-
PDF
- Split View
-
Views
-
Cite
Cite
Richard D McCulloch, Mark D Baker, Analysis of One-Sided Marker Segregation Patterns Resulting From Mammalian Gene Targeting, Genetics, Volume 172, Issue 3, 1 March 2006, Pages 1767–1781, https://doi.org/10.1534/genetics.105.051680
Close - Share Icon Share
Abstract
The double-strand break repair (DSBR) model is currently accepted as the paradigm for acts of double-strand break (DSB) repair that lead to crossing over between homologous sequences. The DSBR model predicts that asymmetric heteroduplex DNA (hDNA) will form on both sides of the DSB (two-sided events; 5:3/5:3 segregation). In contrast, in yeast and mammalian cells, a considerable fraction of recombinants are one sided: they display full conversion (6:2 segregation) or half-conversion (5:3 segregation) on one side of the DSB together with normal 4:4 segregation on the other side of the DSB. Two mechanisms have been proposed to account for these observations: (i) hDNA formation is restricted to one side of the DSB or the other, and (ii) recombination is initially two sided, but hDNA repair directed by Holliday junction cuts restores normal 4:4 segregation on that side of the DSB in which the mismatch is closest to the cut junction initiating repair. In this study, we exploited a well-characterized gene-targeting assay to test the predictions that these mechanisms make with respect to the frequency of recombinants displaying 4:4 marker segregation on one side of the DSB. Unexpectedly, the results do not support the predictions of either mechanism. We propose a derivation of mechanism (ii) in which the nicks arising from Holliday junction cleavage are not equivalent with respect to directing repair of adjacent hDNA, possibly as a result of asynchronous cleavage of the DSBR intermediate.
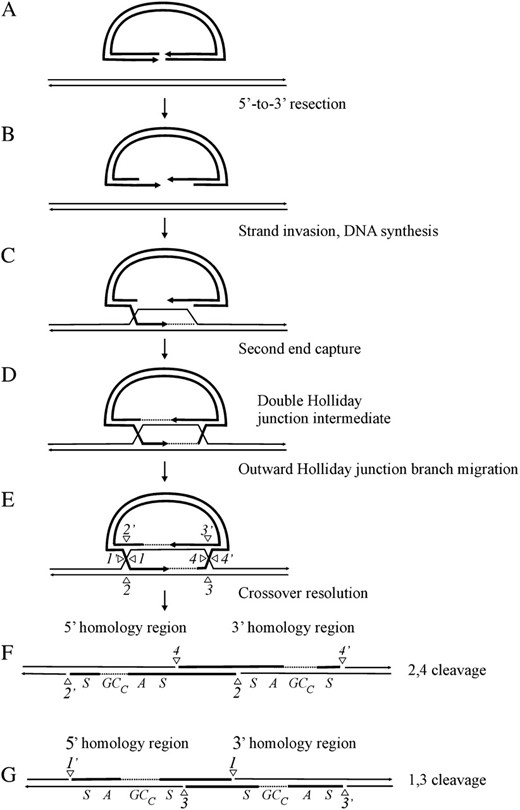
THE double-strand break repair (DSBR) model (Orr-Weaver et al. 1981; Szostak et al. 1983), as revised by Sun et al. (1991), is currently accepted as the paradigm for acts of double-strand break (DSB) repair that lead to crossing over between homologous sequences. The DSBR model explains homologous recombination between a linearized gene-targeting vector and the chromosome (Figure 1). The events involve resection on the two sides of the DSB, invasion by one 3′-end, which primes DNA synthesis leading to the capture of the second 3′-end, and eventually, formation of the double Holliday junction intermediate. If the recombining sequences differ, the initial events of strand invasion and annealing generate asymmetric heteroduplex DNA (hDNA) tracts on opposite strands on the two sides of the DSB. Outward branch migration of the double Holliday junction intermediate will result in asymmetric hDNA being flanked by symmetric hDNA on one or both sides of the DSB. Alternate sense cleavage of the double Holliday junction intermediate in either the 2,4 or 1,3 mode generates the crossover products in Figure 1, F and G, respectively, with the regions undergoing recombination being preserved as 5′ and 3′ homologous repeats on the chromosome. Nicks at positions 2,2′ and 4,4′ in the crossover product in Figure 1F and those at positions 1,1′ and 3,3′ in the crossover product in Figure 1G correspond to the DNA cuts required to resolve Holliday junctions previously located to the left and right of the DSB, respectively. In the crossover products, the positions of gene conversion tracts toward the chromosomal sequence (GCc), asymmetric hDNA (A), and symmetric hDNA (S) in the 5′ and 3′ homology regions are indicated. The crossover products in Figure 1, F and G, differ with respect to the arrangement of asymmetric hDNA (A) and gene conversion (GCc) tracts about the DSB in the two homology regions. Although the canonical DSBR model depicts formation of both crossover products, a bias yielding products predominantly of the 2,4 resolution type (Figure 1F) is observed (Foss et al. 1999; Baker and Birmingham 2001; Merker et al. 2003; Jessop et al. 2005). To account for the bias in recovery of type 2,4 resolution products, Foss et al. (1999) propose that each Holliday junction bears a structural asymmetry resulting from new DNA, or its synthesis, that biases the pair of like strands that are cleaved at each junction: with respect to Figure 1, crossed strands in the Holliday junction to the right of the DSB and noncrossed strands in the Holliday junction to the left of the DSB.
The DSBR model for homologous recombination between a linearized gene-targeting vector and the chromosome. (A) Following DSB formation, (B) 5′-to-3′ resection on the two sides of the DSB leaves long 3′ overhangs. (C) Invasion by one 3′-end primes DNA synthesis leading to D-loop formation, (D) capture of the second 3′-end, and formation of the double Holliday junction intermediate. If the recombining sequences differ, strand invasion generates asymmetric hDNA on opposite strands on the two sides of the DSB. (E) Outward branch migration of the double Holliday junction intermediate will result in asymmetric hDNA being flanked by symmetric hDNA on one or both sides of the DSB. Alternate sense cleavage of the double Holliday junction intermediate in either the 2,4 or 1,3 mode generates the crossover products in F and G, respectively, with the regions undergoing recombination being preserved as 5′ and 3′ homologous repeats on the same chromosome. In the crossover products in F and G, the positions of gene conversion tracts toward the chromosomal sequence (GCc), asymmetric hDNA (A), and symmetric hDNA (S) in the 5′ and 3′ homology regions are indicated. Also, nicks at positions 2,2′ and 4,4′ in the crossover product in F, and those at positions 1,1′ and 3,3′ in the crossover product in G, correspond to the DNA cuts required to resolve Holliday junctions previously located to the left and right of the DSB, respectively.
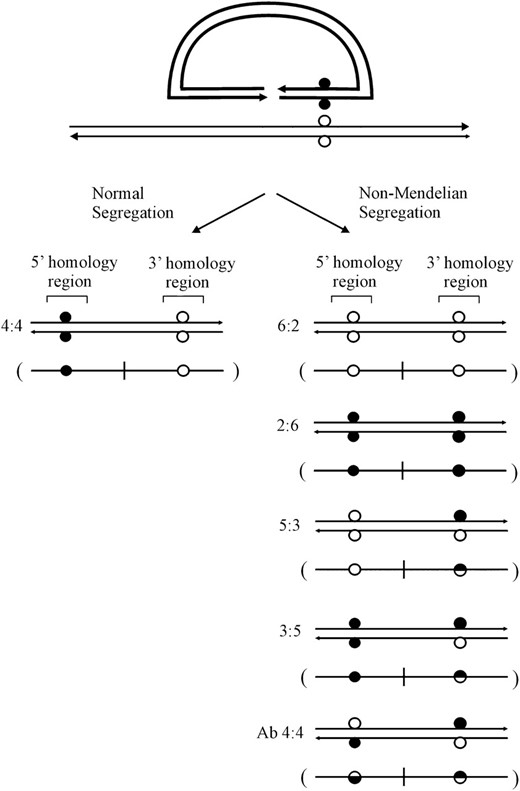
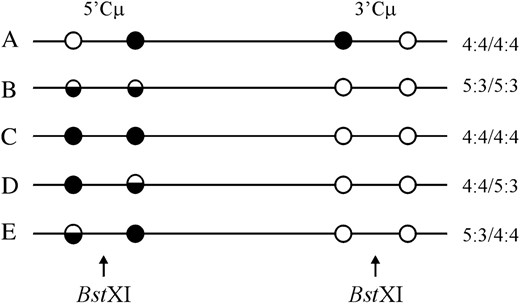
Heteroduplex DNA generated during DSBR may be unrepaired or repaired in favor of sequences in one of the recombining strands. Consequently, different segregation classes are possible in crosses that involve markers flanking a DSB. To reveal heteroduplexes, genetic crosses often utilize small palindromes as markers, since they are semirefractory to repair when in heteroduplex with the wild-type sequence (Nag et al. 1989; Bollag et al. 1992; Donoho et al. 1998; Li and Baker 2000). In describing marker segregation patterns that arise during gene targeting, we have found it convenient (Birmingham et al. 2004) to adopt nomenclature normally used to categorize meiotic recombination events in yeast and fungi (Petes et al. 1991). Figure 2 illustrates possible segregation patterns arising by gene targeting in the case where a small palindrome genetic marker (solid circle) positioned arbitrarily to the right of the vector-borne DSB replaces an endogenous restriction enzyme site (open circle) in the corresponding chromosomal position. For simplicity, in depicting marker patterns, the two DNA strands found in the recombination product are condensed to one illustrative strand (as shown in parentheses). One recombinant type (normal 4:4 segregation) contains a vector-borne marker in one homology region and the chromosomal marker in the other. Any pattern that differs from normal 4:4 segregation is referred to as non-Mendelian segregation (NMS). One class of NMS is full conversion (FC). FC events are denoted 6:2 or 2:6, depending on whether chromosomal or vector-borne markers, respectively, reside in both 5′ and 3′ homology regions. Another form of NMS is half-conversion (HC). HC events denote 5:3 or 3:5 classes in which one allelic position is sectored, while the other contains either the chromosomal or vector-borne marker, respectively. In meiotic recombination, HC is also referred to as postmeiotic segregation (Petes et al. 1991). Another relevant category of NMS is aberrant 4:4 segregation (Ab4:4) in which repair fails for both heteroduplexes generating recombinants sectored at both allelic positions. For HC and Ab4:4 segregation, only one allelic pattern is presented. Although not illustrated, different combinations of the above marker patterns are possible in the two homology regions if a mismatch involving the small palindrome also resides to the left of the DSB.
