-
PDF
- Split View
-
Views
-
Cite
Cite
Jeffrey Linger, Jessica K Tyler, The Yeast Histone Chaperone Chromatin Assembly Factor 1 Protects Against Double-Strand DNA-Damaging Agents, Genetics, Volume 171, Issue 4, 1 December 2005, Pages 1513–1522, https://doi.org/10.1534/genetics.105.043000
Close - Share Icon Share
Abstract
The removal of histones from DNA and their subsequent replacement is likely to be necessary for all processes that require access to the DNA sequence in eukaryotic cells. The histone chaperone chromatin assembly factor 1 (CAF-1) mediates histone H3-H4 assembly during DNA replication and nucleotide excision repair in vitro. We have found that budding yeast deleted for the genes encoding CAF-1 are highly sensitive to double-strand DNA-damaging agents. Our genetic analyses indicate that CAF-1 plays a role in both homologous recombination and nonhomologous end-joining pathways and that the function of CAF-1 during double-strand repair is distinct from that of another histone H3-H4 chaperone, anti-silencing function 1 (ASF1). CAF-1 does not protect the genome by assembling it into a damage-resistant chromatin structure, because induction of CAF-1 after DNA damage is sufficient to restore viability. Furthermore, CAF-1 is not required for repair of the DNA per se or for DNA damage checkpoint function. CAF-1-mediated resistance to DNA damage is dependent on the ability of CAF-1 to bind PCNA, indicating that PCNA may recruit CAF-1 to sites of double-strand DNA repair. We propose that CAF-1 has an essential role in assembling chromatin during double-strand-DNA repair.
DOUBLE-STRAND DNA repair is critical for maintaining genomic integrity. Double-strand DNA breaks (DSBs) are an exceptionally lethal type of DNA damage, where even a single DSB left unrepaired can lead to cell death (Bennett et al. 1993). DSBs are predominantly repaired through two major pathways: homologous recombination and nonhomologous end joining (NHEJ) (Dudas and Chovanec 2004; Dudasova et al. 2004). Homologous recombination is a process in which double-strand DNA damage is repaired using a replication-based mechanism through the use of an undamaged homologous template, whereas NHEJ essentially involves the direct religation of broken DNA ends with very little DNA synthesis and without any requirement for sequence homology. To fully understand these repair processes, we have to focus on chromatin, the template upon which repair occurs.
The eukaryotic nuclear genome is assembled into the nucleoprotein structure termed chromatin. The basic repeating unit of chromatin is the nucleosome, which comprises 147 bp of DNA wrapped around an octamer of histone proteins (two molecules each of histones H3, H4, H2A, and H2B) (Luger et al. 1997). The majority of chromatin is assembled immediately following DNA replication. This is mediated in part by the histone chaperone chromatin assembly factor 1 (CAF-1), which deposits histones H3 and H4 onto newly replicated DNA in vitro (Smith and Stillman 1989). CAF-1 is a heterotrimeric protein that has been highly conserved through evolution (Kaufman et al. 1995, 1997; Tyler et al. 1996; Verreault et al. 1996; Tyler et al. 2001). CAF-1 functions synergistically with another H3–H4 histone chaperone, antisilencing function 1 (ASF1), during the assembly of newly replicated DNA into chromatin in vitro (Tyler et al. 1999). The replication dependence of chromatin assembly by CAF-1 appears to be due to the targeting of CAF-1 to DNA replication forks via its interaction with the proliferating cell nuclear antigen (PCNA) (Shibahara and Stillman 1999). Supporting a role in assembling chromatin following DNA replication in vivo, CAF-1 has been shown to localize to sites of DNA replication in tissue culture cells (Krude 1995). Yeast lacking CAF-1 have global underassembly of their genome into chromatin (Adkins et al. 2004), and reduction of CAF-1 activity in tissue culture cells leads to reduced packaging of the genome into chromatin, DNA replication defects, and arrest in S phase of the cell cycle (Krude 1999; Hoek and Stillman 2003; Ye et al. 2003; Nabatiyan and Krude 2004).
CAF-1 has also been implicated in single-strand DNA repair via the nucleotide excision repair (NER) pathway. NER is used to repair single-strand DNA lesions such as those incurred by exposure to ultraviolet light (UV) (Prakash and Prakash 2000). CAF-1 is capable of assembling chromatin coupled with NER in vitro (Gaillard et al. 1996). CAF-1 has also been localized to DNA templates undergoing NER, in a manner dependent upon PCNA (Moggs et al. 2000; Green and Almouzni 2003). Accordingly, yeast deleted for CAF-1 components are hypersensitive to UV irradiation, indicating the importance of CAF-1 in surviving UV-induced DNA damage (Kaufman et al. 1997).
Given the common theme of CAF-1-mediated DNA synthesis-dependent chromatin assembly during NER and DNA replication, we investigated whether CAF-1 may also play a role in assembling chromatin during double-strand DNA repair. We show here that yeast lacking CAF-1 are sensitive to multiple types of double-strand DNA damage and that resistance to DNA damage is dependent on the ability of CAF-1 to bind PCNA. Interestingly, while CAF-1 mutants have no defect in the ability to repair double-strand DNA lesions, they have a greatly decreased ability to survive a DSB. Furthermore, induction of CAF-1 following DNA damage is sufficient for survival. This suggests a role for CAF-1 in the assembly of chromatin following or during the repair of double-strand DNA damage.
MATERIALS AND METHODS
Yeast strains and media:
All strains were haploid derivatives of W303-1a (Thomas and Rothstein 1989), unless otherwise indicated, and the genotypes are given in Table 1. Deletion mutants were created by one-step PCR-mediated integration, as described previously (Longtine et al. 1998).
