---|---|
| 1 | GUG | − | +++ | ++ | +/− | +++ | +++ | +++ |
| 2 | UUG | − | ++ | ++ | − | +++ | +++ | ++ |
| 3 | AUU | + | +++ | ++ | +/− | +++ | +++ | ++/+++ |
| 4 | ACG | − | ++ | − | − | ++ | ++ | − |
| 5 | UUC | − | − | − | − | − | − | − |
Line . | Reporter . | wt . | G157Rnb101 . | E155Knb131 . | K205T . | L209P . | S219Y . | V223G . |
|---|---|---|---|---|---|---|---|---|
| 1 | GUG | − | +++ | ++ | +/− | +++ | +++ | +++ |
| 2 | UUG | − | ++ | ++ | − | +++ | +++ | ++ |
| 3 | AUU | + | +++ | ++ | +/− | +++ | +++ | ++/+++ |
| 4 | ACG | − | ++ | − | − | ++ | ++ | − |
| 5 | UUC | − | − | − | − | − | − | − |
The GFP intensity was scored under the same guidelines used in Table 2.
Expression of selected non-AUG reporters in worms coexpressing equivalent SUI3 eIF2β mutant genes
Line . | Reporter . | wt . | G157Rnb101 . | E155Knb131 . | K205T . | L209P . | S219Y . | V223G . |
|---|---|---|---|---|---|---|---|---|
| 1 | GUG | − | +++ | ++ | +/− | +++ | +++ | +++ |
| 2 | UUG | − | ++ | ++ | − | +++ | +++ | ++ |
| 3 | AUU | + | +++ | ++ | +/− | +++ | +++ | ++/+++ |
| 4 | ACG | − | ++ | − | − | ++ | ++ | − |
| 5 | UUC | − | − | − | − | − | − | − |
Line . | Reporter . | wt . | G157Rnb101 . | E155Knb131 . | K205T . | L209P . | S219Y . | V223G . |
|---|---|---|---|---|---|---|---|---|
| 1 | GUG | − | +++ | ++ | +/− | +++ | +++ | +++ |
| 2 | UUG | − | ++ | ++ | − | +++ | +++ | ++ |
| 3 | AUU | + | +++ | ++ | +/− | +++ | +++ | ++/+++ |
| 4 | ACG | − | ++ | − | − | ++ | ++ | − |
| 5 | UUC | − | − | − | − | − | − | − |
The GFP intensity was scored under the same guidelines used in Table 2.
Expression of GFP from non-AUG start codon reporters on extrachromosomal arrays coexpressing eIF2β mutants. Each row (A–G) shows fluorescent images from an eIF2β construct with different non-AUG reporters (indicated at the top of image columns). (A) Wild-type eIF2β. (B) iftb-1(nb101)/G157R. (C) iftb-1(nb131)/E155K. (D) K205T. (E) L209P. (F) S219Y. (G) V223G. (H) Relative GFP signals determined by digital image analysis.
These results indicate that the C. elegans eIF2β containing equivalent SUI3 mutations behave similarly to the nb101 and nb131 mutations in permitting translation initiation at several non-AUG codons, unlike in yeast where non-AUG translation occurs mainly from the UUG codon. This appears to be true for all three SUI3 alleles (L209P, S219Y, and V223G) whose mutant phenotypes could be observed by the above transgenic assay. Although only limited codons were assayed, the non-AUG codons that support expression have a common feature in that they differ from the native AUG start codon by 1 base at either the first or the third base position. Similar to what is observed in yeast, these SUI3 mutations did not allow translation initiation from a reporter containing two base changes within the start site.
DISCUSSION
Using a GFP reporter with an altered AUG initiation codon, we recovered C. elegans mutants that allow translation to start at non-AUG codons. By genetic suppression experiments, we showed that these mutants could also initiate translation from a non-AUG codon for an endogenous mRNA. We have determined that two of them have mutations in the gene iftb-1, which encodes the β-subunit of eIF2. The phenotype of these mutants is similar to the yeast SUI3 mutations of eIF2β (Castilho-Valavicius et al. 1992), in that they allow translation to initiate at non-AUG codons. Our analyses with both newly identified and equivalent SUI3 mutants in C. elegans further support the crucial role of eIF2β in AUG start codon selection.
