-
PDF
- Split View
-
Views
-
Cite
Cite
Kristin L M Boylan, Thomas S Hays, The Gene for the Intermediate Chain Subunit of Cytoplasmic Dynein Is Essential in Drosophila, Genetics, Volume 162, Issue 3, 1 November 2002, Pages 1211–1220, https://doi.org/10.1093/genetics/162.3.1211
Close - Share Icon Share
Abstract
The microtubule motor cytoplasmic dynein powers a variety of intracellular transport events that are essential for cellular and developmental processes. A current hypothesis is that the accessory subunits of the dynein complex are important for the specialization of cytoplasmic dynein function. In a genetic approach to understanding the range of dynein functions and the contribution of the different subunits to dynein motor function and regulation, we have identified mutations in the gene for the cytoplasmic dynein intermediate chain, Dic19C. We used a functional Dic transgene in a genetic screen to recover X-linked lethal mutations that require this transgene for viability. Three Dic mutations were identified and characterized. All three Dic alleles result in larval lethality, demonstrating that the intermediate chain serves an essential function in Drosophila. Like a deficiency that removes Dic19C, the Dic mutations dominantly enhance the rough eye phenotype of Glued1, a dominant mutation in the gene for the p150 subunit of the dynactin complex, a dynein activator. Additionally, we used complementation analysis to identify an existing mutation, shortwing (sw), as an allele of the dynein intermediate chain gene. Unlike the Dic alleles isolated de novo, shortwing is homozygous viable and exhibits recessive and temperature-sensitive defects in eye and wing development. These phenotypes are rescued by the wild-type Dic transgene, indicating that shortwing is a viable allele of the dynein intermediate chain gene and revealing a novel role for dynein function during wing development.
CYTOPLASMIC dynein is a minus-end-directed microtubule motor involved in numerous intracellular motility events including retrograde axonal transport, the transport and positioning of vesicles and organelles, spindle assembly and morphogenesis, and nuclear migration. The dynein motor is a large complex composed of two heavy chain polypeptides and numerous intermediate and light chain subunits. The heavy chains compose the ATPase portion of the molecule, providing energy for movement along microtubules through the binding and hydrolysis of ATP (reviewed by Holzbaur and Vallee 1994). Electron microscopy analysis has shown that the heavy chains form two globular heads connected by thin stalks. The intermediate and light chain subunits are present as a complex at the base of the heavy chain stalk where they are in a position to interact with other cellular components and may participate in targeting the motor to specific cargoes (Vallee et al. 1988; King and Witman 1990; Steffen et al. 1996).
A role for the intermediate chain (IC) subunit in the attachment of dynein to cargo was first suggested by structural analysis of axonemal outer arm dynein. In the flagellar axoneme, dynein motor activity drives the sliding of adjacent outer doublet microtubules. As the heavy chain motor subunit moves along one outer doublet, the base of the motor complex remains attached to the adjacent outer doublet. Thus the transported cargo for axonemal dynein is another doublet microtubule attached through the base of the motor complex. Chemical crosslinking studies show that attachment through the base is mediated by direct binding of the intermediate chain subunit and α-tubulin within the A-tubule lattice of the outer doublet microtubule (King et al. 1991).
The homology between axonemal and cytoplasmic dynein intermediate chains has suggested a similar cargo-binding function for the IC subunit of cytoplasmic dynein (Paschal et al. 1992). Subsequently, in vitro binding of the cytoplasmic dynein intermediate chain to the p150-Glued subunit of dynactin was demonstrated in rat brain extracts (Karki and Holzbaur 1995; Vaughan and Vallee 1995). Dynactin, initially identified because of its ability to stimulate dynein-mediated vesicle motility (Schroer and Sheetz 1991), may act to couple dynein to cellular cargoes (reviewed by Karki and Holzbaur 1999). The interaction between the dynein intermediate chain and p150-Glued and the association of the Arp1 subunit of dynactin with the membrane skeleton component spectrin (Holleran et al. 1996) have suggested a model in which dynactin serves as a cargo adapter molecule for dynein attachment to vesicular cargo. Additional studies showing that dynactin function is required during mitosis present the possibility that dynactin may also serve as a cargo adapter for dynein during cell division (Echeverri et al. 1996). Alternatively, the interaction of dynein IC and p150-Glued may affect motor processivity (King and Schroer 2000).
The diversity of cytoplasmic dynein heavy chains is limited, but the multiplicity of accessory subunits is proposed to modulate specific dynein functions. Evidence for the assembly of functionally different dynein complexes has been demonstrated for the dynein light intermediate chain (LIC) and light chain subunits (Tai et al. 1998, 2001; Tynan et al. 2000; Chuang et al. 2001). Two LIC genes have been identified in rat: one that binds to pericentrin and one that does not. In triple overexpression studies, Tynan et al. (2000) showed that the dynein heavy chain could bind to either LIC1 or LIC2, but not to both. Multiple alternatively spliced isoforms of the dynein intermediate chain have been identified (Vaughan and Vallee 1995; Pfister et al. 1996; Nurminsky et al. 1998) and it has been suggested that this isoform diversity contributes to functional specificity, perhaps by the formation of distinct dynein complexes with specific intermediate chain isoforms. Recent reports have also implicated dynein light chain subunits in binding directly to specific cargoes, suggesting that the dynein intermediate chain may act indirectly to modulate cargo attachment by association with specific light chain subunits. For example, the 14-kD light chain was found to bind rhodopsin in the rod cells of the vertebrate retina and may function in turnover of photoreceptor membrane (Tai et al. 1999). Despite these leads, the functional analysis of how accessory subunits might contribute to specifying dynein functions is limited.
As part of a systematic approach to understanding the functions of the intermediate chain subunit in the attachment of dynein to specific cargoes, we have previously cloned and characterized the gene Dic19C from Drosophila (Boylan et al. 2000). We showed that, similar to the dynein heavy chain, the dynein intermediate chain is present as a single gene that is expressed throughout Drosophila development. In addition, we found evidence for a dosage-sensitive interaction between the intermediate chain gene and a mutation in the p150/Glued subunit of dynactin. In this report, we use the Dic transgene in a screen to identify mutations in the dynein intermediate chain gene and investigate functions of the dynein intermediate chain during Drosophila development through analysis of the mutant phenotypes, both alone and in combination with the mutant Glued1.