Strains used in this study
Strains . | Relevant genotype . | Source . |
|---|---|---|
| BOB852 | MATa his3Δ1 leu20 met50 ura3Δ0 | Bob Sclafani |
| BY4741-Y06502 | MATa his3Δ1 leu20 met50 ura3Δ0 cac2∷KAN | Research Genetics |
| JKT0004 | MATa rad52∷TRP1 trp1-1 ura3-1 can1-100 ADE bar1∷LEU2 his3-11 | Ramey et al. (2004) |
| JKT0010 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 | Ramey et al. (2004) |
| JLY030 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 cac1∷LEU2 | This study |
| JLY031 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 | This study |
| JLY060 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 CAC2:FLAG:KAN | This study |
| JLY063 | MATa ade2-1 his3-11 leu2-3 can1-100 trp1-1 ura3-52 ade3∷pGAL-HO bar1∷LEU2 | This study |
| JLY072 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 cac2∷HPH rad52∷TRP1 | This study |
| JLY073 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 yku70∷KAN | This study |
| JLY074 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 cac2∷HPH bar1∷LEU2 yku70∷KAN | This study |
| JLY086 | MATa ade2-1 his3-11 leu2-3 can1-100 trp1-1 ura3-52 ade3∷pGAL-HO bar1∷LEU2 cac2∷KAN | This study |
| JLY087 | MATa ade2-101 leu2-3,112 pol30Δtrp1Δura3-52 cac2∷HPH | This study |
| NWY008 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 cac2∷HPH | This study |
| PY75 | MATa ade2-101 leu2-3,112 pol30Δtrp1Δura3-52 [pBL211 (POL30, URA3)] | Peter Burgers |
| RDY005 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 cac2∷KAN | This study |
| RDY006 | MATα ADE lys2 leu2-3,112 his3-11,15 trp1-1 ura3-1 asf1∷his5+cac2∷KAN | This study |
| RDY026 | MATα cac1∷LEU2 cac2∷KAN hmr∷ADE2 ade2-1 leu2-3 his3-11 trp1-1 ura3-1 can1-100 | This study |
| ROY1246 | MATα ADE2 lys2 leu2-3,112 his3-11,15 trp1-1 ura3-1Δasf1∷his5+ | Tyler (1999) |
| SH018 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 PGAL:CAC2-FLAG-PEST | Zabaronick et al. (2004) |
Strains . | Relevant genotype . | Source . |
|---|---|---|
| BOB852 | MATa his3Δ1 leu20 met50 ura3Δ0 | Bob Sclafani |
| BY4741-Y06502 | MATa his3Δ1 leu20 met50 ura3Δ0 cac2∷KAN | Research Genetics |
| JKT0004 | MATa rad52∷TRP1 trp1-1 ura3-1 can1-100 ADE bar1∷LEU2 his3-11 | Ramey et al. (2004) |
| JKT0010 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 | Ramey et al. (2004) |
| JLY030 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 cac1∷LEU2 | This study |
| JLY031 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 | This study |
| JLY060 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 CAC2:FLAG:KAN | This study |
| JLY063 | MATa ade2-1 his3-11 leu2-3 can1-100 trp1-1 ura3-52 ade3∷pGAL-HO bar1∷LEU2 | This study |
| JLY072 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 cac2∷HPH rad52∷TRP1 | This study |
| JLY073 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 yku70∷KAN | This study |
| JLY074 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 cac2∷HPH bar1∷LEU2 yku70∷KAN | This study |
| JLY086 | MATa ade2-1 his3-11 leu2-3 can1-100 trp1-1 ura3-52 ade3∷pGAL-HO bar1∷LEU2 cac2∷KAN | This study |
| JLY087 | MATa ade2-101 leu2-3,112 pol30Δtrp1Δura3-52 cac2∷HPH | This study |
| NWY008 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 cac2∷HPH | This study |
| PY75 | MATa ade2-101 leu2-3,112 pol30Δtrp1Δura3-52 [pBL211 (POL30, URA3)] | Peter Burgers |
| RDY005 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 cac2∷KAN | This study |
| RDY006 | MATα ADE lys2 leu2-3,112 his3-11,15 trp1-1 ura3-1 asf1∷his5+cac2∷KAN | This study |
| RDY026 | MATα cac1∷LEU2 cac2∷KAN hmr∷ADE2 ade2-1 leu2-3 his3-11 trp1-1 ura3-1 can1-100 | This study |
| ROY1246 | MATα ADE2 lys2 leu2-3,112 his3-11,15 trp1-1 ura3-1Δasf1∷his5+ | Tyler (1999) |
| SH018 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 PGAL:CAC2-FLAG-PEST | Zabaronick et al. (2004) |
Strains used in this study
Strains . | Relevant genotype . | Source . |
|---|---|---|
| BOB852 | MATa his3Δ1 leu20 met50 ura3Δ0 | Bob Sclafani |
| BY4741-Y06502 | MATa his3Δ1 leu20 met50 ura3Δ0 cac2∷KAN | Research Genetics |
| JKT0004 | MATa rad52∷TRP1 trp1-1 ura3-1 can1-100 ADE bar1∷LEU2 his3-11 | Ramey et al. (2004) |
| JKT0010 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 | Ramey et al. (2004) |
| JLY030 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 cac1∷LEU2 | This study |
| JLY031 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 | This study |
| JLY060 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 CAC2:FLAG:KAN | This study |
| JLY063 | MATa ade2-1 his3-11 leu2-3 can1-100 trp1-1 ura3-52 ade3∷pGAL-HO bar1∷LEU2 | This study |
| JLY072 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 cac2∷HPH rad52∷TRP1 | This study |
| JLY073 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 yku70∷KAN | This study |
| JLY074 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 cac2∷HPH bar1∷LEU2 yku70∷KAN | This study |
| JLY086 | MATa ade2-1 his3-11 leu2-3 can1-100 trp1-1 ura3-52 ade3∷pGAL-HO bar1∷LEU2 cac2∷KAN | This study |
| JLY087 | MATa ade2-101 leu2-3,112 pol30Δtrp1Δura3-52 cac2∷HPH | This study |
| NWY008 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 cac2∷HPH | This study |
| PY75 | MATa ade2-101 leu2-3,112 pol30Δtrp1Δura3-52 [pBL211 (POL30, URA3)] | Peter Burgers |
| RDY005 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 cac2∷KAN | This study |
| RDY006 | MATα ADE lys2 leu2-3,112 his3-11,15 trp1-1 ura3-1 asf1∷his5+cac2∷KAN | This study |
| RDY026 | MATα cac1∷LEU2 cac2∷KAN hmr∷ADE2 ade2-1 leu2-3 his3-11 trp1-1 ura3-1 can1-100 | This study |
| ROY1246 | MATα ADE2 lys2 leu2-3,112 his3-11,15 trp1-1 ura3-1Δasf1∷his5+ | Tyler (1999) |
| SH018 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 PGAL:CAC2-FLAG-PEST | Zabaronick et al. (2004) |
Strains . | Relevant genotype . | Source . |
|---|---|---|
| BOB852 | MATa his3Δ1 leu20 met50 ura3Δ0 | Bob Sclafani |
| BY4741-Y06502 | MATa his3Δ1 leu20 met50 ura3Δ0 cac2∷KAN | Research Genetics |
| JKT0004 | MATa rad52∷TRP1 trp1-1 ura3-1 can1-100 ADE bar1∷LEU2 his3-11 | Ramey et al. (2004) |
| JKT0010 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 | Ramey et al. (2004) |
| JLY030 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 cac1∷LEU2 | This study |
| JLY031 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 | This study |
| JLY060 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 CAC2:FLAG:KAN | This study |
| JLY063 | MATa ade2-1 his3-11 leu2-3 can1-100 trp1-1 ura3-52 ade3∷pGAL-HO bar1∷LEU2 | This study |
| JLY072 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 cac2∷HPH rad52∷TRP1 | This study |
| JLY073 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 yku70∷KAN | This study |
| JLY074 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 cac2∷HPH bar1∷LEU2 yku70∷KAN | This study |
| JLY086 | MATa ade2-1 his3-11 leu2-3 can1-100 trp1-1 ura3-52 ade3∷pGAL-HO bar1∷LEU2 cac2∷KAN | This study |
| JLY087 | MATa ade2-101 leu2-3,112 pol30Δtrp1Δura3-52 cac2∷HPH | This study |
| NWY008 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 cac2∷HPH | This study |
| PY75 | MATa ade2-101 leu2-3,112 pol30Δtrp1Δura3-52 [pBL211 (POL30, URA3)] | Peter Burgers |
| RDY005 | MATα ade2-1 leu2-3,112 his3-11 trp1-1 ura3-1 can1-100 cac2∷KAN | This study |
| RDY006 | MATα ADE lys2 leu2-3,112 his3-11,15 trp1-1 ura3-1 asf1∷his5+cac2∷KAN | This study |
| RDY026 | MATα cac1∷LEU2 cac2∷KAN hmr∷ADE2 ade2-1 leu2-3 his3-11 trp1-1 ura3-1 can1-100 | This study |
| ROY1246 | MATα ADE2 lys2 leu2-3,112 his3-11,15 trp1-1 ura3-1Δasf1∷his5+ | Tyler (1999) |
| SH018 | MATa his3-11 leu2-3,112 lys2 trp1-1 ura3-1 bar1∷LEU2 PGAL:CAC2-FLAG-PEST | Zabaronick et al. (2004) |
Plasmids:
Plasmid pGAL-ASF1 was created by inserting a PCR product carrying the ASF1 ORF into the TOPO-TA cloning vector pYES2.1 (Invitrogen, Carlsbad, CA). The POL30-containing plasmids were described previously (Ayyagari et al. 1995). Plasmid pGAL-HO carried the HO endonuclease ORF under the control of the GAL1 promoter (Haber 2000).