An interesting phenomenon of our study came from the expression analyses of non-AUG GFP reporters in iftb-1 and SUI3 equivalent mutants. We found that these mutants allow expression of GFP reporters containing single-base changes at either the first or the third base position within the AUG start codon of GFP mRNA. However, with the exception of the ACG codon-containing reporter, little or no GFP expression was observed in those reporters with second base position changes or simultaneous changes at two base positions. It is possible that two contiguous base-pairing interactions between these non-AUG codons and the anticodon are sufficient to allow translation initiation in these mutants. These observations suggest a model in which eIF2β might be involved in discriminating between two base pairings vs. three base pairings within the translation initiation complex. This is consistent with a previous notion that states that the base-pairing interactions, and not the identity of the bases, are recognized during start codon selection (Cigan et al. 1988; Kolitz et al. 2009).
The SUI3 mutations behave differently in C. elegans than in yeast. SUI3 mutants permit translation from a UUG codon much more efficiently than from other non-AUG codons in yeast (Huang et al. 1997). In our analyses in C. elegans, equivalent SUI3 mutations did not have a marked preference for UUG. Instead, similar to iftb-1 mutants isolated in our C. elegans screens, they permit translation initiation at any non-AUG codons that have one base change either at the first or at the third position of the start codon AUG. The difference may be due to the sequence context near the non-AUG codons, since it is known that the sequence surrounding the native AUG start codon has been shown to influence translation initiation (Kozak 1989). The non-AUG codon reporters used in the yeast studies were constructed by altering the third codon of the His4 gene (Huang et al. 1997). The sequence context flanking this codon may be different from that of the native AUG start codon and thus led to an unusual effect on translation with non-AUG codons. Alternatively, it is possible that the UUG codon might behave differently from other non-AUG codons in yeast, perhaps making special interactions with the anticodon or components of the translation initiation complex (Pestova et al. 2007).
The identification of dominant alleles of eIF2β in yeast and C. elegans provides clues to its specific role in start site AUG recognition, which would be otherwise difficult to study using standard loss-of-function mutants due to its multifaceted roles. Biochemically, eIF2β together with the α- and γ-subunits is known to form an eIF2 heterotrimer that has two distinct roles during translation initiation (Pestova et al. 2007). eIF2 is responsible for loading Met-tRNAi and then delivering it to the preinitiation complex, where it also serves as a critical factor in recognizing the AUG start codon. It appears that the primary defect of these eIF2β mutants occurs at the AUG recognition step, since they are able to support productive translation with non-AUG start codons. This is consistent with the dominant nature of these mutants. In heterozygous organisms, both the mutant and the wild-type eIF2β could deliver Met-tRNAi into separate preinitiation complexes. The mutant-containing complex would permit translation from non-AUG codons while the wild-type-containing complex would initiate translation normally. In contrast, loss-of-function of eIF2β would disrupt the initial step of Met-tRNAi delivery, precluding the analysis of its subtle role in the AUG recognition step. Indeed, yeast deletion studies (Castilho-Valavicius et al. 1992) and knockdown by RNAi in C. elegans (our personal observations) have showed that eIF2β is an essential gene, likely corresponding to a lack of eIF2 heterotrimers. Amid these analyses, it is interesting to note that the iftb-1(nb101) mutation is distinct, in that it is dominant in regard to non-AUG codon translation, but results in a much weaker phenotype in homozygous worms. One explanation for the weaker recessive phenotype is that the nb101 mutation may also cause aberrant initiations of translation from numerous host mRNAs that result in an overall reduction of protein synthesis, including the expression of GFP from non-AUG reporters.
Biochemical and structural studies provide insights into how our newly identified eIF2β mutants might function. eIF2β can be divided into three regions on the basis of its sequence features and interactions with distinct factors. Both the N-terminal Lys stretches and the C-terminal region including the C2–C2 zinc finger domain are required for mRNA binding (Laurino et al. 1999). There is evidence that the N-terminal region also interacts with eIF5 and eIF2B (Asano et al. 1999). The central region, where nb101 and nb131 mutations are located, mediates interactions with eIF2γ. The stringency of this interaction is important for the correct selection of the AUG start codon (Thompson et al. 2000;Hashimoto et al. 2002). This interaction is supported by a crystal structure of P. furiosus aIF2βγ in which residues corresponding to the nb101 and nb131 mutations are found in an interface adjacent to the regions that are in close contact with the γ-subunit (Sokabe et al. 2006). Thus, one possibility is that these two mutations may impair the ability of eIF2β to properly interact with eIF2γ. However, it is also possible that our mutations affect the GTPase activity of eIF2, as is the case for the yeast SUI3 mutations. Each of the yeast SUI3 alleles was found to have a missense mutation in one of six amino acids located in the C-terminal C2–C2 zinc finger domain (Castilho-Valavicius et al. 1992). Two of these mutations have been shown to increase the intrinsic GTPase activity of eIF2, causing premature hydrolysis of GTP and therefore initiation at non-AUG codons (Huang et al. 1997). Since the zinc finger domain is located in close proximity to the central domain (Sokabe et al. 2006; Yatime et al. 2007), it is possible that these two domains control GTPase activity together in response to start codon recognition.