MATERIALS AND METHODS
Fly stocks: Transformation and mutagenesis experiments were performed using the stock Df (1)w67c23, which carries the markers yellow (y) body and white (w) eyes (Lefevre and Green 1972). The construction and characterization of the Dic transgene P(Dic+) is described in Boylan et al. (2000). Briefly, the complete dynein intermediate chain transcription unit with the endogenous promoter was assembled from genomic cosmids and subcloned into the transformation vector pCaSpeR4 (Klemenz et al. 1987). Germline transformants were obtained, and the Dic transgene was determined to be functional by its ability to suppress the rough eye phenotype of the dominant mutation Glued1 (Boylan et al. 2000). The sw1 stock was obtained from the Bloomington Stock Center, as was a stock with the X-linked deficiency Df(1)mal3 (breakpoints 19A1-2; 20E1-F). Df(1)mal3 is maintained in males with the duplication Dp (1;Y)mal106 (breakpoints 1A1; B2 and 18F; 20F4). To facilitate identification of the transgene in rescue experiments, a y w sw chromosome was generated by meiotic recombination; y w sw males were identified on the basis of the shortwing eye and wing phenotype and were tested for lethality over the deficiency Df(1)mal3 or over the strong Dic allele Dic1. The Glued1 stock was provided by Douglas Kankel (Yale University) and is described in Harte and Kankel (1982). Mutant alleles of the dynein heavy chain gene Dhc64C have been described previously (Gepner et al. 1996). Chromosomes and markers are described in Lindsley and Zimm (1992). All flies were raised on standard yeast-cornmeal-agar medium at 25°, unless stated otherwise.
Mutagenesis: y w males were mutagenized with EMS as previously described (Lewis and Bacher 1968). Males were starved for 1 hr and then fed 25 mm EMS in 1% sucrose overnight. Mutagenized males were mass mated to C(1)DX females, with attached-X chromosomes, homozygous for a second chromosome insertion of the Dic transgene P(Dic+). F1 males, carrying a mutagenized X chromosome and heterozygous for the Dic transgene, were individually mated to attached-X virgin females without the Dic transgene. Pair matings were done in test tubes using yeast-sucrose food (Simmons et al. 1980) at 28°. Because initial screening showed that a high percentage of the F1 males were infertile, some of the pair matings (1230 of 7748) were reared at 25° to determine if the fertility of the F1 males could be improved by a lower rearing temperature. In the F2 generation, segregation of the Dic transgene was followed using the mini-w+ eye color marker. F2 populations were screened for the absence of white-eyed males, which would indicate that the Dic transgene was required for viability. Mutant stocks were maintained as males in the presence of the Dic transgene or in females over the X chromosome balancer FM7.
Complementation tests and genetic analyses: To test for complementation between shortwing and the Dic19C alleles, virgin females of the genotype y w Dic-/FM7 were crossed to sw/Y males and examined for the presence of y w Dic-/sw progeny. Crosses were done at 18°, 22°, 25°, and 28° for each Dic19C allele. To show that the phenotypes associated with the Dic-/sw progeny could be rescued by the wild-type Dic transgene, y w sw males heterozygous for a second chromosome insertion of the Dic transgene (genotype y w sw/Y; P(Dic+)/+) were crossed to Dic-/FM7 virgin females. The y w Dic-/sw; P(Dic+)/+ progeny were compared to y w Dic-/sw progeny for number of adults as well as for the presence of eye and wing phenotypes. Again, crosses were done at 18°, 22°, 25°, and 28°. The rescue of the sw mutant phenotype by the Dic transgene was also tested by separately crossing sw/Y males to attached-X females without the Dic transgene and to attached-X females homozygous for a second chromosome insertion of the Dic transgene. For the comparison of Dic1/sw to Df/sw, Dic1/FM7 females were crossed to sw/Y males at 22° and 18°, and sw/FM7 females were crossed to Df(1)mal3/Y Dp(1:Y)mal106 males at 22° and 18°.
Lethal phase analysis: To estimate the lethal stage of the Dic mutants, balanced virgin females for each Dic allele were crossed to wild-type (Oregon-R) males. From this cross, approximately one-quarter of the progeny would be expected to die due to the presence of the Dic mutation. After several days of mating, 2- to 4-hr egg collections were made on grape juice agar plates. Embryos were counted and transferred to fresh plates. After 36 hr, unhatched eggs were counted, and larvae were counted and placed on fresh food in culture vials. Subsequently the numbers of pupae and adult flies from each cross were counted. The total lethality was determined as a percentage of the collected embryos that died before reaching adulthood {%L = [(no. of eggs - no. of adults)/no. of eggs] × 100}. The lethality for each stage of development was determined as a percentage of the total lethality {e.g., % embryonic lethality = [(no. of eggs - no. of larvae)/(no. of eggs - no. of adults)] × 100}.
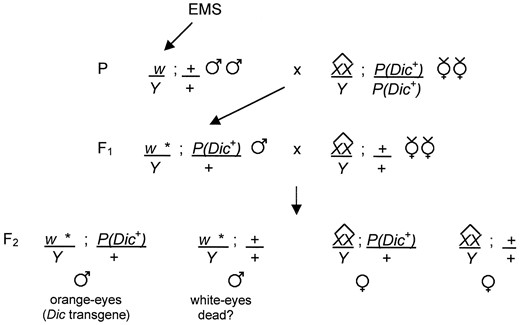
—Strategy for the identification of mutations in the cytoplasmic dynein intermediate chain gene. The screen strategy relies on an autosomal insertion of a dynein intermediate chain transgene P(Dic+), marked with the mini-w+ eye color gene, to rescue lethal mutations in the dynein intermediate chain gene. White-eyed males are mutagenized and mated to attached-X virgin females carrying a homozygous copy of the P(Dic+) transgene inserted on chromosome 2. The attached-X chromosome present in the parental females causes patroclinous inheritance of the mutagenized X chromosome (denoted by an asterisk) from fathers to sons. In the F1 generation, males containing a mutagenized X chromosome and heterozygous for the P(Dic+) transgene are individually mated to attached-X virgin females without the transgene. In the F2 generation, segregation of the transgene allows populations to be scored for the absence of white-eyed males, indicating a mutation in the dynein intermediate chain gene.