Serial dilution analysis:
Strains were grown overnight to midlog phase. Strains were diluted 10-fold serially from a starting concentration of 2 × 108 cells/ml. Cultures were spotted onto the indicated media using a frogging device and allowed to grow for 2 days in the dark at 30°. In experiments using galactose induction, strains were grown in media containing raffinose (YEPR or SC-URA+ Raffinose) as the sole carbon source for 4 hr prior to the start of the experiment.
HO repair viability assay:
Induction of HO in liquid culture was achieved by growing cells in SC-Ura+ 2% raffinose at 30° and adding galactose (final concentration of 2%). Samples were removed after 3 hr of galactose addition, sonicated briefly, and then counted on a hemocytometer to determine cell concentrations, followed by dilution and spreading of ∼400 cells onto SC-Ura+ 2% glucose to repress HO expression and determine the number of viable colonies. The plates were scored after 2 days of growth at 30°.
PCR analysis of mating-type switching:
Primers flanking the HO site in the MAT locus were used to determine mating type by PCR amplification as described previously (Ramey et al. 2004). Cultures were grown overnight in raffinose-containing medium. Galactose and glucose were added to 2% at the times indicated in the figure legends. Primers to the RAD27 gene were included in the multiplex PCR as an internal control for normalization. The number of PCR cycles for producing amplification in the linear range was determined empirically for each primer set. PCR products were resolved on a 2% agarose gel and stained with ethidium bromide. The ratio of the MAT products to the control product was quantified using Labworks (GelPro4.0, Media Cybernetics, LP).
Pulsed-field gel electrophoresis:
Methyl methanesulfonate (MMS) was added to cultures of logarithmically growing yeast to a final concentration of 0.07% for 30 min. Cells were pelleted and washed twice with YEPD and then allowed to recover in YEPD. Samples were taken prior to the addition of MMS, immediately following the MMS treatment, and then at the indicated times following removal of MMS from the media. Preparation of chromosome-sized DNA embedded in agarose was performed as previously described (Ramey et al. 2004). Electrophoresis was performed using a Bio-Rad CHEF-DR II pulsed-field electrophoresis system. Samples were resolved on a 0.5% agarose gel at 6 V/cm for 24 hr. The initial switch time was 60 sec and the final switch time was 120 sec. The gel was stained with ethidium bromide for 30 min and subsequently destained for 3 hr.
Microscopic analysis of cells:
Logarithmically growing cultures were spread onto YEPD and YEPD+ 2 μg/ml Zeocin plates and incubated at 30°. At the indicated times, cells were washed from the plate with water. For DAPI staining, cells were suspended in phosphate-buffered saline (PBS) with 2.5 μg/ml DAPI and incubated for 30 min prior to visualization. For viability staining (Molecular Probes, LIVE/DEAD yeast viability kit) the manufacturer's protocol was followed exactly. FUN1 was used at a 1:1000 dilution and Calcofluor White M2R was used at a 1:2000 dilution. Fields of cells were scored as either alive or dead. Dead cells were further classified by bud size: unbudded, small to medium, or large bud. Visualization was performed with an Eclipse E800 fluorescence microscope (Nikon, Garden City, NY), equipped with a CoolSnap HQ camera (Photometrics), and Meta-Morph analysis software.
RESULTS
CAF-1 yeast mutants are sensitive to double-strand DNA-damaging agents:
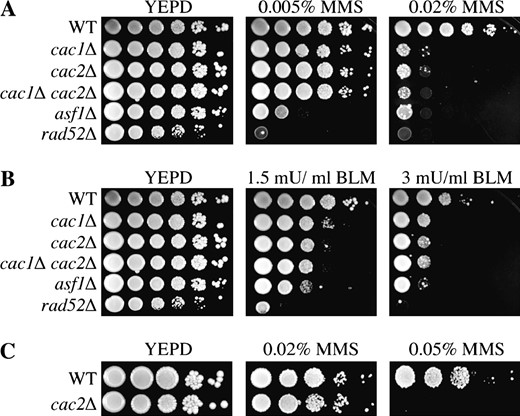
The Saccharomyces cerevisiae CAF-1 complex is encoded by the genes CAC1, CAC2, and CAC3 (chromatin assembly complex 1–3). Consistent with the lack of sensitivity of cac mutants to gamma irradiation (Kaufman et al. 1997), we previously reported that cac1 mutants are insensitive to double-strand DNA-damaging drugs (Tyler et al. 1999). However, we have since learned that the strain deleted for CAC1 (cac1Δ) used in our previous analyses contained a dominant suppressor mutation providing resistance to double-strand DNA-damaging agents (data not shown). Having crossed the suppressor out of our strains, we wanted to characterize the sensitivity of cac mutants to double-strand DNA-damaging agents. Using serial dilution analysis, we found that cac1Δ and cac2Δ strains were equally sensitive in a dose-dependent manner to the DNA-alkylating agent MMS, which results in double- and single-strand DNA damage (Schwartz 1989) (Figure 1A), and to the radiomimetic drug bleomycin (BLM), which generates double-strand DNA damage (Povirk 1996) (Figure 1B). Note that cac mutants are less sensitive to MMS and BLM than a rad52Δ strain, where the RAD52 gene is absolutely required for homologous recombination (Figure 1, A and B). Additionally, CAF-1 mutants are less sensitive than a strain deleted for another H3-H4 histone chaperone ASF1 (asf1Δ), which has also been shown to be sensitive to DSB-inducing agents (Tyler et al. 1999). Furthermore, the cac1Δ cac2Δ double mutant appears no more sensitive than either of the single cac mutants (Figure 1, A and B), suggesting that the sensitivity is due to the loss of function of the CAF-1 complex. We also observe hypersensitivity of cac mutants to double-strand DNA-damaging drugs in different strain backgrounds (Figure 1C). These results suggest that the chromatin assembly factor CAF-1 plays a role in cell survival following double-strand DNA repair.