Marker segregation patterns. The diagram depicts possible segregation patterns that arise in recombinants generated by crossover gene targeting in the case where the genetic marker is positioned arbitrarily to the right of the vector-borne DSB. Open circles represent the chromosomal marker, while solid circles represent the vector marker. Half-solid circles represent sectored sites. For simplicity, the two DNA strands found in the crossover product are condensed to one illustrative strand (as shown in parentheses). A full description of the marker segregation patterns is presented in the text.
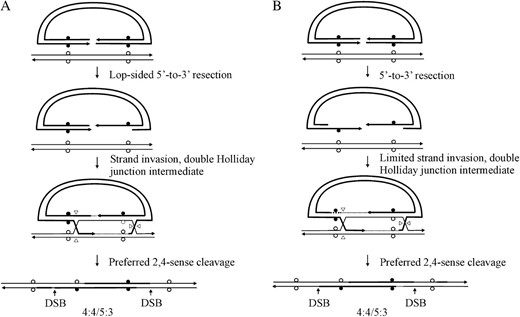
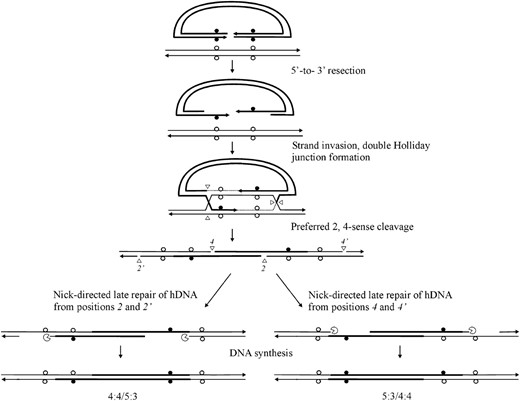
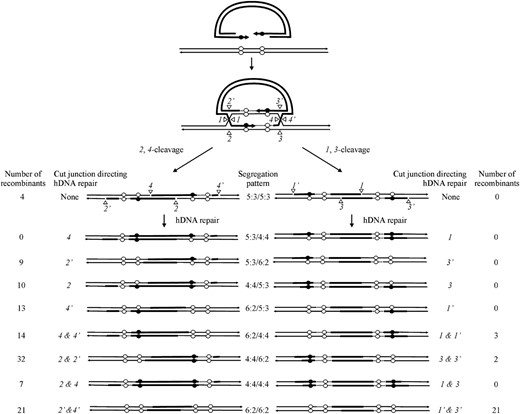
For crosses involving palindrome markers flanking the DSB, the initial strand invasion and annealing events depicted in Figure 1 predict asymmetric hDNA on the two sides of the DSB (5:3/5:3 segregation; also referred to as two-sided HC events). However, this expectation is not fulfilled in studies of meiotic recombination in yeast (Porter et al. 1993; Gilbertson and Stahl 1996; Foss et al. 1999; Merker et al. 2003; Jessop et al. 2005) or mammalian gene targeting (Baker and Birmingham 2001; Birmingham et al. 2004). Instead, a considerable fraction of recombination events are one sided; that is, an FC (6:2) or HC (5:3) event on one side of the DSB is usually found in conjunction with normal 4:4 segregation on the other side of the DSB. Two DSBR mechanisms have been proposed to account for the discrepancy in expected (two-sided) and observed (one-sided) results, and these are described below in the context of a gene-targeting reaction. The first mechanism proposes that one-sided events reflect a recombination process that limits hDNA formation to <300 bp on one side of the DSB, through either lop-sided DSB resection (Porter et al. 1993) (Figure 3A) or a process that limits strand invasion or DNA synthesis on one side of the DSB (Merker et al. 2003; Jessop et al. 2005) (Figure 3B). Thus, if flanking palindrome markers are >300 bp from the DSB, this first mechanism would be expected to yield a similar frequency of normal 4:4 marker segregation on the two sides of the DSB. The second mechanism proposes that recombination events are initially two sided (5:3/5:3 segregation), but hDNA repair on one side of the DSB frequently restores the parental 4:4 marker segregation (Foss et al. 1999) (Figure 4). Restorational repair is directed by nicks that arise from cleavage of the double Holliday junction intermediate. In Figure 4, preferred sense cleavage of the Holliday junction to the left and right of the DSB generates nicks at positions 2,2′ and 4,4′, respectively, which direct repair of adjacent mismatches in hDNA (nick-directed late repair of hDNA). An implication of the restorational repair mechanism of Foss et al. (1999) is a competition between the two cut Holliday junctions for repair of an adjacent mismatch in hDNA: the closer the mismatch is to a cut junction, the greater is the likelihood that its repair will be directed by that junction. Therefore, this mechanism is expected to favor normal 4:4 segregation on the side of the DSB in which the flanking mismatch is closest to the cut Holliday junction.
Mechanisms limiting hDNA formation on one side of the DSB. (A) Lop-sided DSB resection (adapted from Porter et al. 1993, Figure 7) and (B) limited strand invasion (adapted from Jessop et al. 2005, Figure 6). The diagrams illustrate normal 4:4 segregation positioned arbitrarily to the left of the DSB.
Restorational repair of hDNA. The DSBR model (Figure 1) generates hDNA on both sides of the DSB (5:3/5:3 segregation). Preferred sense cleavage (2,4-mode) generates nicks in the crossover intermediate at positions 2,2′ and 4,4. Nick-directed repair of hDNA can restore normal 4:4 marker segregation pattern on one or the other side of the DSB (adapted from Foss et al. 1999, Figure 5). The proximity of the mismatch to a cut junction increases the likelihood that its repair will be directed by that junction.
In this study, we tested the predictions that these mechanisms make with respect to the frequency of recombinants displaying 4:4 marker segregation on one side of the DSB. Our strategy exploits a well-characterized gene-targeting assay, which examines in vivo homologous recombination between a linearized plasmid vector and a mammalian chromosome (Baker et al. 1988; Ng and Baker 1998). The study makes use of four gene-targeting vectors, each bearing a common DSB site. In the vectors, identical small palindrome genetic markers replace endogenous restriction enzyme sites located 598 and 439 bp to the left and right of the DSB, respectively. The vectors differ with respect to the amount of homology flanking the DSB, although in each case the homology is well above the minimum of 1 kb that is required for efficient gene targeting (Shulman et al. 1990; Hasty et al. 1991). In two vectors, homology on each side of the DSB is similar, but one vector has more total homology than the other. In a third vector, the amount of homology to the left of the DSB is significantly less than that on the right, while in the last vector, the opposite is true. Limited hDNA formation mechanisms (Porter et al. 1993; Merker et al. 2003; Jessop et al. 2005) predict that the frequency of recombinants displaying normal 4:4 marker segregation on one or the other side of the DSB should be similar with each vector. In contrast, the restorational repair mechanism of Foss et al. (1999) predicts a bias in normal 4:4 segregation to the left and right of the DSB in recombinants generated with gene-targeting vectors where homology is restricted in that way, while a similar frequency of normal 4:4 segregation on one or the other side of the DSB is expected in recombinants generated with the other two vectors. Unexpectedly, the results did not match predictions of either mechanism. Instead, we observed a strong bias favoring normal 4:4 segregation to the left of the DSB in recombinants generated with the two gene-targeting vectors with limited homology to the left of the DSB, while recombinants generated with the other two gene-targeting vectors show no bias at all. These results suggest that, like the model presented by Foss et al. (1999), nicks generated by Holliday junction cleavage direct repair of adjacent hDNA, but contrary to the restorational repair model, the nicks are not equivalent in directing hDNA repair. Instead, nicks 2 and/or 2′ generated by cleavage of the Holliday junction to the left of the DSB are more often responsible for directing hDNA repair than are nicks 4 and 4′ generated by cleavage of the Holliday junction to the right of the DSB. As an explanation for the bias in normal 4:4 segregation, we propose a mechanism of recombination that involves asynchronous cleavage of the double Holliday junction intermediate.
MATERIALS AND METHODS
Cell lines and plasmids:
The Sp6/HL mouse hybridoma cell line was used as the recipient in the gene-targeting studies. As described previously (Köhler et al. 1982; Baker et al. 1988), Sp6/HL bears a single copy of the trinitrophenyl (TNP)-specific chromosomal immunoglobulin μ heavy chain gene, which includes the μ-gene constant (Cμ) region that serves as the target for homologous recombination with transfected vector DNA. The origin of this cell line, along with the conditions used for cell culture, has been described previously (Köhler and Shulman 1980; Köhler et al. 1982).