Phenotypic analysis: Wild-type and mutant larvae were dissected in phosphate-buffered saline (PBS) as described in Gindhardt et al. (1998). Filleted larvae were fixed in 4% formaldehyde in PBS + 0.1% Triton X-100 (PBT) for 30 min at room temperature and washed with several changes of PBT followed by blocking in antibody incubation buffer (PBT + 2% bovine serum albumin) for 1 hr at room temperature. All primary antibody incubations were performed overnight at 4°, and secondary antibody incubations were performed for 2 hr at room temperature. Primary antibodies used were mouse monoclonal anti-cysteine string protein, 1:100 (Zinsmaier et al. 1994), and affinity-purified rabbit polyclonal anti-Drosophila kinesin heavy chain, 1:2000 (Cytoskeleton, Denver). Samples were washed for 1-2 hr in three changes of PBT after antibody incubations. The final wash was in 80% glycerol in PBT. Stained larvae were mounted in PermaFluor mounting medium (ThermoShandon, Pittsburgh).
Drosophila heads were dehydrated in an ethanol series as described in Carthew and Rubin (1990) and prepared for scanning electron microscopy by critical point drying and sputter coating with gold, using a Fullam sputter coat device (Ernest F. Fullam, Schenectady, NY). Images were recorded on film (type 55; Polaroid, Technical Imaging Products, Cambridge, MA).
Wings were dissected from adult flies, mounted in methylsalicylate and Canada balsam, and examined by brightfield microscopy with a Nikon Eclipse E800 microscope equipped with a ×4 objective. Digital images were collected using a CoolCam liquid-cooled three color CCD camera (Cool Camera Company, Decatur, GA) and Image Pro Plus software (Media Cybernetics, Silver Springs, MD).
RESULTS
Recovery of Dic mutations: We have previously shown that the cytoplasmic dynein heavy chain subunit is expressed throughout development (Li et al. 1994) and that mutations in the unique Dhc64C gene that encodes this subunit result in larval lethality (Gepner et al. 1996). Similarly, the dynein intermediate chain is the product of a single gene, Dic19C, which is expressed throughout development (Boylan et al. 2000). We reasoned that mutations in this gene would also be lethal. Thus we used a modified F2 screen to recover mutations in flies that were rescued by a wild-type Dic transgene. As shown in Figure 1, we screened for F2 populations in which the Dic transgene was required for viability at 28°. The progeny of 3006 fertile F1 males were examined, and three lethal mutations were identified. To retest these mutations, males carrying the mutant X chromosome and a single copy of the Dic transgene were crossed to attached-X females without a Dic transgene. Analysis of the resultant progeny showed that for each of the three mutations, the Dic transgene was required for viability at 28° (Table 1). The mutations were all tested for viability at lower temperatures (18°, 22°, and 25°) and were found to be inviable at all temperatures (data not shown). Consequently, none of the mutations identified are temperature sensitive.
We previously identified the cytological location of the dynein intermediate chain gene as polytene region 19C (Boylan et al. 2000). As expected, all mutants identified in the screen were found to be lethal over Df(1)mal3, a deficiency of polytene region 19 that removes the intermediate chain gene (Table 2). To determine whether any interallelic complementation occurs between the Dic mutations, inter se crosses were performed. All three heteroallelic combinations of Dic alleles failed to complement for adult viability.
Transgene rescue of X-linked lethal mutations
| . | F2 progeny classes . | ||
|---|---|---|---|
| Dic allele . | Dicm/Y; +/+ ♂ . | Dicm/Y; P(Dic+)/+ ♂ . | XX/Y; +/+ and XX/Y; P(Dic+)/+ ♀a . |
| Dic1 | 0 | 44 | 111 |
| Dic2 | 2b | 111 | 117 |
| Dic3 | 0 | 41 | 104 |
| . | F2 progeny classes . | ||
|---|---|---|---|
| Dic allele . | Dicm/Y; +/+ ♂ . | Dicm/Y; P(Dic+)/+ ♂ . | XX/Y; +/+ and XX/Y; P(Dic+)/+ ♀a . |
| Dic1 | 0 | 44 | 111 |
| Dic2 | 2b | 111 | 117 |
| Dic3 | 0 | 41 | 104 |
X-linked lethal mutations (Dicm) were tested for rescue by the Dic transgene P(Dic+).
Female classes with and without the Dic transgene were counted together. The presence or absence of the transgene cannot be scored in the attached-X females due to their wild-type eye color.
These were determined to be P(Dic+) flies that had lost the mini-w+ marker.
Transgene rescue of X-linked lethal mutations
| . | F2 progeny classes . | ||
|---|---|---|---|
| Dic allele . | Dicm/Y; +/+ ♂ . | Dicm/Y; P(Dic+)/+ ♂ . | XX/Y; +/+ and XX/Y; P(Dic+)/+ ♀a . |
| Dic1 | 0 | 44 | 111 |
| Dic2 | 2b | 111 | 117 |
| Dic3 | 0 | 41 | 104 |
| . | F2 progeny classes . | ||
|---|---|---|---|
| Dic allele . | Dicm/Y; +/+ ♂ . | Dicm/Y; P(Dic+)/+ ♂ . | XX/Y; +/+ and XX/Y; P(Dic+)/+ ♀a . |
| Dic1 | 0 | 44 | 111 |
| Dic2 | 2b | 111 | 117 |
| Dic3 | 0 | 41 | 104 |
X-linked lethal mutations (Dicm) were tested for rescue by the Dic transgene P(Dic+).
Female classes with and without the Dic transgene were counted together. The presence or absence of the transgene cannot be scored in the attached-X females due to their wild-type eye color.
These were determined to be P(Dic+) flies that had lost the mini-w+ marker.