In conclusion, dominant mutations in eIF2β provide unique tools to analyze the mechanism of AUG recognition that would be otherwise difficult to study due to the involvement of many essential proteins and RNAs. The phenotypes of these mutants support the involvement of eIF2β in recognizing proper base-pairing interactions between the mRNA codons and the initiator anti-codon. Our analyses are consistent with a model that eIF2β plays a role in the discrimination of near cognate codons from the true AUG initiation codon, which is an essential mechanism in start codon selection (Pestova et al. 2007).
Footnotes
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.115485/DC1.
Footnotes
Communicating editor: K. Kemphues
Acknowledgements
We gratefully acknowledge the encouragement and support of Donald Comb and New England Biolabs. We thank Tilde Carlow, Ana Egana, Brendan Galvin, Bill Jack, Liz Li, Chris Noren, Lise Raleigh, and Richard Roberts for critical reading of the manuscript and David Hill for technical assistance. We thank Bill Wood for providing the unc-62(ct344) strain. Some C. elegans strains were obtained from the Caenorhabditis Genetics Center, which is funded by the National Center for Research Resources of the National Institutes of Health.
References
Algire, M. A., D. Maag and J. R. Lorsch,
Asano, K., T. Krishnamoorthy, L. Phan, G. D. Pavitt and A. G. Hinnebusch,
Bitinaite, J., M. Rubino, K. H. Varma, I. Schildkraut, R. Vaisvila et al.,
Blumenthal, T.,
Blumenthal, T., and K. Steward,
Castilho-Valavicius, B., G. M. Thompson and T. F. Donahue,
Cheung, Y. N., D. Maag, S. F. Mitchell, C. A. Fekete, M. A. Algire et al.,
Cigan, A. M., L. Feng and T. F. Donahue,
Cigan, A. M., E. K. Pabich, L. Feng and T. F. Donahue,
Das, S., R. Ghosh and U. Maitra,
Davis, M. W., M. Hammarlund, T. Harrach, P. Hullett, S. Olsen et al.,
De Stasio, E. A., and S. Dorman,
Donahue, T. F., A. M. Cigan, E. K. Pabich and B. C. Valavicius,
Dopf, J., and T. M. Horiagon,
Hashimoto, N. N., L. S. Carnevalli and B. A. Castilho,
Hinnebusch, A. G., T. E. Dever and K. Asano,
Huang, H. K., H. Yoon, E. M. Hannig and T. F. Donahue,
Kolitz, S. E., J. E. Takacs and J. R. Lorsch,
Kozak, M.,
Krause, M., and D. Hirsh,
Laurino, J. P., G. M. Thompson, E. Pacheco and B. A. Castilho,
Li, X., G. Zhang, N. Ngo, X. Zhao, S. R. Kain et al.,
Paulin, F. E., L. E. Campbell, K. O'Brien, J. Loughlin and C. G. Proud,
Pestova, T. V., J. R. Lorsch and C. U. T. Hellen,
Rhoads, R. E., T. D. Dinkova and N. L. Korneeva,
Sokabe, M., M. Yao, N. Sakai, S. Toya and I. Tanaka,
Thompson, G. M., E. Pacheco, E. O. Melo and B. A. Castilho,
Van Auken, K., D. Weaver, B. Robertson, M. Sundaram, T. Saldi et al.,
Waters, J. C.,
Willis, J. H., E. Munro, R. Lyczak and B. Bowerman,
Wood, W. B.,
Yatime, L., Y. Mechulam, S. Blanquet and E. Schmitt,
Yoon, H. J., and T. F. Donahue,