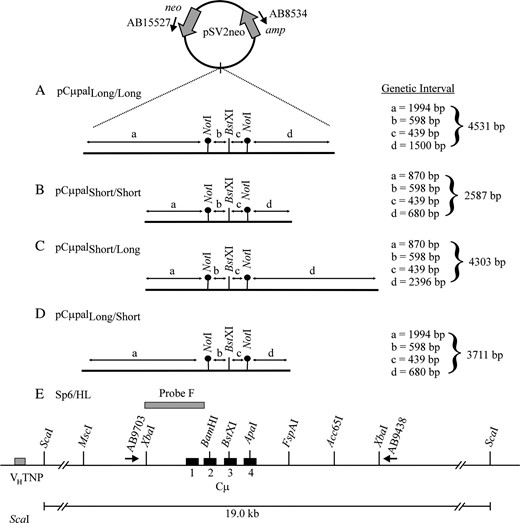
Four gene-targeting vectors were constructed (Figure 5, A–D). Each vector is a derivative of pSV2neo (Southern and Berg 1982) from which the 372-bp NsiI/NdeI fragment spanning the SV40 early region enhancer was deleted (Ng and Baker 1999a). As described previously (Bautista and Shulman 1993; Ng and Baker 1998), enhancer-trap vectors significantly enrich for gene-targeting events because cis-acting sequences in the chromosomal μ-locus permit neo gene expression. The Cμ region of homology was inserted into a SalI site that replaced the unique EcoRI site in pSV2neo. The vector-borne Cμ region was engineered so that unique BamHI and ApaI sites were replaced with palindrome insertions as described (Baker and Birmingham 2001). The palindrome contains a novel NotI site (indicated by boldface type) (5′-GTACTGTATGTGCGGCCGCACATACAGTAC-3′) to facilitate discrimination between sequences of vector and chromosomal origin (Baker and Birmingham 2001). In addition to the common palindrome insertions, each vector contains a unique BstXI site for creating the vector-borne DSB. Following vector linearization at BstXI, the NotI palindrome insertions reside 598 and 439 bp to the left and right of the DSB, respectively. The enhancer-trap gene-targeting vectors differ solely in the amount of homology that they share with the Sp6/HL chromosomal Cμ region (Figure 5E) and are designated according to how much homology resides on the two sides of the DSB: pCμpalLong/Long contains a 4531-bp MscI/Acc65I fragment (Figure 5A), pCμpalShort/Short contains a 2587-bp XbaI/FspAI fragment (Figure 5B), pCμpalShort/Long contains a 4303-bp XbaI/XbaI fragment (Figure 5C), and pCμpalLong/Short contains a 3711-bp MscI/FspAI fragment (Figure 5D). Plasmid propagation, purification, and manipulation were performed as described (Sambrook et al. 1989; Baker and Birmingham 2001).
Gene-targeting vectors and chromosomal target locus. The structures of the four enhancer-trap gene-targeting vectors (A–D) and recipient Sp6/HL chromosomal μ-gene (E) are illustrated. Each vector is an SV40 enhancer-deleted derivative of pSV2neo. A segment homologous to the immunoglobulin Cμ region was cloned into a SalI site that replaces the unique EcoRI site in pSV2neo. The gene-targeting vectors (A–D) contain the same BstXI site for introducing the vector-borne DSB. Unique NotI palindrome markers replace endogenous BamHI and ApaI sites located 598 and 439 bp to the left and right of the BstXI site, denoted by genetic intervals b and c, respectively. The vectors differ in the amount of DNA between the palindromes and the border of shared homology to the chromosomal μ-locus (as indicated in genetic intervals a and d). Restriction enzyme sites that define the homology borders in the various gene-targeting vectors are indicated in the recipient Sp6/HL chromosomal μ-gene, as are the VHTNP and Cμ region exons. The relative positions of primers AB9703 and AB9438 specific to the chromosomal μ-gene and primers AB15527 and AB8534 specific for the pSV2neo vector backbone are shown. The position of the 870-bp XbaI/BamHI probe F fragment used in Southern analysis of the endogenous 19.0-kb ScaI chromosomal μ-gene fragment is indicated.
Recombinant isolation and characterization:
BstXI-linearized gene-targeting vector was electroporated into Sp6/HL hybridoma cells as described previously (Baker et al. 1988). The enhancer-trap nature of the targeting vectors permitted independent G418R transformants to be isolated by limited dilution cloning as detailed elsewhere (Ng and Baker 1999a; Baker and Birmingham 2001). Genomic DNA was prepared from G418R transformants by SDS–proteinase K digestion (Gross-Bellard et al. 1973).
The G418R transformants were screened to cull cell lines in which the chromosomal μ-locus was unmodified by targeted vector insertion. This was accomplished by PCR amplification using μ-gene-specific primer pairs that reside outside the vector-borne Cμ region of homology (not illustrated): for G418R transformants generated with pCμpalLong/Long, the forward primer AB9931 (5′-GCAAGAGTGAGTAGAGCTGGCTGG-3′) and reverse primer AB17390 (5′-GGTTCGGTTCTGTCTGCACTACTC-3′) were used to detect a 7483-bp endogenous μ-gene product; for G418R transformants generated with pCμpalShort/Short, the forward primer AB9703 (5′-CTACTTGAGAAGCCAGGATCTAGG-3′) and reverse primer AB13713 (5′-CTGTGAGTGGTTGTGTGTCTATGC-3′) were used to detect a 2746-bp endogenous μ-gene product; for G418R transformants generated with pCμpalLong/Short, the forward primer AB9931 and reverse primer AB9438 (5′-GTACCATCAGACTGCACTGTTCCA-3′) were used to generate a 5682-bp endogenous μ-gene product; and for G418R transformants generated with pCμpalShort/Long, the forward primer AB9703 and reverse primer AB9438 were used to generate a 4622-bp endogenous μ-gene product. Cell lines in which an endogenous μ-gene PCR product was not observed were saved as putative examples of targeted G418R recombinants. These cell lines were examined by Southern analysis (Sambrook et al. 1989) to identify the chromosomal Cμ region duplication that characterizes targeted recombinants generated by vector insertion.
To determine Cμ region marker segregation patterns, the 5′ and 3′ Cμ regions in each recombinant were amplified with primer pairs AB9703/AB8534 (5′-CTTACCGCTGTTGAGATCCAGT-3′) and AB15527 (5′-CCTTGTGGTCAGTGTTCATCTGCT-3′)/AB9438, respectively, and the PCR products were tested separately for their sensitivity or resistance to cleavage with NotI (diagnostic for the vector-borne palindrome marker), BamHI, and ApaI (both diagnostic of the endogenous chromosomal markers). PCR amplification was performed using the Roche long template PCR kit (Roche Diagnostics, Laval, Quebec). Restriction enzymes were purchased from New England Biolabs (Mississauga, Ontario) and used according to the manufacturer's specifications.
RESULTS
Recombinant isolation:
In this study, we tested predictions of DSBR mechanisms involving (i) limited hDNA formation (Porter et al. 1993; Merker et al. 2003; Jessop et al. 2005) and (ii) restorational repair of hDNA (Foss et al. 1999) with regard to the frequency of recombinants bearing normal 4:4 marker segregation on one side of the DSB (one-sided recombinants). The experimental system exploits a well-characterized gene-targeting assay, which examines in vivo homologous recombination between a linearized, enhancer-trap plasmid vector and the single-copy chromosomal immunoglobulin (Ig) μ-locus in mouse hybridoma cells (Baker et al. 1988; Ng and Baker 1998).
Four enhancer-trap gene-targeting vectors were constructed (Figure 5, A–D). Each vector is a pSV2neo derivative devoid of the SV40 early region enhancer facilitating neo gene expression (Southern and Berg 1982) and containing a fragment of homology from the Sp6/HL hybridoma chromosomal Ig μ-gene constant (Cμ) region (Figure 5E). In each vector, the Cμ region of homology contains two NotI palindrome markers that replace endogenous BamHI and ApaI sites located 598 and 439 bp, respectively, from the unique BstXI site of vector linearization. Since small palindromes are semirefractory to mismatch repair in yeast (Nag et al. 1989) and mammalian cells (Bollag et al. 1992; Donoho et al. 1998; Li and Baker 2000), their segregation patterns provide information about hDNA formation in recombination intermediates. The vectors differ in the amount of homology that they share with the chromosomal Ig Cμ region, although in each it is well above the ∼1-kb minimum required to effect mammalian gene targeting (Shulman et al. 1990; Hasty et al. 1991). The vector pCμpalLong/Long contains 4531 bp of total homology distributed approximately evenly on the two sides of the DSB (Figure 5A). This is also true of pCμpalShort/Short, although, in comparison, the total amount of Cμ region homology is lower (2587 bp) (Figure 5B). The vectors pCμpalShort/Long and pCμpalLong/Short contain 4303 and 3711 bp of Cμ region homology, respectively, but to the left of the DSB in pCμpalShort/Long, and to the right of the DSB in pCμpalLong/Short, there is significantly less homology than on the other side (Figure 5, C and D).