Dic mutants exhibit larval lethality: To establish the time of development at which zygotic expression of the dynein intermediate chain is required, we examined the stage at which flies hemizygous for mutant Dic died. For each Dic mutant allele, females with one mutant gene and one wild-type gene were crossed to wild-type males. From this cross, approximately one-quarter of the progeny would be expected to die due to the presence of the Dic mutation. The stage of lethality was determined by counting the numbers of larvae, pupae, and adults resulting from the embryos collected for each cross (Figure 2). For all three Dic alleles, the mutations resulted in lethality predominantly at the larval stage. The weakest Dic allele recovered in the screen (Dic2) lives to the third instar larval stage. These larvae exhibit a crawling defect that results in complete paralysis with the heads of the larvae poking up out of the food (Figure 2). Previous work has shown that mutations affecting either anterograde or retrograde axonal transport display abnormal larval crawling behavior and paralysis and the accumulation of vesicles and organelles within the axon (Hurd and Saxton 1996; Gindhardt et al. 1998; Bowman et al. 1999; Martin et al. 1999). To determine if the larval paralysis phenotype of the Dic2 mutation resulted from a defect in axonal transport, we looked for the presence of axonal “cargo jams” in Dic2 mutant larvae using antibodies to the anterograde motor, kinesin, and a synaptic vesicle component, cysteine string protein (Zinsmaier et al. 1994). Similar to mutations in the kinesin heavy and light chains, as well as the dynein heavy chain, we observed accumulations of cargoes in the segmental nerves of Dic2 mutant larvae that were recognized by both antibodies (data not shown).
Dic mutations fail to complement a deficiency that removes polytene region 19C
| . | Progeny classes (total no. of adults)a . | |||
|---|---|---|---|---|
| Dic allele . | Dicm/Df ♀ . | Dicm/YDp ♂ . | Dicm/FM7 ♀ . | FM7/YDp ♂ . |
| Dic1 | 0 | 76 | 92 | 77 |
| Dic2 | 0 | 106 | 108 | 98 |
| Dic3 | 0 | 125 | 154 | 133 |
| swb | 0 | 119 | NA | NA |
| . | Progeny classes (total no. of adults)a . | |||
|---|---|---|---|---|
| Dic allele . | Dicm/Df ♀ . | Dicm/YDp ♂ . | Dicm/FM7 ♀ . | FM7/YDp ♂ . |
| Dic1 | 0 | 76 | 92 | 77 |
| Dic2 | 0 | 106 | 108 | 98 |
| Dic3 | 0 | 125 | 154 | 133 |
| swb | 0 | 119 | NA | NA |
Balanced Dic alleles (Dicm) were mated to Df(1)mal3/Dp(1:Y)mal106 males.
Homozygous shortwing (sw) virgin females were mated to Df(1)mal3/Dp(1:Y)mal106 males. NA, not applicable.
Dic mutations fail to complement a deficiency that removes polytene region 19C
| . | Progeny classes (total no. of adults)a . | |||
|---|---|---|---|---|
| Dic allele . | Dicm/Df ♀ . | Dicm/YDp ♂ . | Dicm/FM7 ♀ . | FM7/YDp ♂ . |
| Dic1 | 0 | 76 | 92 | 77 |
| Dic2 | 0 | 106 | 108 | 98 |
| Dic3 | 0 | 125 | 154 | 133 |
| swb | 0 | 119 | NA | NA |
| . | Progeny classes (total no. of adults)a . | |||
|---|---|---|---|---|
| Dic allele . | Dicm/Df ♀ . | Dicm/YDp ♂ . | Dicm/FM7 ♀ . | FM7/YDp ♂ . |
| Dic1 | 0 | 76 | 92 | 77 |
| Dic2 | 0 | 106 | 108 | 98 |
| Dic3 | 0 | 125 | 154 | 133 |
| swb | 0 | 119 | NA | NA |
Balanced Dic alleles (Dicm) were mated to Df(1)mal3/Dp(1:Y)mal106 males.
Homozygous shortwing (sw) virgin females were mated to Df(1)mal3/Dp(1:Y)mal106 males. NA, not applicable.
shortwing is allelic to Dic19C: In addition to the de novo isolation of mutations in the dynein intermediate chain gene, we examined the database for existing mutations in the region of the Dic gene that might represent additional Dic alleles. One candidate, the recessive mutant shortwing (sw), was mapped by linkage analysis to the X chromosome between positions 63.5 and 64 (Eker 1935). Further analysis of deficiencies in the region positioned the sw locus at polytene segment 19B (Lefevre 1981; Paradi et al. 1983). We previously showed that the Dic gene is also located near this region (Boylan et al. 2000). The sw mutant was originally identified in 1932, arising spontaneously in a stock of the second chromosome mutant short-bristle (Eker 1935). The sw phenotype was described as pleiotropic, with defects in eye and wing development at 25°. sw mutants have eyes that are variably smaller than those of wild type, elliptically shaped, and rough. The wing phenotype is also variable. The wings may have incisions of the medial, lateral, and posterior margins and may be reduced in size. Additionally, portions of the longitudinal or cross veins may be missing, veins may be bifurcated, or additional vein material may be present. Wings may also be outheld from the body. Previous work has shown that mutations in the dynein heavy chain gene, Dhc64C, also result in a rough eye phenotype (McGrail et al. 1995; Gepner et al. 1996). Taken together, the similar location of the Dic and sw mutations and the eye phenotype of other known components of the dynein complex suggested that sw might be allelic to Dic19C. To verify the chromosomal location of the shortwing mutation, sw virgin females were crossed to males carrying a deficiency that removes the dynein intermediate chain gene. No sw/Df progeny were recovered at 25° (Table 2) or 28° (data not shown). Some sw/Df flies were viable at 22° (9/167), and at 18° sw/Df females represent 39% of the progeny (n = 107). These results indicate that, although the sw mutation is homozygous viable, sw is lethal over a deficiency that removes the dynein intermediate chain gene and the lethality is temperature sensitive.