CAF-1 mutants are sensitive to agents that induce double-strand DNA breaks. W303-1a strains JLY31 (WT), JLY030 (cac1Δ), RDY005 (cac2Δ), RDY026 (cac1Δ cac2Δ), ROY1246 (asf1Δ), and JKT0004 (rad52Δ) were tested for their ability to grow on media supplemented with (A) MMS and (B) BLM by 10-fold serial dilution analysis. (C) S288c strains BOB852 (WT) and BY4741-YML102WΔ (cac2Δ) were tested for their ability to grow on media supplemented with MMS by 10-fold serial dilution analysis.
CAF-1 and ASF1 have independent roles in providing resistance to double-strand DNA-damaging agents:
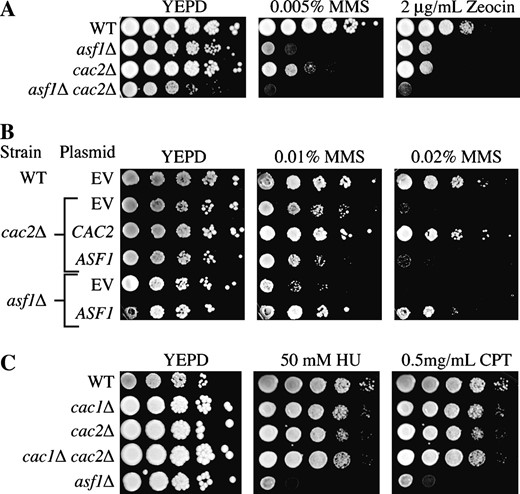
To further investigate the function of CAF-1 in double-strand DNA repair, we compared its disruption to mutants of another histone H3-H4 chaperone, ASF1, whose deletion results in a hypersensitivity to DSB-inducing agents (Singer et al. 1998; Tyler et al. 1999; Ramey et al. 2004). To determine whether ASF1 and CAF-1 function in the same genetic pathway to provide resistance to double-strand DNA-damaging agents, we deleted ASF1 with CAC2. We found that the asf1Δ cac2Δ double mutants were even more sensitive to MMS and Zeocin (a radiomimetic in the bleomycin family that generates double-strand DNA lesions) than either single mutant (Figure 2A). This result indicates that CAF-1 and ASF1 are playing distinct or partially overlapping roles in response to double-strand DNA damage.
CAF-1 and Asf1 have independent functions in maintaining viability in response to double-strand DNA damage. (A) Strains JLY31 (WT), ROY1246 (asf1Δ), RDY005 (cac2Δ), and RDY006 (asf1Δ cac2Δ) were tested for their ability to grow on media supplemented with MMS and Zeocin by 10-fold serial dilution analysis. (B) Strains JLY31 (WT), ROY1246 (asf1Δ), and RDY005 (cac2Δ) containing plasmids pYES2.1 (EV) and pYES2.1 with either the CAC2 or the ASF1 open-reading frame cloned downstream of the GAL1 promoter (CAC2 and ASF1, respectively) were grown overnight in SC −Ura supplemented with 2% galactose and then 10-fold diluted serially onto SC −Ura with 2% galactose and increasing concentrations of MMS. (C) Strains JLY31 (WT), JLY30 (cac1Δ), RDY005 (cac2Δ), RDY026 (cac1Δ cac2Δ), and ROY1246 (asf1Δ) were tested for their ability to grow on media supplemented with HU and CPT by 10-fold serial dilution analysis.
To further analyze the relationship between CAF-1 and ASF1 in providing resistance to double-strand DNA damage, we tested whether overexpression of ASF1 could rescue the DNA damage sensitivity of a CAF-1 mutant. While the overexpression of ASF1 was capable of rescuing a strain deleted for ASF1, it did not alleviate the sensitivity of the cac2Δ mutant (Figure 2B). This result indicates that ASF1 and the CAF-1 complex provide resistance to double-strand DNA damage via distinct mechanisms.
Given CAF-1's proposed role in the assembly of chromatin during DNA replication, we investigated whether cac mutants were sensitive to replicational stress. To do this, we tested sensitivity to hydroxyurea (HU) and camptothecin (CPT). Hydroxyurea is a ribonucleotide reductase inhibitor that acts to block the production of dNTPs, ultimately leading to the stalling of replication forks (Slater 1973). Camptothecin inhibits the religation activity of topoisomerase-I leading to the formation of double-strand DNA breaks specifically during DNA replication (Hryciw et al. 2002). Interestingly, while asf1Δ strains were sensitive to both camptothecin and hydroxyurea, neither cac1Δ nor cac2Δ strains showed any sensitivity to these drugs relative to an isogenic wild-type strain (Figure 2C). Even at higher doses of hydroxyurea and camptothecin, neither cac1Δ nor cac2Δ strains showed enhanced sensitivity when compared to the wild-type strain (data not shown). This result indicates that the sensitivity of CAF-1 mutants to DNA-damaging agents may be directly related to a role for CAF-1 in double-strand DNA repair, as opposed to a role during DNA replication.
CAF-1 is required for viability following double-strand DNA repair via both homologous recombination and NHEJ:
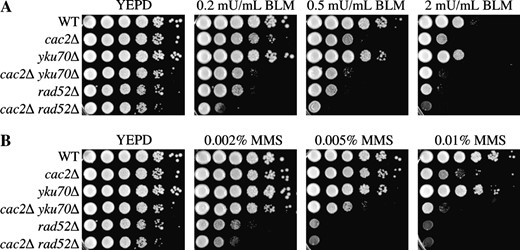
We wanted to ascertain whether CAF-1 was involved in DSB repair by homologous recombination or NHEJ. To do this, we deleted RAD52 or YKU70, genes essential for homologous recombination and NHEJ, respectively (Dudas and Chovanec 2004; Dudasova et al. 2004), in strains previously deleted for cac2Δ. We found that the rad52Δ cac2Δ double-mutant strain exhibits more sensitivity to bleomycin and MMS than either the rad52Δ or the cac2Δ single-mutant strains (Figure 3, A and B). Since RAD52 is absolutely required for homologous recombination, this additive effect of the cac2Δ mutation indicates that CAF-1's role in providing resistance to DSBs does not operate entirely through the homologous recombination pathway. Similarly, the yku70Δ cac2Δ double mutant showed more sensitivity than either of the single-mutant counterparts (Figure 3), suggesting that CAF-1's role in DSB repair does not reside entirely within the NHEJ pathway. These results indicate that CAF-1 contributes to cell viability following double-strand DNA damage via both the homologous recombination and NHEJ pathways.