The BstXI-linearized vectors were electroporated separately into Sp6/HL mouse hybridoma cells as described (Baker and Birmingham 2001), and independent G418R transformants originating from single cells were recovered by limited dilution cloning procedures (Ng and Baker 1999a). In total, 24 electroporations yielded 3372 independent G418R transformants, which were saved for analysis (Table 1). PCR screening using μ-gene-specific primers was used to cull nontargeted transformants bearing an unmodified chromosomal Cμ region (data not shown) (for primer details, refer to materials and methods). The remaining cell lines were screened by Southern analysis of ScaI-digested genomic DNA using Cμ-specific probe F (data not shown) to verify loss of the endogenous 19.0-kb ScaI Cμ region fragment (Figure 5E) and gain of the specific 5′ and 3′ ScaI Cμ region fragments that characterize the targeted chromosomal μ-locus in the recombinants (Figure 6). In all, these procedures identified 172 targeted G418R recombinants bearing the Cμ region duplication (Table 1). The gene-targeting frequencies are in accord with previous studies in which vectors of similar homology were used (Baker et al. 1988; Birmingham et al. 2004). The remaining 3200 transformants were divided among random transformants (3169 cell lines), targeted recombinants in which two copies of the vector were integrated in the chromosomal μ-locus (9 cell lines), and cell lines in which the endogenous μ-locus bore an abnormal structure (22 cell lines), perhaps as a result of illegitimate vector integration. All of these transformant types have been observed previously in other studies (Baker and Read 1993; Ng and Baker 1999a,b).
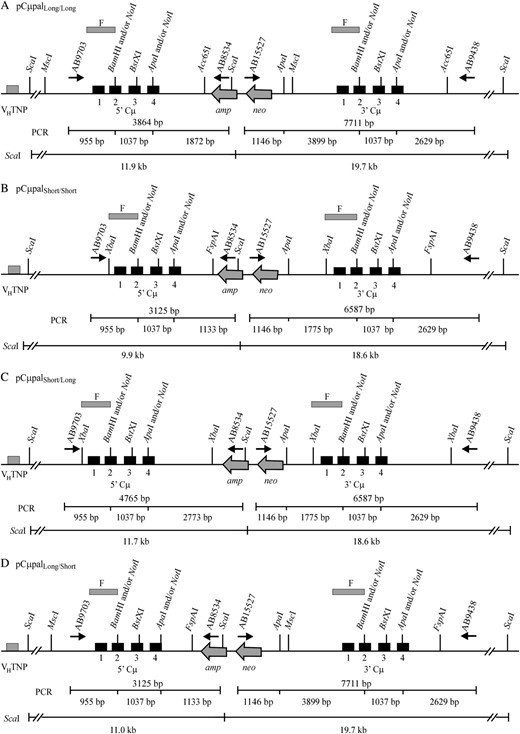
Recombinant μ-gene structures. (A–D) The 5′ and 3′ Cμ region duplications that result from homologous recombination between the recipient Sp6/HL chromosomal μ-gene and the four enhancer-trap gene-targeting vectors. PCR primers AB9703/AB8534 were used to amplify the recombinant 5′ Cμ region, while primer pair AB15527/AB9438 was used to amplify the recombinant 3′ Cμ region. The position of each PCR product is denoted by a horizontal line below the chromosomal μ-gene structure. The total size of each PCR product is above each line, while a restriction map for the enzymes NotI, BamHI, and ApaI is below each line. Also illustrated are the fragment sizes expected in Southern analysis of the various recombinant μ-genes following digestion of genomic DNA with ScaI and hybridization with probe F (Figure 5E).
Gene targeting efficiencies of different gene targeting vectors
Targeting vector . | Electroporations (2 × 107 cells) . | Transformants screened . | Targeted lines . | Relative targeting frequencya (%) . | Absolute targeting frequencyb . |
|---|---|---|---|---|---|
| pCμpalLong/Long | 6 | 659 | 47 | 7.1 | 3.9 × 10−7 |
| pCμpalShort/Short | 6 | 1072 | 41 | 3.8 | 3.4 × 10−7 |
| pCμpalLong/Short | 8 | 1112 | 50 | 4.5 | 3.1 × 10−7 |
| pCμpalShort/Long | 4 | 529 | 34 | 6.4 | 4.2 × 10−7 |
| Totals | 24 | 3372 | 172 |
Targeting vector . | Electroporations (2 × 107 cells) . | Transformants screened . | Targeted lines . | Relative targeting frequencya (%) . | Absolute targeting frequencyb . |
|---|---|---|---|---|---|
| pCμpalLong/Long | 6 | 659 | 47 | 7.1 | 3.9 × 10−7 |
| pCμpalShort/Short | 6 | 1072 | 41 | 3.8 | 3.4 × 10−7 |
| pCμpalLong/Short | 8 | 1112 | 50 | 4.5 | 3.1 × 10−7 |
| pCμpalShort/Long | 4 | 529 | 34 | 6.4 | 4.2 × 10−7 |
| Totals | 24 | 3372 | 172 |
Number of targeted lines/number of transformants screened.
Number of targeted lines/total number of cells electroporated.
Gene targeting efficiencies of different gene targeting vectors
Targeting vector . | Electroporations (2 × 107 cells) . | Transformants screened . | Targeted lines . | Relative targeting frequencya (%) . | Absolute targeting frequencyb . |
|---|---|---|---|---|---|
| pCμpalLong/Long | 6 | 659 | 47 | 7.1 | 3.9 × 10−7 |
| pCμpalShort/Short | 6 | 1072 | 41 | 3.8 | 3.4 × 10−7 |
| pCμpalLong/Short | 8 | 1112 | 50 | 4.5 | 3.1 × 10−7 |
| pCμpalShort/Long | 4 | 529 | 34 | 6.4 | 4.2 × 10−7 |
| Totals | 24 | 3372 | 172 |
Targeting vector . | Electroporations (2 × 107 cells) . | Transformants screened . | Targeted lines . | Relative targeting frequencya (%) . | Absolute targeting frequencyb . |
|---|---|---|---|---|---|
| pCμpalLong/Long | 6 | 659 | 47 | 7.1 | 3.9 × 10−7 |
| pCμpalShort/Short | 6 | 1072 | 41 | 3.8 | 3.4 × 10−7 |
| pCμpalLong/Short | 8 | 1112 | 50 | 4.5 | 3.1 × 10−7 |
| pCμpalShort/Long | 4 | 529 | 34 | 6.4 | 4.2 × 10−7 |
| Totals | 24 | 3372 | 172 |
Number of targeted lines/number of transformants screened.
Number of targeted lines/total number of cells electroporated.
Assignment of Cμ region genetic markers:
The 5′ and 3′ Cμ region marker patterns in the 172 targeted recombinants were determined according to PCR and gel analysis methods described previously (Ng and Baker 1999a; Baker and Birmingham 2001). As indicated in Figure 5, primers AB15527 and AB8534 are specific for the pSV2neo backbone of each gene-targeting vector, while primers AB9703 and AB9438 are specific for the chromosomal μ-gene. Thus, the crossing-over event that links vector-borne and chromosomal μ-gene sequences permits the 5′ and 3′ Cμ regions in each recombinant to be amplified with the primer pairs AB9703/AB8534 and AB15527/AB9438, respectively (Figure 6). The PCR products differ in size for recombinants generated with each gene-targeting vector (5′ Cμ region: 3125 bp for pCμpalLong/Short and pCμpalShort/Short, 3864 bp for pCμpalLong/Long, and 4765 bp for pCμpalShort/Long; 3′ Cμ region: 6587 bp for pCμpalShort/Short and pCμpalShort/Long and 7711 bp for pCμpalLong/Long and pCμpalLong/Short). The marker positions in the 5′ and 3′ Cμ region PCR products were tested separately for their sensitivity or resistance to digestion with NotI (diagnostic of the vector-borne palindrome), BamHI, and ApaI (both diagnostic of the chromosomal Cμ region markers). The resulting fragments were resolved by standard gel electrophoresis and markers were assigned to individual Cμ region positions by comparing the observed fragment sizes with those predicted from the 5′ and 3′ Cμ region restriction maps (Figure 6).