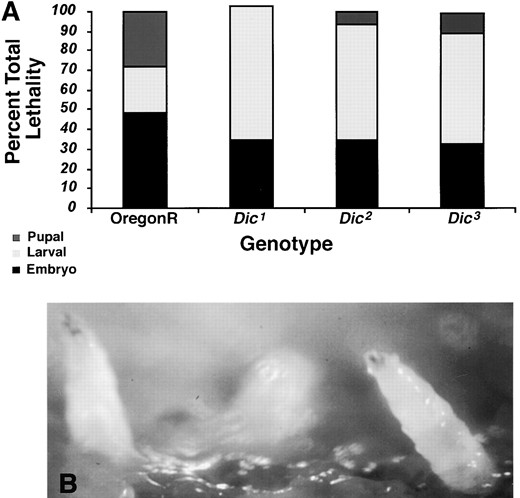
—The Dic mutants exhibit larval lethality. (A) Histogram summarizing results of the lethal phase analysis for Dic mutants. The percentage of the total lethality is plotted for each stage. Oregon-R (n = 576), Dic1 (n = 357), Dic2 (n = 328), and Dic3 (n = 390). (B) Dic2 mutant larvae live to the third instar stage, but display a larval crawling phenotype and become paralyzed with their heads poking out of the food.
To determine whether the nonlethal mutant shortwing is allelic to the lethal Dic19C mutations, we first conducted a complementation analysis. For each Dic19C allele, balanced females were crossed to sw/Y males at 18°, 22°, 25°, and 28°. As shown in Table 3, sw fails to complement the Dic1 allele for viability at 25° and 28°. At 22° and 18° some sw/Dic1 flies are viable; however, viability is reduced compared to the sibling class. sw in combination with Dic3 also shows reduced viability at 22°, 25°, and 28°. In addition to reduced viability, sw flies in combination with these two Dic alleles display abnormal wing and eye development, similar to the phenotype of sw mutant males (Figures 3 and 4). The weakest of the Dic19C lethal alleles, Dic2, complements sw for viability at all temperatures; however, at 28° the sw/Dic2 flies have a visible wing and eye phenotype. These results show that sw fails to complement mutations in Dic19C and suggests that the sw mutation is an allele of the dynein intermediate chain gene. Moreover, the failure of these mutants to complement is temperature sensitive and varies with the strength of the lethal Dic allele.
To demonstrate rescue of the sw eye and wing phenotypes by the Dic transgene, sw mutants were tested using a modification of the original screen for Dic mutations. sw/Y males were separately crossed either to attached-X females homozygous for a second chromosome insertion of the Dic transgene or to attached-X females without the Dic transgene, at 25° and 28°. In the absence of the Dic transgene at 28°, many of the sw males had a visible sw phenotype; however, none of the flies displayed a wing or eye phenotype in the presence of the Dic transgene (Figure 4), showing that the sw phenotype is rescued by the Dic transgene. Furthermore, although sw/Y males are viable at both 25° and 28°, they appear to be present in reduced numbers in the absence of the Dic transgene [43% of the progeny (n = 239) compared to 55% in the presence of the Dic transgene (n = 285)], suggesting that in addition to the eye and wing phenotype, the sw mutation also affects viability.
Complementation tests
| . | . | Progeny classes (total no. of adults)a . | |||
|---|---|---|---|---|---|
| Dic allele . | Temp. . | Dicm/sw ♀ . | Dicm/Y ♂ . | sw/FM7 ♀ . | FM7/Y ♂ . |
| Dic1 | 28° | 0 | 0 | 24 | 9 |
| 25° | 0 | 0 | 72 | 53 | |
| 22° | 14 | 0 | 91 | 46 | |
| 18° | 36 | 0 | 55 | 24 | |
| Dic2 | 28° | 55 | 0 | 52 | 42 |
| 25° | 74 | 0 | 91 | 61 | |
| 22° | 69 | 0 | 59 | 66 | |
| 18° | 61 | 0 | 61 | 31 | |
| Dic3 | 28° | 2 | 0 | 63 | 71 |
| 25° | 12 | 0 | 63 | 62 | |
| 22° | 33 | 0 | 87 | 56 | |
| 18° | 56 | 0 | 58 | 37 | |
| . | . | Progeny classes (total no. of adults)a . | |||
|---|---|---|---|---|---|
| Dic allele . | Temp. . | Dicm/sw ♀ . | Dicm/Y ♂ . | sw/FM7 ♀ . | FM7/Y ♂ . |
| Dic1 | 28° | 0 | 0 | 24 | 9 |
| 25° | 0 | 0 | 72 | 53 | |
| 22° | 14 | 0 | 91 | 46 | |
| 18° | 36 | 0 | 55 | 24 | |
| Dic2 | 28° | 55 | 0 | 52 | 42 |
| 25° | 74 | 0 | 91 | 61 | |
| 22° | 69 | 0 | 59 | 66 | |
| 18° | 61 | 0 | 61 | 31 | |
| Dic3 | 28° | 2 | 0 | 63 | 71 |
| 25° | 12 | 0 | 63 | 62 | |
| 22° | 33 | 0 | 87 | 56 | |
| 18° | 56 | 0 | 58 | 37 | |
Lethal Dic alleles (Dicm) were tested for complementation with the viable allele shortwing (sw).
Complementation tests
| . | . | Progeny classes (total no. of adults)a . | |||
|---|---|---|---|---|---|
| Dic allele . | Temp. . | Dicm/sw ♀ . | Dicm/Y ♂ . | sw/FM7 ♀ . | FM7/Y ♂ . |
| Dic1 | 28° | 0 | 0 | 24 | 9 |
| 25° | 0 | 0 | 72 | 53 | |
| 22° | 14 | 0 | 91 | 46 | |
| 18° | 36 | 0 | 55 | 24 | |
| Dic2 | 28° | 55 | 0 | 52 | 42 |
| 25° | 74 | 0 | 91 | 61 | |
| 22° | 69 | 0 | 59 | 66 | |
| 18° | 61 | 0 | 61 | 31 | |
| Dic3 | 28° | 2 | 0 | 63 | 71 |
| 25° | 12 | 0 | 63 | 62 | |
| 22° | 33 | 0 | 87 | 56 | |
| 18° | 56 | 0 | 58 | 37 | |
| . | . | Progeny classes (total no. of adults)a . | |||
|---|---|---|---|---|---|
| Dic allele . | Temp. . | Dicm/sw ♀ . | Dicm/Y ♂ . | sw/FM7 ♀ . | FM7/Y ♂ . |
| Dic1 | 28° | 0 | 0 | 24 | 9 |
| 25° | 0 | 0 | 72 | 53 | |
| 22° | 14 | 0 | 91 | 46 | |
| 18° | 36 | 0 | 55 | 24 | |
| Dic2 | 28° | 55 | 0 | 52 | 42 |
| 25° | 74 | 0 | 91 | 61 | |
| 22° | 69 | 0 | 59 | 66 | |
| 18° | 61 | 0 | 61 | 31 | |
| Dic3 | 28° | 2 | 0 | 63 | 71 |
| 25° | 12 | 0 | 63 | 62 | |
| 22° | 33 | 0 | 87 | 56 | |
| 18° | 56 | 0 | 58 | 37 | |
Lethal Dic alleles (Dicm) were tested for complementation with the viable allele shortwing (sw).