CAF-1 contributes to both homologous recombination and NHEJ. Strains JKT0010 (WT), NWY008 (cac2Δ), JKT0004 (rad52Δ), JLY072 (cac2Δ rad52Δ), JLY073 (yku70Δ), and JLY074 (cac2Δ yku70Δ) were tested for their ability to grow on media supplemented with (A) bleomycin or (B) MMS by 10-fold serial dilution analysis.
To gain further insight into the role of CAF-1 in homologous recombination, we sought to inflict double-strand DNA damage that would predominantly be repaired by homologous recombination. To generate a double-strand break that is repaired by homologous recombination, we induced the HO endonuclease under control of the GAL1 promoter. The HO endonuclease creates a unique site-specific DSB in the MAT locus that is repaired through homologous recombination 98% of the time (Lee et al. 1999) using either the HMR or the HML locus as the donor template. After a 3-hr induction of HO endonuclease, we inhibited expression of the endonuclease to allow repair to occur and then tested viability. We found that viability was reduced to 76% (±2.7 SD) for wild-type cells, 50% (±9.1 SD) for the cac2Δ strain, and 8% (±1.7 SD) for the rad52Δ strain. This result indicates that CAF-1 contributes to viability during repair of the HO lesion via the homologous recombination pathway. Taken together, our data support a role for CAF-1 in both the homologous recombination and NHEJ pathways of DSB repair.
CAF-1 mutants show no apparent defect in repair of double-strand DNA breaks:
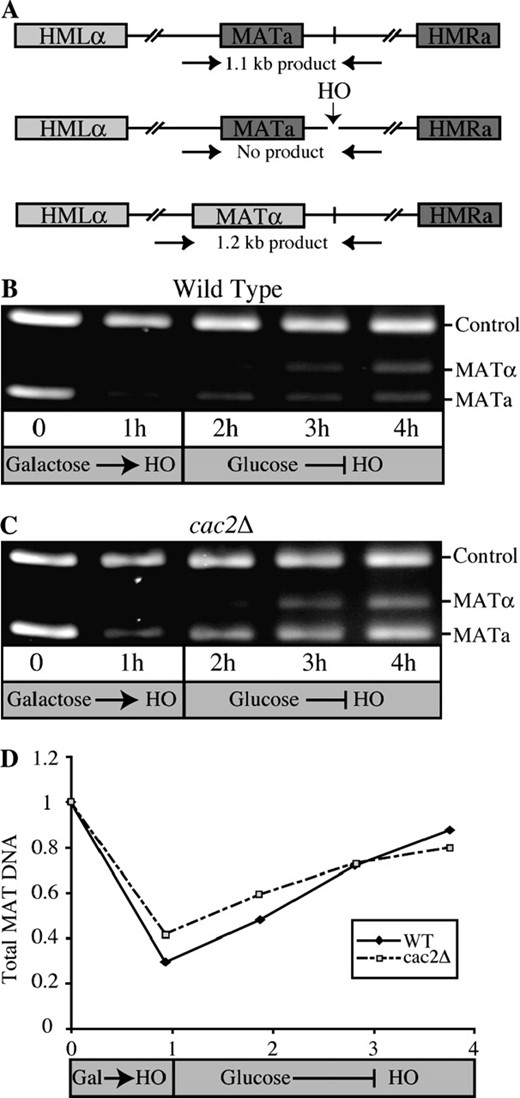
To determine whether the decreased ability of CAF-1 mutants to survive a double-strand break resulted from a reduced ability to repair the DNA, we measured repair of a DSB. Repair of the HO lesion was quantified using PCR amplification of genomic DNA with primers flanking the HO recognition site as indicated in Figure 4A. The HO lesion was generated by adding galactose to induce the HO endonuclease at time 0, followed by the addition of glucose after 1 hr to repress HO transcription and to allow homologous recombination to occur. Cutting and repair was evident in both the wild-type and cac2Δ strain by quantifying the amount of MAT product at each time point, normalized to the internal PCR control (Figure 4, B–D). Furthermore, the kinetics and efficiency of repair were not significantly different between the wild-type and cac2Δ strain in four independent experiments (Figure 4D). This result indicates that while CAF-1 mutants are sensitive to the double-strand break induced by the HO endonuclease, this sensitivity does not arise from the failure to repair the DNA lesion per se.
CAF-1 mutants are proficient in the repair of a single double-strand break. (A) Schematic of the PCR-based system for monitoring the repair of the HO lesion. Primers flanking the HO site in the MAT locus yield the indicated PCR products in MATa, MATα, and HO cut yeast. (B) Gel electrophoresis analysis of HO cutting and repair in wild-type yeast. PCR products were obtained from strain JLY063 (wild type) at the indicated times after induction of the HO endonuclease by galactose addition or repression of the HO endonuclease by glucose addition. An ethidium bromide-stained 2% agarose gel is shown. (C) Gel electrophoresis analysis of HO cutting and repair in CAF-1 mutant yeast. The analysis shown in B was repeated using strain JLY086 (cac2Δ). (D) Quantification of HO cutting and repair in wild-type and CAF-1 mutant yeast. The ratio of both of the MAT products to the control product from the data in B and C was quantified using Labworks (GelPro4.0, Media Cybernetics, LP) and was normalized to 1 at T = 0.
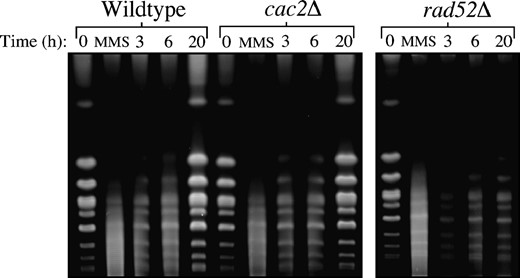
Since the repair of the HO lesion is a unique and specialized process in the cell, we wanted to confirm that CAF-1 mutants were capable of repairing gross chromosomal damage incurred by the DNA-damaging agent MMS. We used pulsed-field gel electrophoresis to monitor MMS-induced DSBs and the subsequent repair in wild-type, cac2Δ, and rad52Δ strains (Figure 5) (Ramey et al. 2004). In brief, logarithmically growing cultures were exposed to MMS for 30 min and then were allowed to recover for up to 20 hr. Upon MMS treatment, the bands representing intact chromosomes disappear and are replaced by a lower-molecular-weight smear, indicating that double-strand DNA damage has occurred. During the 20-hr recovery, full-length chromosomes reappear at a similar rate in both wild-type and cac2Δ strains indicating that cac2Δ strain has no defect in the repair of gross chromosomal double-strand DNA damage. In contrast, the rate of repair in the rad52Δ strain is severely impeded, most notably at the latest time point (Figure 5). Taken together, these results indicate that CAF-1 is not required for repair of the DNA lesion per se.
CAF-1 mutants have no defect in the repair of gross chromosomal DNA damage as measured by pulsed-field gel electrophoresis. Strains JKT0010 (wild type), NWY008 (cac2Δ), and JKT0004 (rad52Δ) were grown to midlog phase, and then MMS was added to final concentration of 0.07% for 30 min. Following MMS treatment, cells were washed and resuspended in YEPD and allowed to recover for up to 20 hr with samples for pulsed-field gel electrophoresis taken at the indicated time points.