Classification of recombinants:
As in previous experiments where the gene-targeting vectors contained markers flanking the DSB site (Birmingham et al. 2004), multiple classes of recombinants were identified in this study. A full listing of marker assignations for recombinants generated with each gene-targeting vector is presented as supplemental information at http://www.genetics.org/supplemental/. In categorizing the marker segregation patterns, we have adopted nomenclature normally reserved for describing meiotic recombination in eight-spored fungi (Petes et al. 1991). As in Figure 2, the supplemental information at http://www.genetics.org/supplemental/ incorporates a condensed single-stranded format for genetic markers in the two-stranded recombination product. The ordering of marker segregation classes/categories in the supplemental information at http://www.genetics.org/supplemental/ is preserved in the summary of this information in Table 2
Marker segregation patterns
Marker segregation . | . | . | . | . | |
|---|---|---|---|---|---|
| Class no. . | Type . | pCμpalLong/Long . | pCμpalShort/Short . | pCμpalLong/Short . | pCμpalShort/Long . |
| 1 | 5:3/5:3 | 1 | 1 | 1 | 1 |
| 2 | 4:4/5:3 | 4 | 3 | 2 | 1 |
| 3 | 4:4/6:2 | 6 | 10 | 9 | 9 |
| 4 | 4:4/3:5 | 1 | 2 | 0 | 2 |
| 5 | 4:4/2:6 | 0 | 2 | 0 | 0 |
| 6 | 4:4/Ab4:4 | 1 | 0 | 0 | 1 |
| 7 | 6:2/4:4 | 7 | 2 | 6 | 2 |
| 8 | 3:5/4:4 | 0 | 0 | 1 | 0 |
| 9 | 2:6/4:4 | 2 | 2 | 1 | 0 |
| 10 | 6:2/5:3 | 4 | 3 | 3 | 3 |
| 11 | 5:3/6:2 | 1 | 2 | 4 | 2 |
| 12 | 6:2/6:2 | 7 | 3 | 10 | 1 |
| 13 | 4:4/4:4 | 1 | 2 | 4 | 0 |
| 14 | 3:5/6:2 | 2 | 0 | 0 | 0 |
| 15 | 6:2/3:5 | 1 | 0 | 0 | 0 |
| 16 | 2:6/6:2 | 0 | 0 | 1 | 1 |
| 17 | 5:3/2:6 | 0 | 0 | 0 | 1 |
| 18 | 2:6/2:6 | 0 | 1 | 0 | 0 |
| 19 | 2:6/5:3 | 0 | 1 | 0 | 0 |
| 20 | 5:3/Ab4:4 | 1 | 0 | 1 | 1 |
| 21 | 3:5/Ab4:4 | 0 | 1 | 0 | 0 |
| 22 | 2:6/Ab4:4 | 1 | 0 | 0 | 0 |
| 23 | Othera | ||||
| a | 5:3/5:3 | 1 | 0 | 0 | 2 |
| b | 4:4/4:4 | 1 | 4 | 5 | 2 |
| c | 4:4/5:3 | 3 | 2 | 1 | 5 |
| d | 5:3/4:4 | 1 | 0 | 0 | 0 |
| e | No marker | 1 | 0 | 1 | 0 |
| 47 | 41 | 50 | 34 | ||
Marker segregation . | . | . | . | . | |
|---|---|---|---|---|---|
| Class no. . | Type . | pCμpalLong/Long . | pCμpalShort/Short . | pCμpalLong/Short . | pCμpalShort/Long . |
| 1 | 5:3/5:3 | 1 | 1 | 1 | 1 |
| 2 | 4:4/5:3 | 4 | 3 | 2 | 1 |
| 3 | 4:4/6:2 | 6 | 10 | 9 | 9 |
| 4 | 4:4/3:5 | 1 | 2 | 0 | 2 |
| 5 | 4:4/2:6 | 0 | 2 | 0 | 0 |
| 6 | 4:4/Ab4:4 | 1 | 0 | 0 | 1 |
| 7 | 6:2/4:4 | 7 | 2 | 6 | 2 |
| 8 | 3:5/4:4 | 0 | 0 | 1 | 0 |
| 9 | 2:6/4:4 | 2 | 2 | 1 | 0 |
| 10 | 6:2/5:3 | 4 | 3 | 3 | 3 |
| 11 | 5:3/6:2 | 1 | 2 | 4 | 2 |
| 12 | 6:2/6:2 | 7 | 3 | 10 | 1 |
| 13 | 4:4/4:4 | 1 | 2 | 4 | 0 |
| 14 | 3:5/6:2 | 2 | 0 | 0 | 0 |
| 15 | 6:2/3:5 | 1 | 0 | 0 | 0 |
| 16 | 2:6/6:2 | 0 | 0 | 1 | 1 |
| 17 | 5:3/2:6 | 0 | 0 | 0 | 1 |
| 18 | 2:6/2:6 | 0 | 1 | 0 | 0 |
| 19 | 2:6/5:3 | 0 | 1 | 0 | 0 |
| 20 | 5:3/Ab4:4 | 1 | 0 | 1 | 1 |
| 21 | 3:5/Ab4:4 | 0 | 1 | 0 | 0 |
| 22 | 2:6/Ab4:4 | 1 | 0 | 0 | 0 |
| 23 | Othera | ||||
| a | 5:3/5:3 | 1 | 0 | 0 | 2 |
| b | 4:4/4:4 | 1 | 4 | 5 | 2 |
| c | 4:4/5:3 | 3 | 2 | 1 | 5 |
| d | 5:3/4:4 | 1 | 0 | 0 | 0 |
| e | No marker | 1 | 0 | 1 | 0 |
| 47 | 41 | 50 | 34 | ||
Situations where the marker pattern differs from that expected by the DSBR model (Figure 1).
Marker segregation patterns
Marker segregation . | . | . | . | . | |
|---|---|---|---|---|---|
| Class no. . | Type . | pCμpalLong/Long . | pCμpalShort/Short . | pCμpalLong/Short . | pCμpalShort/Long . |
| 1 | 5:3/5:3 | 1 | 1 | 1 | 1 |
| 2 | 4:4/5:3 | 4 | 3 | 2 | 1 |
| 3 | 4:4/6:2 | 6 | 10 | 9 | 9 |
| 4 | 4:4/3:5 | 1 | 2 | 0 | 2 |
| 5 | 4:4/2:6 | 0 | 2 | 0 | 0 |
| 6 | 4:4/Ab4:4 | 1 | 0 | 0 | 1 |
| 7 | 6:2/4:4 | 7 | 2 | 6 | 2 |
| 8 | 3:5/4:4 | 0 | 0 | 1 | 0 |
| 9 | 2:6/4:4 | 2 | 2 | 1 | 0 |
| 10 | 6:2/5:3 | 4 | 3 | 3 | 3 |
| 11 | 5:3/6:2 | 1 | 2 | 4 | 2 |
| 12 | 6:2/6:2 | 7 | 3 | 10 | 1 |
| 13 | 4:4/4:4 | 1 | 2 | 4 | 0 |
| 14 | 3:5/6:2 | 2 | 0 | 0 | 0 |
| 15 | 6:2/3:5 | 1 | 0 | 0 | 0 |
| 16 | 2:6/6:2 | 0 | 0 | 1 | 1 |
| 17 | 5:3/2:6 | 0 | 0 | 0 | 1 |
| 18 | 2:6/2:6 | 0 | 1 | 0 | 0 |
| 19 | 2:6/5:3 | 0 | 1 | 0 | 0 |
| 20 | 5:3/Ab4:4 | 1 | 0 | 1 | 1 |
| 21 | 3:5/Ab4:4 | 0 | 1 | 0 | 0 |
| 22 | 2:6/Ab4:4 | 1 | 0 | 0 | 0 |
| 23 | Othera | ||||
| a | 5:3/5:3 | 1 | 0 | 0 | 2 |
| b | 4:4/4:4 | 1 | 4 | 5 | 2 |
| c | 4:4/5:3 | 3 | 2 | 1 | 5 |
| d | 5:3/4:4 | 1 | 0 | 0 | 0 |
| e | No marker | 1 | 0 | 1 | 0 |
| 47 | 41 | 50 | 34 | ||
Marker segregation . | . | . | . | . | |
|---|---|---|---|---|---|
| Class no. . | Type . | pCμpalLong/Long . | pCμpalShort/Short . | pCμpalLong/Short . | pCμpalShort/Long . |
| 1 | 5:3/5:3 | 1 | 1 | 1 | 1 |
| 2 | 4:4/5:3 | 4 | 3 | 2 | 1 |
| 3 | 4:4/6:2 | 6 | 10 | 9 | 9 |
| 4 | 4:4/3:5 | 1 | 2 | 0 | 2 |
| 5 | 4:4/2:6 | 0 | 2 | 0 | 0 |
| 6 | 4:4/Ab4:4 | 1 | 0 | 0 | 1 |
| 7 | 6:2/4:4 | 7 | 2 | 6 | 2 |
| 8 | 3:5/4:4 | 0 | 0 | 1 | 0 |
| 9 | 2:6/4:4 | 2 | 2 | 1 | 0 |
| 10 | 6:2/5:3 | 4 | 3 | 3 | 3 |
| 11 | 5:3/6:2 | 1 | 2 | 4 | 2 |
| 12 | 6:2/6:2 | 7 | 3 | 10 | 1 |
| 13 | 4:4/4:4 | 1 | 2 | 4 | 0 |
| 14 | 3:5/6:2 | 2 | 0 | 0 | 0 |
| 15 | 6:2/3:5 | 1 | 0 | 0 | 0 |
| 16 | 2:6/6:2 | 0 | 0 | 1 | 1 |
| 17 | 5:3/2:6 | 0 | 0 | 0 | 1 |
| 18 | 2:6/2:6 | 0 | 1 | 0 | 0 |
| 19 | 2:6/5:3 | 0 | 1 | 0 | 0 |
| 20 | 5:3/Ab4:4 | 1 | 0 | 1 | 1 |
| 21 | 3:5/Ab4:4 | 0 | 1 | 0 | 0 |
| 22 | 2:6/Ab4:4 | 1 | 0 | 0 | 0 |
| 23 | Othera | ||||
| a | 5:3/5:3 | 1 | 0 | 0 | 2 |
| b | 4:4/4:4 | 1 | 4 | 5 | 2 |
| c | 4:4/5:3 | 3 | 2 | 1 | 5 |
| d | 5:3/4:4 | 1 | 0 | 0 | 0 |
| e | No marker | 1 | 0 | 1 | 0 |
| 47 | 41 | 50 | 34 | ||
Situations where the marker pattern differs from that expected by the DSBR model (Figure 1).
.
The following terminology and simplifying assumptions were used: (1) recombination is presumed to initiate at the BstXI vector-borne DSB; (2) assimilation of single-stranded DNA into the unbroken chromosome is continuous beginning from the 3′-end of the DSB; (3) mismatches involving the NotI-palindrome marker are equally correctable; (4) when hDNA forms on one or both sides of the DSB, Holliday junction resolution occurs at or beyond the end of the hDNA tract; and (5) hDNA repair is used to denote mismatch rectification where the repair system is unknown.