Similarly, the lethal and visible phenotypes associated with the Dic alleles in trans-heterozygous combination with sw are also rescued by the Dic transgene. To demonstrate the transgene rescue of the lethal Dic alleles over sw, the complementation tests were repeated using y w sw males heterozygous for a second chromosome insertion of the Dic transgene (genotype y w sw/Y; P(Dic+)/+). The results of these crosses are shown in Table 4. For all Dic alleles in trans-heterozygous combination with sw, the lethal and visible phenotypes are rescued by the Dic transgene. The identification of sw as an allele of the dynein intermediate chain is also supported by the observation that the levels of the intermediate chain polypeptide appear reduced in the mutant background (data not shown). However, neither the analysis of genomic DNA nor immunoblots have suggested that the Dic mutants produce aberrant truncated gene products.
Dic mutations interact with a mutation in dynactin: As a simple test for interactions between the Dic mutations and other components of dynein or dynactin, the Dic mutations were crossed to recessive lethal alleles of the dynein heavy chain gene, Dhc64C, and mutations in the gene for the p150-Glued subunit of dynactin (Glued gene). No dominant interactions were observed between any of the lethal Dic alleles and recessive alleles of the dynein heavy chain gene or recessive alleles of the Glued locus. We have previously shown that the dominant mutation Glued1 exhibits a dosage-sensitive interaction with the dynein intermediate chain gene. A deficiency that removes the Dic19C gene dominantly enhances the rough eye phenotype of Glued1, and a duplication of the Dic region suppresses the rough eye phenotype (Boylan et al. 2000). Similar to the deficiency, all three lethal Dic alleles were found to be dominant enhancers of the rough eye phenotype of Glued1 (Figure 5). To determine whether the nonlethal Dic allele sw also shows a genetic interaction with Glued1, homozygous sw females were crossed to Gl1Sb/TM6B, D males. As shown in Table 5, sw females in trans-heterozygous combination with Glued1 are viable at all temperatures tested. However, these flies have an enhanced rough eye compared with the Glued1 mutation alone (Figure 5), and the enhanced phenotype is reversed in the presence of the Dic transgene. In males in which only mutant Dic is present, the combination of sw and Glued1 is lethal.
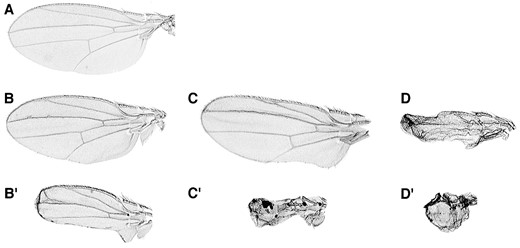
—The lethal Dic alleles fail to complement the sw wing phenotype. Examples of the variable wing phenotype of the lethal Dic alleles in combination with the viable allele, shortwing (sw). Shown are mild and severe wing defects for each allelic combination. (A) The wing phenotype is rescued by a copy of the wild-type Dic transgene. (B and B′) Dic2/sw displays a mild wing phenotype at 28°. The Dic3/sw (C and C′) and Dic1/sw (D and D′) flies have a more severe wing phenotype and have reduced viability compared to Dic2/sw.
—The rough eye phenotype caused by shortwing is rescued by the Dic transgene. (A) Scanning electron micrograph (SEM) showing the rough eye phenotype of sw/Y. (B) SEM of an sw eye in the presence of the Dic transgene [genotype sw/Y; P(Dic+)/+]. (C) The wing phenotype of shortwing is variable, including defects in wing size and venation and incised margins. Arrow, bifurcated wing vein; arrowheads, ectopic vein material. (D) The wing defects are rescued by the wild-type Dic transgene [genotype sw/Y; P(Dic+)/+].
DISCUSSION
Our results provide the first direct evidence of an essential function for the intermediate chain subunit of cytoplasmic dynein. Previous analysis of dynein heavy chain mutations in mouse and Drosophila has demonstrated that dynein function is essential in these organisms (Gepner et al. 1996; Harada et al. 1998). In yeast and filamentous fungi, however, dynein heavy chain mutations are not lethal, but have defects in nuclear migration and spindle orientation (Eshel et al. 1993; Li et al. 1993; Plamann et al. 1994; Xiang et al. 1994). Similar to the yeast mutations in the heavy chain, mutations in the Saccharomyces cerevisiae dynein intermediate chain gene (pac11) are not lethal, but are synthetic lethal in combination with mutations in the kinesin gene, cin8 (Geiser et al. 1997). Attempts to generate dynein heavy chain and intermediate chain knockouts by homologous recombination in Dictyostelium failed, consistent with an essential function for both subunits (Koonce and Knecht 1998; Ma et al. 1999). In Dictyostelium, mutants overexpressing truncations of the Dic gene by a conditional promoter exhibited defects in Golgi dispersion, interphase microtubule organization, cell division, and centrosome replication and separation (Ma et al. 1999). Whether the intermediate chain subunit is required for all dynein functions is not known.