CAF-1 mutants are sensitive to transient exposure to double-strand DNA-damaging agents:
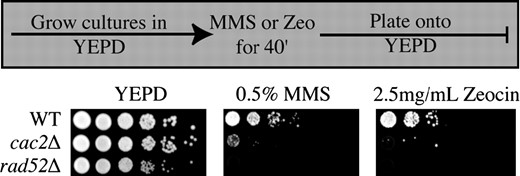
One possibility for explaining the fact that CAF-1 mutants can repair their DNA damage, yet die, is that they may have delayed recovery from the DNA damage checkpoint (Vaze et al. 2002). In this case, CAF-1 mutants would be sensitive to prolonged exposure to DNA-damaging agents, but not to transient exposure. In support of this idea, CAF-1 mutants are not sensitive to gamma-irradiation, a transient source of DNA damage (Kaufman et al. 1997). To test further whether CAF-1 mutants are sensitive to transient exposure to DNA-damaging agents, we exposed cac2Δ cultures to MMS or Zeocin, followed by monitoring viability on YEPD plates (Figure 6). We found that cac2Δ mutants are indeed sensitive to transient exposure to MMS and Zeocin, as compared to their isogenic wild-type controls. Furthermore, analysis of the DNA content of cultures of cac2Δ yeast exposed transiently to MMS indicated that the yeast accumulate with a G2/M content (due to activation of the DNA damage checkpoint), and following removal of MMS, the cac2Δ yeast re-entered the cell cycle with the same kinetics as wild-type yeast (supplemental Figure 1 at http://www.genetics.org/supplemental/). Taken together, these data indicate that the sensitivity of CAF-1 mutant yeast to double-strand DNA-damaging agents is not due to failure to arrest or to a delayed exit from the DNA damage cell-cycle checkpoint.
CAF-1 mutants are sensitive to transient exposure to double-strand DNA-damaging agents. A total of 0.5% MMS final or 2.5 mg/ml Zeocin was added to cultures of strains JKT0010 (WT), NWY008 (cac2Δ), and JKT0004 (rad52Δ) and then washed out and plated in 10-fold serial dilutions onto YEPD.
CAF-1 mutants die at metaphase of the cell cycle following exposure to double-strand DNA-damaging agents:
To gain further insight into why CAF-1 mutants die following exposure to double-strand DNA-damaging agents, we examined the terminal morphology of cac2Δ yeast upon treatment with Zeocin. Using a concentration of Zeocin that had little effect on the viability of wild-type cells, we found that in contrast to wild-type cells, most of the cac2Δ cells arrested as large budded cells with a single mass of DNA at the bud neck within 9 hr (Figure 7). Furthermore, examining wild-type and cac2Δ cells by viability staining (Molecular Probes LIVE/DEAD yeast viability kit) revealed that while approximately half of the wild-type cells that died were large budded, virtually all of the dead cac2Δ cells were large budded (Figure 7). This result suggests that the loss of CAF-1 results in arrest and subsequent cell death in metaphase following exposure to double-strand DNA-damaging agents.
CAF-1 mutants arrest and die in metaphase of the cell cycle in response to double-strand DNA damage. Strains JKT0010 (wild type) and NWY0008 (cac2Δ) were plated onto YEPD and Zeocin and photographed after 0 (YEPD), 9, or 24 hr on Zeocin. Typical bright field images are shown, and the boxes represent the area zoomed in for the DAPI staining shown below. All images were taken using a ×100 objective. The total number of dead cells in each culture, and the distribution of those dead cells by bud size, is given below the appropriate images.
CAF-1 functions after DNA damage exposure to provide resistance to DSBs:
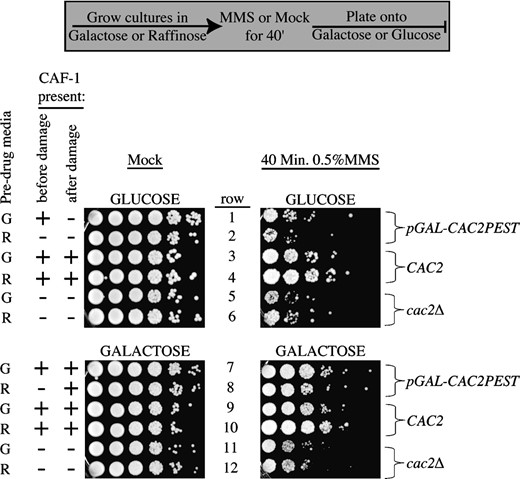
One possible reason why CAF-1 mutants could be sensitive to double-strand DNA damage is that their overly open chromatin structure (Adkins and Tyler 2004) could render the DNA more accessible to DNA-damaging agents, resulting in more DNA lesions. To test this idea, we asked whether CAF-1 had to be present before and/or after DNA damage to provide resistance to double-strand DNA-damaging agents. We utilized a destabilized form of the Cac2 protein expressed from the GAL1 promoter that has a PEST degradation domain fused to its C terminus (Zabaronick and Tyler 2005). Induction of the Cac2-PEST protein to the same levels as the endogenous Cac2 protein generates a phenotypically normal yeast strain and enables rapid degradation of Cac2-PEST upon transcriptional repression (by addition of glucose) (Zabaronick and Tyler 2005). We grew cultures of the wild-type, cac2Δ, and pGAL CAC2-PEST strains in raffinose or galactose, followed by a 40-min exposure to MMS or mock treated, and then plated onto glucose or galactose containing plates to examine viability. When grown in raffinose, no Cac2-PEST protein was detectable and the yeast was phenotypically identical to cac2Δ yeast (data not shown). We found that the presence of Cac2-PEST only before the MMS treatment (Figure 8, row 1) did not provide significant resistance to double-strand DNA-damaging agents (Figure 8). This result indicates that the CAF-1-assembled chromatin structure does not protect against increased numbers of DNA lesions being generated. By contrast, expressing Cac2-PEST only after the MMS treatment (Figure 8, row 8) was sufficient to provide resistance to MMS (Figure 8). This result indicates that the function of CAF-1 that provides resistance to double-strand DNA-damaging agents is mediated during and/or after DNA repair.
CAF-1 is required only after DNA damage to provide resistance. Strains SH018 (pGAL-CAC2PEST), JLY060 (CAC2), and NWY008 (cac2Δ) were grown overnight in rich media supplemented with either 2% raffinose (R) or 2% galactose (G) to midlog phase. Cultures were either supplemented with 0.5% MMS or mock treated for 40 min. Cells were spun down and washed three times in water before plating 10-fold serial dilutions onto plates containing glucose or galactose.