High frequency of one-sided events:
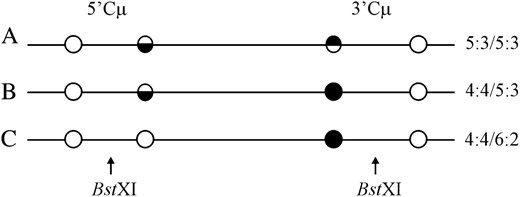
The DSBR model (Figure 1) predicts that hDNA in recombinants should be two-sided; that is, a HC event should reside on both sides of the DSB (5:3/5:3 segregation) as in Figure 7A. Instead, this configuration of hDNA is rare, being observed in only 4/172 recombinants (∼2%) (segregation class 1 in Table 2). In contrast to the paucity of the 5:3/5:3 class, a substantial fraction of recombinants generated with each of the cut vectors are one sided: they display normal 4:4 segregation on one side of the DSB, while the palindrome marker on the other side of the DSB displays some form of NMS (segregation classes 2–9 in Table 2). Recombinants displaying “one-sidedness” range from 38–51% for the various gene-targeting vectors used. Of these, normal 4:4 segregation on one side of the DSB accompanied by a HC (5:3) or a FC (6:2) event on the other side of the DSB are the most frequent, accounting for 71–89% of the total one-sided events (segregation classes 2, 3, and 7 in Table 2, as represented in Figure 7, B and C).
DSBR marker patterns. Examples of marker patterns observed in recombinants generated by crossover gene targeting include the segregation categories (A) 5:3/5:3, (B) 4:4/5:3, and (C) 4:4/6:2. As in Figure 2, a single illustrative strand is used for simplicity. Open circles represent chromosomal (BamHI or ApaI) markers, while solid circles represent the vector-borne palindrome containing the NotI site. Half-solid circles represent sectored sites. The site of the recombination-initiating DSB at BstXI is shown. Refer to the text for details.
One-sided events occur preferentially to the left of the DSB:
Further inspection of the distribution of one-sided recombinants in segregation classes 2–9 reveals that 81% and 87% of the normal 4:4 segregation events occur to the left of the DSB in recombinants generated with the vectors pCμpalShort/Short and pCμpalShort/Long, respectively. According to chi-square analysis, these values are significantly different from the equivalence expected if normal 4:4 segregation events were equally likely to occur on the two sides of the DSB (0.005 < P < 0.001). Although not as pronounced, 57 and 58% of the one-sided events also occur to the left of the DSB in recombinants generated with vectors pCμpalLong/Long and pCμpalLong/Short, respectively, but according to χ2 analysis, these frequencies are not significantly different from equivalence (0.75 < P < 0.50).
The above results are at variance with the predictions of the two mechanisms that have been proposed to explain the origin of one-sided events, namely (i) limited hDNA formation on one side of the DSB (Figure 3) (Porter et al. 1993; Merker et al. 2003; Jessop et al. 2005) and (ii) restorational repair of hDNA (Figure 4) (Foss et al. 1999). As explained in the Introduction, mechanism (i) predicts a similar frequency of one-sided events on the two sides of the DSB in recombinants generated with all four of the gene-targeting vectors, while mechanism (ii) predicts a bias favoring one-sided events on the side of the DSB in which the mismatch is nearest the cut junction initiating repair. In this study, this dictates a bias in one-sided events on the left and right side of the DSB in vectors pCμpalShort/Long and pCμpalLong/Short, respectively, and an approximately equivalent distribution of one-sided events on both sides of the DSB in recombinants generated with vectors pCμpalShort/Short and pCμpalLong/Long.
How might the bias in normal 4:4 segregation that is observed to the left of the DSB in recombinants generated with the vectors pCμpalShort/Short and pCμpalShort/Long be achieved? The bias is unlikely to be the result of insufficient homology that restricts hDNA formation to the left of the DSB, since there should be no bias in recombinants generated with the pCμpalShort/Short gene-targeting vector. Also, if homology restriction were a factor, a bias favoring normal 4:4 segregation to the right of the DSB would be expected in recombinants generated with the gene-targeting vector pCμpalLong/Short. Homology restriction is also expected to influence recombination efficiency. However, as indicated in Figure 5, A–D, each vector contains more than the ∼1-kb minimum homology required to promote recombination, and all target the chromosome with similar efficiency (Table 1). As another mechanism, it could be argued that limited hDNA formation (Figure 3) accounts for the left orientation bias, but only in the circumstance where homology is restricted to the left of the DSB. For example, a sequence that inhibits hDNA formation or that limits DSB resection may reside to the left of the DSB and become active when homology is reduced. While possible, the formal demands of this mechanism appear to make it less tenable. In addition, there is also concern that a mechanism inhibiting hDNA formation may lower recombination efficiency, which is not the case in this system. A third possibility, namely that a loop-specific repair system is involved, also seems unlikely since it would not be expected to generate the specific marker segregation patterns observed.
Instead, we offer the following explanation, taking advantage of Figure 4 as a visual aid. Preferred sense, 2,4-cleavage of a double Holliday junction intermediate in which hDNA has formed asymmetrically on the two sides of the DSB (two HC events: 5:3/5:3 segregation) generates the crossover product (Foss et al. 1999; Baker and Birmingham 2001; Merker et al. 2003; Jessop et al. 2005). Nicks at positions 2,2′ and 4,4′ correspond to the cut strands of Holliday junctions located to the left and right of the DSB, respectively. Nick-directed repair of hDNA can result in restoration of a marker (normal 4:4 segregation) on the same side of the DSB as the cut junction initiating repair (Alani et al. 1994; Schwacha and Kleckner 1995; Foss et al. 1999). Thus, nick-directed repair from junction cutting at position 2 generates recombinants in which normal 4:4 segregation is favored on the left of the DSB. Similarly, hDNA repair directed by junction cutting at position 4 generates recombinants in which normal 4:4 segregation is favored on the right of the DSB. Thus, in this study, the bias in normal 4:4 segregation to the left of the DSB in recombinants generated with gene-targeting vectors pCμpalShort/Long and pCμpalShort/Short is consistent with nick-directed repair of hDNA initiated by junction cutting at position 2. Therefore, our data suggest that the junction cuts arising from Holliday junction cleavage may not be equivalent with respect to their ability to direct repair of mismatches in adjacent hDNA.
A model for one-sided events based on asynchronous Holliday junction cleavage:
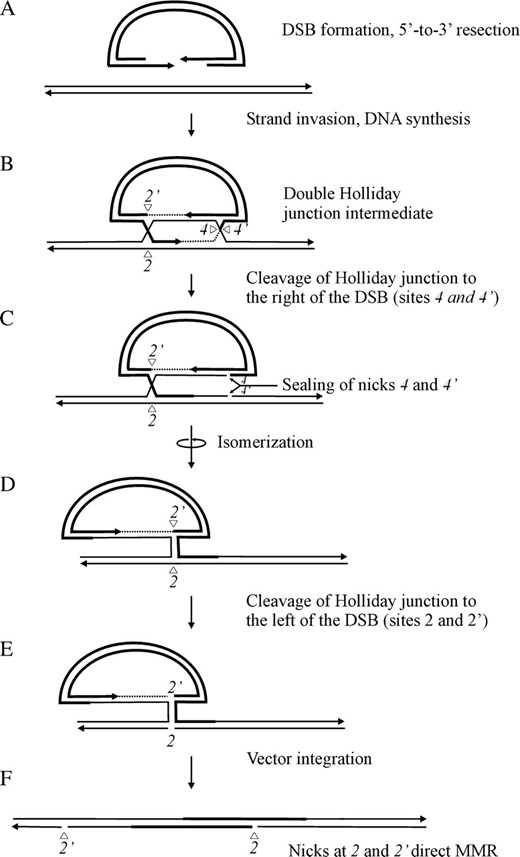
An enhanced nick-directed repair activity from junction cutting at position 2 can be explained by the proposal that cleavage of the two Holliday junctions is frequently asynchronous. A model encapsulating this idea is presented in Figure 8. Illustrations in Figure 8, A and B, condense events depicted in Figure 1, A–E. For simplicity, a fully formed double Holliday junction intermediate is presented in Figure 8B. However, this need not imply that that two Holliday junctions are formed simultaneously, since it is possible that one junction forms before the other. The model proposes that the crossed strands in the Holliday junction to the right of the DSB are frequently the first to be cleaved, generating nicks at positions 4 and 4′ (Figure 8C). We note that Holliday junction resolution may not formally require cutting of both crossed DNA strands in the right junction; in the event that the newly synthesized DNA tract primed from the invading 3′ strand remains unligated (Stahl et al. 2004), a nick would already be present at position 4′, and therefore only the uncut DNA strand at position 4 would need to be cleaved. Following cleavage, we propose that the nicks at positions 4 and 4′ are ligated. Ligation is required for crossover to proceed and also limits the opportunity for nick-directed repair of hDNA from positions 4 and 4′, reducing the number of normal 4:4 segregation events to the right of the DSB. The ligated intermediate is free to isomerize (Figure 8D). Cleavage of the remaining Holliday junction, producing nicks at positions 2 and 2′ (Figure 8E), permits vector integration (Figure 8F). Since the top (5′-to-3′) strand is continuous, incorporation of the vector into the chromosome is expected to occur whether the nicks in the complementary strand at positions 2 and 2′ have been ligated or not. Thus, they may have a long half-life, with greater opportunity to direct hDNA repair. Heteroduplex DNA repair from the nick at position 2 can account for the above-noted bias in normal 4:4 segregation events to the left of the DSB. As above, a nick in the newly synthesized DNA tract at position 2′ may already be present (Stahl et al. 2004), in which case, vector integration would require only cleavage of the unbroken strand at position 2.
Proposed model for one-sided recombinants. The diagrams in A and B condense events in Figure 1, A–E. In the model, one Holliday junction in the fully formed DSBR intermediate is cleaved first, in this case, the right junction, followed by ligation of the nicks (4 and 4′) created during junction resolution. Following isomerization of the intermediate, cleavage of the left Holliday junction permits vector integration. Nicks (2 and 2′) created by cleavage of the left Holliday junction may remain unligated and available to direct repair of adjacent hDNA. For further detail, refer to the text.