Transgene rescue of sw/Dicm
| . | . | Progeny classes (total no. of adults)a . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp. . | Dic allele . | Dicm/y w sw; +/+ ♀ . | Dicm/y w sw; P(Dic+)/+ ♀ . | Dicm/Y; +/+ ♂ . | Dicm/Y; P(Dic+)/+ ♂ . | FM7/y w sw; +/+ ♀ . | FM7/y w sw; P(Dic+)/+ ♀ . | FM7/Y; +/+ ♂ . | FM7/Y; P(Dic+)/+ ♂ . |
| 25° | Dic1 | 0 | 50 | 0 | 54 | 63 | 62 | 47 | 28 |
| 25° | Dic3 | 2 | 59 | 0 | 44 | 64 | 56 | 56 | 59 |
| 25° | Dic2 | 35b | 35 | 0 | 49 | 30 | 33 | 18 | 27 |
| 28° | Dic2 | 1c | 45 | 0 | 43 | 30 | 49 | 31 | 34 |
| 28° | sw | 10d | 24 | 1 | 32 | 23 | 31 | 25 | 22 |
| . | . | Progeny classes (total no. of adults)a . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp. . | Dic allele . | Dicm/y w sw; +/+ ♀ . | Dicm/y w sw; P(Dic+)/+ ♀ . | Dicm/Y; +/+ ♂ . | Dicm/Y; P(Dic+)/+ ♂ . | FM7/y w sw; +/+ ♀ . | FM7/y w sw; P(Dic+)/+ ♀ . | FM7/Y; +/+ ♂ . | FM7/Y; P(Dic+)/+ ♂ . |
| 25° | Dic1 | 0 | 50 | 0 | 54 | 63 | 62 | 47 | 28 |
| 25° | Dic3 | 2 | 59 | 0 | 44 | 64 | 56 | 56 | 59 |
| 25° | Dic2 | 35b | 35 | 0 | 49 | 30 | 33 | 18 | 27 |
| 28° | Dic2 | 1c | 45 | 0 | 43 | 30 | 49 | 31 | 34 |
| 28° | sw | 10d | 24 | 1 | 32 | 23 | 31 | 25 | 22 |
Dic mutant alleles (Dicm).
Some flies with mild wing phenotype.
Severe wing phenotype.
Rough eyes, reduced fertility, mild wing phenotype.
Transgene rescue of sw/Dicm
| . | . | Progeny classes (total no. of adults)a . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp. . | Dic allele . | Dicm/y w sw; +/+ ♀ . | Dicm/y w sw; P(Dic+)/+ ♀ . | Dicm/Y; +/+ ♂ . | Dicm/Y; P(Dic+)/+ ♂ . | FM7/y w sw; +/+ ♀ . | FM7/y w sw; P(Dic+)/+ ♀ . | FM7/Y; +/+ ♂ . | FM7/Y; P(Dic+)/+ ♂ . |
| 25° | Dic1 | 0 | 50 | 0 | 54 | 63 | 62 | 47 | 28 |
| 25° | Dic3 | 2 | 59 | 0 | 44 | 64 | 56 | 56 | 59 |
| 25° | Dic2 | 35b | 35 | 0 | 49 | 30 | 33 | 18 | 27 |
| 28° | Dic2 | 1c | 45 | 0 | 43 | 30 | 49 | 31 | 34 |
| 28° | sw | 10d | 24 | 1 | 32 | 23 | 31 | 25 | 22 |
| . | . | Progeny classes (total no. of adults)a . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp. . | Dic allele . | Dicm/y w sw; +/+ ♀ . | Dicm/y w sw; P(Dic+)/+ ♀ . | Dicm/Y; +/+ ♂ . | Dicm/Y; P(Dic+)/+ ♂ . | FM7/y w sw; +/+ ♀ . | FM7/y w sw; P(Dic+)/+ ♀ . | FM7/Y; +/+ ♂ . | FM7/Y; P(Dic+)/+ ♂ . |
| 25° | Dic1 | 0 | 50 | 0 | 54 | 63 | 62 | 47 | 28 |
| 25° | Dic3 | 2 | 59 | 0 | 44 | 64 | 56 | 56 | 59 |
| 25° | Dic2 | 35b | 35 | 0 | 49 | 30 | 33 | 18 | 27 |
| 28° | Dic2 | 1c | 45 | 0 | 43 | 30 | 49 | 31 | 34 |
| 28° | sw | 10d | 24 | 1 | 32 | 23 | 31 | 25 | 22 |
Dic mutant alleles (Dicm).
Some flies with mild wing phenotype.
Severe wing phenotype.
Rough eyes, reduced fertility, mild wing phenotype.
To identify mutations in the dynein intermediate chain gene, we used a modification of the screen reported by Fehon et al. (1997) in which they isolated mutations for a region of the X chromosome using a cosmid transgene as an autosomally linked duplication of the X chromosome. By substituting a transgene containing a single transcription unit for a larger genomic cosmid transgene, we were able to identify mutations within a single complementation group. Screening the progeny of ∼3000 fertile F1 males identified three mutations in the dynein intermediate chain gene. This recovery rate corresponds well with previous predictions of one lethal mutation for every 1000 F1 progeny scored (Greenspan 1997); however, it is approximately one-third the number of mutations recovered in the screen by Fehon et al. (1997). One consequence of screening by this method is that only X chromosomes with single lethal hits in the Dic gene will be identified. Chromosomes containing multiple lethal hits will not be recovered. While this obviates the need to remove secondsite lethals from the mutagenized chromosome, it could reduce the number of Dic mutations identified.
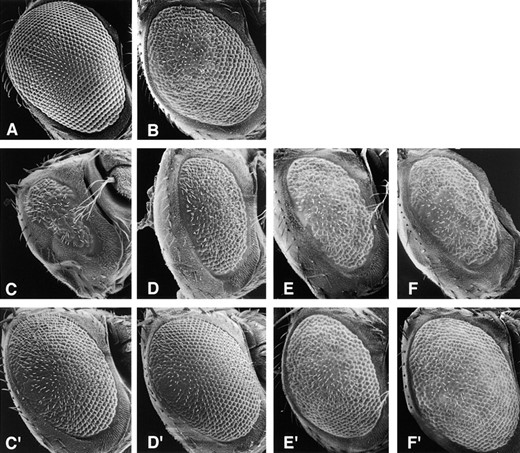
—Mutations in the dynein intermediate chain gene enhance the rough eye phenotype of Glued1. Scanning electron micrographs of Drosophila heads showing (A) a wild-type eye and (B) the dominant rough eye phenotype of Glued1. The Glued1 phenotype is dominantly enhanced by Dic mutant alleles (C) Dic1, (D) Dic2, (E) Dic3, and (F) sw. The enhanced rough eye is reversed by the addition of the Dic transgene, P(Dic+), shown in C′, D′, E′, and F′. Genotypes: (A) wild type, (B) Gl1/+, (C) Dic1/+; Gl1/+, (C′) Dic1/+; P(Dic+)/+; Gl1/+, (D) Dic2/+; Gl1/+, (D′) Dic2/+; P(Dic+)/+; Gl1/+, (E) Dic3/+; Gl1/+, (E′) Dic3/+; P(Dic+)/ +; Gl1/+, (F) sw/+; Gl1/+, and (F′) sw/+; P(Dic+)/+; Gl1/+.