CAF-1-mediated resistance to double-strand DNA-damaging agents is dependent on the ability of CAF-1 to bind PCNA:
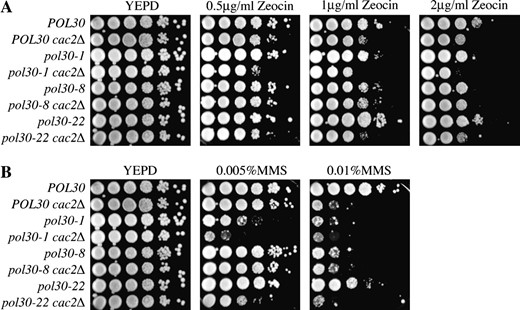
Given that CAF-1 is recruited to assemble newly replicated DNA into chromatin via its interaction with PCNA, we asked whether CAF-1's interaction with PCNA was important for resistance to double-strand DNA-damaging agents. PCNA is required for both homologous recombination and NHEJ (Balajee and Geard 2001). If the reason that CAF-1 mutants are hypersensitive to DSBs is due to a direct role in the repair process, and this role is mediated by an interaction through PCNA, then mutations of PCNA that specifically disrupt the CAF-1-PCNA interaction should result in the same degree of drug sensitivity as a CAF-1 mutant. One allele of PCNA (pol30-8; R61A, D63A) exhibits a severely reduced capacity to bind to CAF-1 (Zhang et al. 2000). Importantly, this mutation is far removed from regions known to interact with other replication proteins and the PCNA trimer interface domains, and this mutant PCNA is capable of binding Polδ, Polϵ, and the RFC clamp-loader (Ayyagari et al. 1995). We also tested two PCNA alleles, pol30-1 (E3A, K5A) and pol30-22 (D256A, E257A), which retain their ability to interact with CAF-1 yet fail to bind to other proteins involved in DNA synthesis (Ayyagari et al. 1995; Zhang et al. 2000). Using yeast expressing either the wild type or mutant PCNA as the sole copy of PCNA, we found that the pol30-8 and pol30-1 yeast were sensitive to a similar extent as cac2Δ to double-strand DNA-damaging agents, while the pol30-22 yeast were not sensitive (Figure 9). However, upon analysis of double mutants of cac2Δ and the PCNA alleles, the cac2Δ pol30-8 strain was equally sensitive as both the cac2Δ and the pol30-8 mutations alone (Figure 9). This result indicates that the sensitivity of the pol30-8 mutant to double-strand DNA-damaging agents is due to the inability of the mutant PCNA to bind the CAF-1 complex. This result was specific to the pol30-8 allele that disrupts the interaction with CAF-1, because deletion of cac2 in strains with the pol30-22 and pol30-1 alleles resulted in increased sensitivity to double-strand DNA damage as compared to the single mutations (Figure 9). These experiments suggest that the function of CAF-1 in providing resistance to double-strand DNA-damaging agents requires PCNA-mediated recruitment of CAF-1 to the sites of DNA synthesis.
CAF-1's interaction with PCNA is required to resist the lethality of double-strand DNA damage. Isogenic PY75 (pol30Δ) and JLY087 (pol30Δ cac2Δ) strains carrying plasmids POL30, pol30-1, pol30-8, and pol30-22 as indicated were tested by serial dilution analysis for their ability to grow at the nonpermissive temperature on media supplemented with (A) Zeocin and (B) MMS.
DISCUSSION
We have shown for the first time that the function of CAF-1 is important for viability following double-strand DNA repair. Furthermore, CAF-1 functions genetically in both homologous recombination and NHEJ, and its function during double-strand DNA repair is dependent on the interaction between CAF-1 and PCNA. Given the biochemical role of CAF-1 in DNA synthesis-coupled chromatin assembly, we propose that CAF-1 mediates chromatin assembly during double-strand DNA repair in vivo.
We have observed sensitivity of CAF-1 mutants to a variety of double-strand DNA-damaging agents. A systematic analysis of the yeast deletion collection also isolated the cac2Δ as a mutant sensitive to MMS (Chang et al. 2002). Similarly, mutants of the genes encoding Arabidopsis CAF-1, fas1, and fas2 are sensitive to MMS (Takeda et al. 2004). Given the role of CAF-1 in chromatin assembly during NER of single-strand lesions and the sensitivity of CAF-1 mutants to UV irradiation (Gaillard et al. 1996; Kaufman et al. 1997; Martini et al. 1998; Green and Almouzni 2003), it was important to establish that the sensitivity of CAF-1 mutants to MMS, BLM, and Zeocin was not due to the single-strand DNA lesions that these drugs are known to generate in addition to double-strand lesions. As such, we found that CAF-1 mutants are also sensitive to induction of the HO endonuclease that generate a single double-strand DNA lesion (Figure 3), showing that CAF-1 mutants are indeed sensitive to double-strand DNA breaks. Furthermore, deletion of CAF-1 leads to an increase in gross chromosomal rearrangements, which result from double-strand DNA lesions (Myung et al. 2003).
Mutants of the genes encoding the CAF-1 complex have been reported to show no sensitivity to gamma irradiation (Kaufman et al. 1997). In agreement, we have been unable to find a dose of gamma irradiation high enough to affect viability of CAF-1 mutants (data not shown). This might reflect a fundamental difference in the genomic toxicity of gamma irradiation vs. MMS, BLM, Zeocin, and the HO endonuclease to which we find cac mutants to be sensitive here. Alternatively, due to the resistance of the yeast cell wall to gamma irradiation, it may not be possible to generate gamma irradiation at a rate high enough to reach the threshold above which CAF-1 mutants may show sensitivity. It is noteworthy that the amounts of MMS and Zeocin that we needed to use to observe sensitivity of CAF-1 mutants to transient DNA damage were quite substantial (Figure 6).
Somewhat surprisingly, CAF-1 is implicated not only in homologous recombination, but also in NHEJ. This is surprising because NHEJ does not involve significant DNA synthesis and the chromatin assembly function of CAF-1 is typically coupled with DNA synthesis. However, it is entirely possible that nucleosomes may be removed to enable access of the repair machinery during NHEJ and therefore would need to be replaced after NHEJ, and CAF-1 may mediate one or both of these processes. Given our genetic results, it is likely that PCNA is recruiting CAF-1 to sites of double-strand DNA repair. PCNA is required for both homologous recombination and NHEJ (Holmes and Haber 1999; Pospiech et al. 2001) and is known to recruit CAF-1 to sites of ongoing DNA synthesis during NER (Gaillard et al. 1996; Martini et al. 1998; Moggs et al. 2000; Green and Almouzni 2003) and replication (Shibahara and Stillman 1999; Zhang et al. 2000). Although we have not been technically able to localize CAF-1 to the vicinity of sites of DNA repair by chromatin immunoprecipitation analysis, a control experiment in a previous study has in fact shown that CAF-1 is recruited to DNA with double-strand breaks in vitro (Mello et al. 2002). Furthermore, CAF-1 has been shown to bind to the RecQ family helicase BLM and to colocalize with BLM to foci coinciding with sites of DNA synthesis following treatment with the alkylating agent MNNG (Jiao et al. 2004). As such, all the available evidence indicates that CAF-1 localizes to sites of double-strand DNA repair.