A threshold distance for nick-directed restorational repair:
When the data in Table 2 are considered together with the model in Figure 8, a distance constraint with respect to the capacity of the nick at position 2 to direct restorational repair is suggested. The strong bias favoring normal 4:4 segregation to the left of the DSB in recombinants generated with the gene-targeting vectors pCμpalShort/Short and pCμpalShort/Long suggests prominent restorational repair when the palindrome is located 870 bp from the homology border (genetic interval “a” in Figure 5, B and C). However, when the distance between the homology border and the palindrome mismatch is 1994 bp, such as in recombinants generated with the gene-targeting vectors pCμpalLong/Long and pCμpalLong/Short (genetic interval “a” in Figure 5, A and D), an approximately equivalent distribution of normal 4:4 segregation events on the two sides of the DSB is observed. We interpret these data to mean that a threshold distance between 870 and 1994 bp represents the limit for an excision tract initiating from a nick at position 2 to effectively remove the palindrome mismatch.
The DSBR model predicts that palindrome markers on the two sides of the DSB should segregate 5:3/5:3 except when repair of hDNA intervenes (Figure 1) (Foss et al. 1999). As indicated above, nick-directed repair from position 2 is consistent with the bias in normal 4:4 segregation to the left of the DSB in those recombinants in which the distance from the initiating nick to the palindrome mismatch is within the threshold. However, what repair system acts to remove palindrome mismatches when the threshold distance from the nick to the palindrome is exceeded? Two mechanisms are possible: (i) junction cuts (or, possibly, unligated 3′-ends; Stahl et al. 2004) at positions 2′ and 4′ direct removal of DSB proximal palindromes (early repair of hDNA) (McGill et al. 1989; Haber et al. 1993; Foss et al. 1999) and (ii) palindromes are removed by localized (nick-independent) repair (Weiss and Wilson 1987; Bollag et al. 1992; Bill et al. 2001). With regard to mechanism (i), nicks at positions 2′ and 4′ would be located at variable distances from the palindrome mismatches due to the different lengths of homology in the four gene-targeting vectors used in generating the recombinants. Assuming that the same threshold distance limits hDNA repair initiated from these nicks, a competition among nicks at 2′, 2, and 4′ to direct repair of adjacent hDNA would exist. If we analyze segregation classes 2, 3, 7, and 10–12 (recombinants that are conveniently explained by hDNA repair of a 5:3/5:3 intermediate), then we observe that, apart from markers to the left of the DSB in recombinants generated with vectors pCμpalShort/Short and pCμpalShort/Long (where restoration outnumbers conversion), conversion outnumbers restoration (68–32%). Thus, there appears to be a competition between nick-directed removal of the palindrome at position 2 and early repair of the palindrome from positions 2′ and 4′. This result is not expected from a localized (nick-independent) repair process [mechanism (ii) above], since this mechanism would be expected to act uniformly on all palindrome mismatches that exceed the threshold distance. Thus, the results favor the interpretation that when the threshold distance for restorational repair is exceeded, early repair of hDNA may occur.
In summary, the unique finding of this study is that one-sided recombinants display an unexpected bias favoring normal 4:4 segregation to the left of the DSB. This result is consistent with a greater efficiency of nick-directed restorational repair from position 2 of a cleaved Holliday junction to the left of the DSB. This bias is compatible with asynchronous cleavage of the double Holliday junction intermediate (Figure 8).
The remaining recombinants in this study (segregation classes 13–23) display features that can also be explained within the framework of the DSBR model in Figure 8. Many of the characteristics of these recombinants have been described in previous studies (Birmingham et al. 2004), and therefore they are described briefly, for completeness, in the appendix.
DISCUSSION
In this study, we analyzed marker segregation patterns produced during crossover gene targeting in mitotic mammalian cells. We observe a bias in normal 4:4 segregation events for markers located to the left of the DSB (one-sided events) in two of the four gene-targeting vectors utilized. Neither model previously proposed to account for the prevalence of one-sided events in yeast meiosis, that is, limited hDNA formation (Porter et al. 1993; Merker et al. 2003; Jessop et al. 2005) or restorational repair of hDNA (Foss et al. 1999), satisfactorily explains the bias observed in this study. Instead, we propose the DSBR model in Figure 8 in which the two Holliday junctions are cut in the favored sense (2,4-cleavage), but in an asynchronous fashion. The crossed strands in the Holliday junction that would be formed through D-loop formation and DNA synthesis primed by the first invading 3′ strand are cut, and the nicks at positions 4 and 4′ are ligated. Following this, the noncrossed strands in the second Holliday junction are cut, but since strand ligation is likely not a prerequisite for crossover, the nicks at positions 2 and 2′ may remain unligated. Unligated nicks at these positions provide a greater opportunity for initiating hDNA repair. Therefore, we suggest that asynchronous cleavage provides a mechanism to restrict the capacity for nick-directed repair of hDNA from the cut Holliday junction to the right of the DSB, while favoring nick-directed repair of hDNA from the cut Holliday junction to the left of the DSB. When the affected mismatch is close to the left cut junction initiating repair, an increased frequency of normal 4:4 segregation is observed on that side of the DSB. Gilbertson and Stahl (1996) also invoked asynchronous Holliday junction cleavage in the context of explaining the relationship between FC and crossing over during meiotic recombination in yeast.
We can think of three reasons why Holliday junction cleavage might be asynchronous. One possibility is that formation of the two Holliday junctions is separated temporally. In the event that Holliday junction formation stimulates Holliday junction cutting, the Holliday junction formed initially is expected to be the first cleaved. This might occur as follows. Strand invasion and DNA synthesis on one side of the DSB extrudes a D loop that eventually results in capture of the noninvading 3′-end on the other side of the DSB. Conceivably, D-loop capture of the second 3′-end results in a Holliday junction forming first on that side of the DSB. Later, DNA synthesis primed by the second 3′-end establishes a Holliday junction on the other side of the DSB. Thus, in the context of the model in Figure 8, the Holliday junction formed initially to the right of the DSB is the first cleaved. This model is attractive because the driving force in forming the first Holliday junction, namely DNA synthesis from the invading 3′ strand, is also considered important in determining the pair of like strands in each Holliday junction that are cut in preferred sense cleavage of the double Holliday junction intermediate (2,4 mode) (Foss et al. 1999). A second possibility is that there is functional “cross-talk” between the proteins that carry out Holliday junction resolution, which prevents cleavage from occurring simultaneously. This mechanism and the one above are not mutually exclusive. A third possibility is that Holliday junction resolution occurs preferentially at a specific sequence in a fashion similar to that proposed for Holliday junction cleavage in Escherichia coli (Shah et al. 1994). In this way, outward branch migration of the two Holliday junctions would fashion a sort of race, where cleavage of the DSBR intermediate occurs when the resolution consensus sequence is reached. Thus, if the consensus sequence is closer to the DSB in one flanking region of homology than in the other, asynchronous Holliday junction cleavage would be observed.
The events depicted in Figure 8 may be specific to crossover resolution during gene targeting, and this may explain the discrepancy in the distribution of one-sided events in this study and previous studies of meiotic recombination in yeast (Porter et al. 1993; Merker et al. 2003; Jessop et al. 2005). We note that ligation of nicks at positions 2 and 2′ may not be required for vector integration, and this may provide greater opportunity for hDNA repair to initiate from these junctions, producing the bias that we observe in restorational repair to the left of the DSB. In contrast, crossover between meiotic chromosomes depends on proper ligation of all DNA strands, and in its absence, chromosome dysjunction may be abnormal. Accordingly, the junction cuts arising from Holliday junction cleavage during meiotic recombination may not display the same differences with respect to their ability to direct repair of adjacent hDNA as in the gene-targeting studies described here. It is possible that asynchronous Holliday junction cleavage does occur during crossover resolution in meiosis (Gilbertson and Stahl 1996), but the bias in restorational repair that would reveal it is obscured by the requirement for nick ligation. In the event that crossover resolution during meiotic recombination does not involve asynchronous cleavage of the double Holliday junction intermediate, other differences, such as the manner in which the recombination-initiating DSB is introduced, may account for the discrepancy in generating one-sided recombinants. In meiotic recombination, programmed DSB formation requires the Spo11 protein, which, in turn, remains bound to the newly created DSB and seems to regulate end-processing (Neale et al. 2005). In contrast, in mitotic gene targeting, the DSB is preformed, and therefore end-processing may be regulated in a manner unlike that in Spo11-catalyzed meiotic DSB formation. Since end-processing may influence hDNA formation, it may contribute to the differences in marker segregation patterns that are observed between meiotic recombination and mitotic gene targeting.
In the event that asymmetric Holliday junction cleavage is a more general feature of resolving the DSBR intermediate during crossover resolution, might it serve a physiological role? Perhaps asynchronous Holliday junction cleavage provides the means to prevent potentially deleterious chromosomal rearrangements. If both Holliday junctions were cleaved simultaneously, four nicks could potentially be generated, providing opportunities for deletion or rearrangement of DNA sequences. Asynchronous Holliday junction cleavage may provide a mechanism for limiting the number of breaks that occur in the intermediate at one time, reducing the chance of undesirable rearrangements. Asynchronous Holliday junction cleavage might also facilitate the “decision” to resolve a recombination intermediate as either a crossover or noncrossover product. That is, following cleavage of one Holliday junction, either the intermediate could be unwound to generate a noncrossover product or the DNA strands in the second Holliday junction could be cleaved in the opposite sense to generate a crossover product (Stahl et al. 2004).
APPENDIX: OTHER FEATURES ASSOCIATED WITH CROSSING OVER
Crossover at or near the DSB:
Of the 172 recombinants (4%), 7 revealed normal 4:4 marker segregation on both sides of the DSB (segregation class 13 in Table 2 as represented in Figure A1A). The simplest explanation for these recombinants is that a crossover event has occurred at or near the DSB, and flanking palindromes are not included in hDNA. However, we cannot exclude the possibility that restorational repair of a 5:3/5:3 DSBR intermediate may occasionally be directed by the cut strands of Holliday junctions to the left and right of the DSB at positions 2 and 4 (Figure 1).
Additional DSBR marker patterns. Examples of additional marker segregation patterns generated during crossover gene targeting include (A) normal 4:4/4:4 segregation, as well as nonstandard (B) 5:3/5:3, (C) 4:4/4:4, (D) 4:4/5:3, and (E) 5:3/4:4 segregations. Additional details are in the text.
Symmetric hDNA on one side of the DSB:
In several recombinants, the marker patterns are consistent with formation of symmetric hDNA on one side of the DSB. Symmetric hDNA is likely formed as a consequence of outward Holliday junction branch migration that results in the swapping of vector and chromosomal DNA strands of like polarity between the recombining duplexes (Figure 1). For the double Holliday junction intermediate to include markers in both duplexes on one side of the DSB, 5′-to-3′ resection of the vector-borne DSB must not have included the palindrome marker on one side of the DSB. Figure 2 serves as a visual aid to interpreting segregation patterns that signify symmetric hDNA, using as an example the case where the genetic marker is positioned arbitrarily to the right of the DSB. The failure to repair mismatches in symmetric hDNA results in Ab4:4 marker segregation. A signature of repair acting on symmetric hDNA is HC (3:5) and FC (2:6) marker segregation patterns. The above marker patterns are visible to the left or right of the DSB in segregation classes 4–6, 8, 9, 14, and 16–20 (Table 2). Thus, recombinants that bear evidence of symmetric hDNA on one side of the DSB represent 25/172 or ∼15% of the total recombinants analyzed.
hDNA formation on the two sides of the DSB:
Formation of hDNA on the two sides of the DSB (5:3/5:3 segregation), as predicted in the canonical DSBR model (Figure 1), is observed in segregation class 1. In other cases, two-sided hDNA formation is suggested from the patterns of marker repair. Again, using the patterns in Figure 2 as a guide, these include FC (2:6) for markers on both sides of the DSB (segregation class 18), FC (2:6) for one marker and HC (5:3) for the other (segregation class 19), and Ab4:4 on one side of the DSB and either a HC (5:3 or 3:5) or a FC (2:6) event on the other side of the DSB (segregation classes 20–22). Furthermore, the recombinants in segregation classes 18, 21, and 22 are highly likely to have been derived from intermediates in which symmetric hDNA resided on both sides of the DSB.
Other recombination events:
In other recombinants, nonstandard marker configurations were apparent. These are grouped collectively in segregation class 23, a–e. Class 23a displays 5:3/5:3 segregation, but it differs from the pattern predicted by the DSBR model (Figure 1) in that hDNA has formed in one homology region on both sides of the DSB. Figure A1B illustrates an example of this pattern in which unrepaired hDNA resides in the 5′ Cμ region, while both markers in the 3′ Cμ region have undergone FC. Recombinants displaying the reverse pattern are also observed (supplemental information at http://www.genetics.org/supplemental/). Recombinants in class 23b display a nonstandard 4:4. An example of this pattern is presented in Figure A1C. The supplemental information at http://www.genetics.org/supplemental/ presents other recombinants in which the order of 5′ and 3′ Cμ region markers in this nonstandard 4:4 configuration is reversed. In class 23c, a nonstandard 4:4 marker segregation is observed on one side of the DSB together with an HC (5:3) event on the other side of the DSB. In class 23d, a standard 4:4 is observed together with a nonstandard 5:3 on the other side of the DSB. Examples of these patterns are in Figure A1, D and E. The recombinants in classes 23a–d are consistent with a DSBR intermediate in which hDNA repair may have occurred on both sides of the DSB in one homology region. In the last class (class 23e), a single Cμ region position in two recombinants is devoid of either the vector-borne palindrome or the corresponding endogenous marker. The frequency of such site-loss variants is low: of the total of 688 marker positions examined in the 172 recombinants, site loss was observed in only 2 of 688 positions (∼0.2%). A similarly low frequency of site-loss variants (∼1%) was observed in previous gene-targeting studies (Baker and Birmingham 2001; Birmingham et al. 2004). In each case, the DNA sequencing results of Birmingham et al. (2004) reveal deletion of approximately one-half of the palindrome sequence, possibly as a result of improper repair of a palindrome mismatch in hDNA.
Preferred sense cleavage of the double Holliday junction intermediate:
The results of this study confirm previous observations that crossover resolution of the DSBR intermediate displays a bias favoring the 2,4-cleavage mode (Gilbertson and Stahl 1996; Foss et al. 1999; Baker and Birmingham 2001; Merker et al. 2003; Jessop et al. 2005). As shown in Figure A2, the 2,4- and 1,3-cleavage modes are predicted to generate two types of recombinants displaying 5:3/5:3 marker segregation, distinguishable on the basis of the position of hDNA (Foss et al. 1999; Baker and Birmingham 2001). In the event of hDNA repair, the 5:3/5:3 marker patterns can be altered to yield a total of eight possible segregation classes. Excluding those recombinants displaying FC events on both sides of the DSB (6:2/6:2 segregation), which are consistent with either resolution mode, the 5:3/5:3 segregation patterns together with the seven remaining segregation classes are informative for distinguishing the mode of Holliday junction cleavage. Figure A2 summarizes the supplemental information at http://www.genetics.org/supplemental/ with respect to the number of recombinants whose marker patterns correspond to the eight informative segregation classes. The results reveal 89 recombinants whose marker segregation patterns are consistent with 2,4-cleavage of the double Holliday junction intermediate, compared to only 5 recombinants whose marker patterns can be explained by the 1,3-cleavage mode. Therefore, we take this information as additional evidence for preferential 2,4 cleavage of the DSBR intermediate.
Preferred sense cleavage of the double Holliday junction intermediate. The DSBR model (Figure 1) predicts that, following Holliday junction resolution, a 5:3/5:3 recombinant will be formed. Depending on the mode of resolution (2,4- or 1,3-cleavage), the 5:3/5:3 recombinant will differ with respect to the position of hDNA. Nicks generated following junction resolution (2,2′ and 4,4′ or 1,1′ and 3,3′) are able to direct repair of adjacent hDNA. Nick-directed hDNA repair can produce the indicated marker segregation patterns. The number of recombinants whose marker patterns correspond to those predicted by nick-directed repair is indicated. The number of recombinants whose marker patterns are consistent with repair of a 5:3/5:3 intermediate formed by 2,4-junction cleavage is in excess of those predicted by a 1,3-cleavage mode.
Footnotes
Communicating editor: L. S. Symington
Acknowledgement
We thank members of the Baker laboratory for helpful discussions and appreciate the comments of two anonymous reviewers that helped improve the manuscript. This work was supported by an Ontario Graduate Scholarship to R.D.M. and by an operating grant from the Canadian Institutes of Health Research to M.D.B.
References
Alani, E., R. A. Reenan and R. D. Kolodner,
Baker, M. D., and E. C. Birmingham,
Baker, M. D., and L. R. Read,
Baker, M. D., N. Pennell, L. Bosnoyan and M. J. Shulman,
Bautista, D., and M. J. Shulman,
Bill, C. A., D. G. Taghian, W. A. Duran and J. A. Nickoloff,
Birmingham, E. C., S. A. Lee, R. D. McCulloch and M. D. Baker,
Bollag, R. J., D. R. Elwood, E. D. Tobin, A. R. Godwin and R. M. Liskay,
Donoho, G., M. Jasin and P. Berg,
Foss, H. M., K. J. Hillers and F. W. Stahl,
Gilbertson, L. A., and F. W. Stahl,
Gross-Bellard, M., P. Oudet and P. Chambon,
Haber, J. E., B. L. Ray, J. M. Kolb and C. I. White,
Hasty, P., J. Rivera-Perez and A. Bradley,
Jessop, L., T. Allers and M. Lichten,
Köhler, G., and M. J. Shulman,
Köhler, G., M. J. Potash, H. Lehrach and M. J. Shulman,
Li, J., and M. D. Baker,
McGill, C., B. Shafer and J. Strathern,
Merker, J. D., M. Dominska and T. D. Petes,
Nag, D. K., M. A. White and T. D. Petes,
Neale, M. J., J. Pan and S. Keeney,
Ng, P., and M. D. Baker,
Ng, P., and M. D. Baker,
Ng, P., and M. D. Baker,
Orr-Weaver, T. L., J. W. Szostak and R. J. Rothstein,
Porter, S. E., M. A. White and T. D. Petes,
Sambrook, J. E., E. F. Fritsch and T. Maniatis,
Schwacha, A., and N. Kleckner,
Shah, R., R. J. Bennett and S. C. West,
Shulman, M. J., L. Nissen and C. Collins,
Southern, P. J., and P. Berg,
Stahl, F. W., H. M. Foss, L. S. Young, R. H. Borts, M. F. Abdullah et al.,
Sun, H., D. Treco and J. W. Szostak,
Szostak, J. W., T. L. Orr-Weaver, R. J. Rothstein and F. W. Stahl,
Weiss, U., and J. H. Wilson,