In contrast to the situation in human cells, the function of yeast CAF-1 during replication-coupled chromatin assembly does not appear to protect cells from genomic instability. A dominant negative form of CAF-1 leads to increased levels of endogenous DNA lesions and activation of the DNA damage checkpoint (Ye et al. 2003). However, yeast lacking CAF-1 have no cell-cycle defect and do not activate their DNA damage checkpoint at higher levels than wild-type yeast in response to endogenous DNA damage that occurs during DNA replication. In support of this, CAF-1 mutants are not sensitive to agents that induce DNA replicational stress and double-strand DNA breaks during DNA replication (Figure 2). This is in marked contrast to mutants of another histone H3–H4 chaperone, ASF1, which show increased genomic instability resulting from problems during DNA replication (Prado et al. 2004; Ramey et al. 2004).
That cells lacking CAF-1 die upon exposure to UV (Kaufman et al. 1997) or double-strand DNA-damaging agents is unexpected, because one would not predict that the inability to assemble chromatin structure after the completion of DNA repair would affect the efficiency of DNA repair and, hence, viability. Indeed, we find that CAF-1 mutants can repair a DSB as efficiently as wild-type yeast. This leads us to speculate that the inviability of CAF-1 mutants in response to DNA-damaging agents may reflect the importance of restoring the local chromatin structure following the repair of the DNA lesion. One possibility is that following repair of the DNA, restoration of the local chromatin structure is needed to facilitate cell-cycle checkpoint release. However, we find that CAF-1 mutants are fully competent in activating and inactivating their DNA damage checkpoint in response to DNA damage and after DNA repair, respectively. Another possibility is that the open chromatin structure in CAF-1 mutants (Adkins and Tyler 2004) leads to nonequivalent doses of damaging agents resulting in more DNA lesions in a CAF-1 mutant as compared to a wild-type strain. This does not appear to be the case, because induction of CAF-1 after exposure to DNA-damaging agents is sufficient to restore resistance to double-strand DNA-damaging agents (Figure 8). Alternatively, when cells are exposed to elevated levels of DNA damage, the lack of proper chromatin assembly following double-strand repair may leave the chromatin in an even higher state of disarray, leaving the cell sensitized to further DNA damage.
Given the sensitivity of CAF-1 mutants to DNA-damaging agents, and given CAF-1's role in chromatin assembly during DNA replication, it was somewhat surprising that CAF-1 mutants were not sensitive to replicational stress-inducing agents such as hydroxyurea and camptothecin (Figure 2). The chromatin assembly activity of CAF-1 during double-strand DNA repair is likely to involve, at most, kilobases of DNA, while its chromatin assembly activity during DNA replication would involve entire chromosomes. Therefore, if inefficient chromatin assembly following DSB repair is leading to cell death, one would expect that the failure to assemble chromatin would be at least as, if not more, deleterious to cell survival during DNA replication than during DSB repair. One possibility that may explain this paradox is that during DNA replication redundant factors that are able to compensate for the lack of chromatin assembly activity in CAF-1 mutants exist. However, in the absence of CAF-1-mediated chromatin assembly during DSB repair, cells may not employ additional chromatin assembly factors, such that the disruption of the CAF-1 complex may be more difficult to compensate for in DSB repair than during DNA replication. Future studies will be required to determine why CAF-1-mediated chromatin assembly is important for viability following double-strand and single-strand DNA repair.
Footnotes
Communicating editor: J. Tamkun
Acknowledgement
The authors thank Melissa Adkins, Rachel Alvestad, Christine English, Paul Megee, Josh Ramey, Laura Schulz, and Beth Tamburini for critical reading of this manuscript and Peter Burgers for the generous gift of PCNA alleles. This work was supported by a grant from the National Institutes of Health (CA95641) to J.K.T. J.K.T. is a Leukemia & Lymphoma Society scholar.
References
Adkins, M. W., S. R. Howar and J. K. Tyler,
Adkins, M. W., and J. K. Tyler,
Ayyagari, R., K. J. Impellizzeri, B. L. Yoder, S. L. Gary and P. M. Burgers,
Balajee, A. S., and C. R. Geard,
Bennett, C. B., A. L. Lewis, K. K. Baldwin and M. A. Resnick,
Chang, M., M. Bellaoui, C. Boone and G. W. Brown,
Dudas, A., and M. Chovanec,
Dudasova, Z., A. Dudas and M. Chovanec,
Gaillard, P. H., E. M. Martini, P. D. Kaufman, B. Stillman, E. Moustacchi et al.,
Green, C. M., and G. Almouzni,
Haber, J. E.,
Hoek, M., and B. Stillman,
Holmes, A. M., and J. E. Haber,
Hryciw, T., M. Tang, T. Fontanie and W. Xiao,
Jiao, R., C. Z. Bachrati, G. Pedrazzi, P. Kuster, M. Petkovic et al.,
Kaufman, P. D., R. Kobayashi, N. Kessler and B. Stillman,
Kaufman, P. D., R. Kobayashi and B. Stillman,
Krude, T.,
Krude, T.,
Lee, S. E., F. Paques, J. Sylvan and J. E. Haber,
Longtine, M. S., A. McKenzie, 3rd, D. J. Demarini, N. G. Shah, A. Wach et al.,
Luger, K., A. W. Mader, R. K. Richmond, D. F. Sargent and T. J. Richmond,
Martini, E., D. M. Roche, K. Marheineke, A. Verreault and G. Almouzni,
Mello, J. A., H. H. Sillje, D. M. Roche, D. B. Kirschner, E. A. Nigg et al.,
Moggs, J. G., P. Grandi, J. P. Quivy, Z. O. Jonsson, U. Hubscher et al.,
Myung, K., V. Pennaneach, E. S. Kats and R. D. Kolodner,
Nabatiyan, A., and T. Krude,
Pospiech, H., A. K. Rytkonen and J. E. Syvaoja,
Povirk, L. F.,
Prado, F., F. Cortes-Ledesma and A. Aguilera,
Prakash, S., and L. Prakash,
Ramey, C. J., S. Howar, M. Adkins, J. Linger, J. Spicer et al.,
Schwartz, J. L.,
Shibahara, K., and B. Stillman,
Singer, M. S., A. Kahana, A. J. Wolf, L. L. Meisinger, S. E. Peterson et al.,
Slater, M. L.,
Smith, S., and B. Stillman,
Takeda, S., Z. Tadele, I. Hofmann, A. V. Probst, K. J. Angelis et al.,
Thomas, B. J., and R. Rothstein,
Tyler, J. K., M. Bulger, R. T. Kamakaka, R. Kobayashi and J. T. Kadonaga,
Tyler, J. K., C. R. Adams, S. R. Chen, R. Kobayashi, R. T. Kamakaka et al.,
Tyler, J. K., K. A. Collins, J. Prasad-Sinha, E. Amiott, M. Bulger et al.,
Vaze, M. B., A. Pellicioli, S. E. Lee, G. Ira, G. Liberi et al.,
Verreault, A., P. D. Kaufman, R. Kobayashi and B. Stillman,
Ye, X., A. A. Franco, H. Santos, D. M. Nelson, P. D. Kaufman et al.,
Zabaronick, S. R., and J. K. Tyler,
Zhang, Z., K. Shibahara and B. Stillman,