shortwing interacts with Glued1
| . | Progeny classes (total no. of adults) . | |||
|---|---|---|---|---|
| Temp. . | sw/+; Gl1 Sb/+ ♀ . | sw/Y; Gl1 Sb/+ ♂ . | sw/Y; +/TM6B, D ♂ . | sw/+; +/TM6B, D ♀ . |
| 28° | 63 | 0 | 19 | 68 |
| 25° | 58 | 0 | 42 | 88 |
| 22° | 54 | 0 | 27 | 78 |
| 18° | 25 | 0 | 32 | 36 |
| . | Progeny classes (total no. of adults) . | |||
|---|---|---|---|---|
| Temp. . | sw/+; Gl1 Sb/+ ♀ . | sw/Y; Gl1 Sb/+ ♂ . | sw/Y; +/TM6B, D ♂ . | sw/+; +/TM6B, D ♀ . |
| 28° | 63 | 0 | 19 | 68 |
| 25° | 58 | 0 | 42 | 88 |
| 22° | 54 | 0 | 27 | 78 |
| 18° | 25 | 0 | 32 | 36 |
shortwing interacts with Glued1
| . | Progeny classes (total no. of adults) . | |||
|---|---|---|---|---|
| Temp. . | sw/+; Gl1 Sb/+ ♀ . | sw/Y; Gl1 Sb/+ ♂ . | sw/Y; +/TM6B, D ♂ . | sw/+; +/TM6B, D ♀ . |
| 28° | 63 | 0 | 19 | 68 |
| 25° | 58 | 0 | 42 | 88 |
| 22° | 54 | 0 | 27 | 78 |
| 18° | 25 | 0 | 32 | 36 |
| . | Progeny classes (total no. of adults) . | |||
|---|---|---|---|---|
| Temp. . | sw/+; Gl1 Sb/+ ♀ . | sw/Y; Gl1 Sb/+ ♂ . | sw/Y; +/TM6B, D ♂ . | sw/+; +/TM6B, D ♀ . |
| 28° | 63 | 0 | 19 | 68 |
| 25° | 58 | 0 | 42 | 88 |
| 22° | 54 | 0 | 27 | 78 |
| 18° | 25 | 0 | 32 | 36 |
The lethal phase analysis shows that the Dic mutations result in larval lethality. A similar lethal phase has been observed for mutations in the dynein heavy chain. Strong alleles of the dynein heavy chain (Dhc64C) die as first instar larvae, and somatic clone analysis of Dhc64C mutations demonstrates that dynein function is required for cell viability (Gepner et al. 1996). These results suggest that the maternal contribution of dynein is sufficient to allow the completion of embryogenesis without a zygotic contribution of gene product. Although all three Dic alleles die as larvae, the weakest Dic allele (Dic2) lives to a late larval stage, while two Dic alleles (Dic1 and Dic3) appear to die as first instar larvae. Although none of the Dic alleles identified appears to be a null allele, the relative efficiency of screening for Dic mutants should allow for identification of additional alleles.
In addition to lethality, one of the Dic mutations displays a larval crawling defect. This may result from progressive larval paralysis, as the mutant larvae become stiff with their heads poking out of the food like spikes. Similar crawling and paralysis phenotypes have been identified in mutations in the kinesin heavy chain and kinesin light chain genes (Hurd and Saxton 1996; Gindhardt et al. 1998). The kinesin mutant larvae show axonal organelle jams, suggesting that the larval paralysis is due to a defect in axonal transport. Mutations in the dynein heavy chain (Dhc64C) have also been shown to disrupt axonal transport, causing larval paralysis (Martin et al. 1999). Additionally, screens for mutants with sluggish larval crawling behavior identified a gene, roadblock, in which the larval crawling phenotype was shown to be due to a mutation in the dynein light chain LC7. The roadblock mutants also accumulate axonal cargo and, additionally, have severe axonal loss and nerve degeneration (Bowman et al. 1999). Not surprisingly, we also observed accumulations of cargo in the axons of Dic mutant larvae, suggesting that the larval paralysis is due to a defect in axonal transport.
The genetic analysis of the mutant sw strongly suggests that it represents a viable allele of the cytoplasmic dynein intermediate chain gene, Dic19C. sw fails to complement the lethal Dic alleles in a temperature-sensitive manner. A copy of the wild-type Dic transgene rescues the lethal and visible phenotypes resulting from noncomplementation of sw and the Dic alleles. In addition, the complementation behavior of sw with the lethal Dic alleles provides a way to gauge the relative strength of the lethal alleles. For example, Figure 3 shows a range of wing phenotypes for the combinations of sw with the lethal Dic alleles from mild (Dic2/sw) to severe (Dic1/sw). The weakest lethal allele, Dic2, fully complements sw at 25°, but fails to complement the sw wing and eye phenotype at 28°. The stronger alleles, Dic3 and Dic1, fail to complement sw for viability at 25°. At lower temperatures, Dic1/sw and Dic3/sw adults are viable, but exhibit the sw eye phenotype and display severe defects in wing development. By this test, the allele Dic1 is the strongest of the lethal alleles, although comparison of Dic1/sw to Df/sw suggests that Dic1 is not a null allele.
Using a deficiency that removes the intermediate chain locus and a Dic genomic transgene (Boylan et al. 2000), we have previously shown that the rough eye phenotype of the Glued1 mutation is sensitive to the dosage of the dynein intermediate chain gene. The Glued1 mutation was initially identified on the basis of the dominant rough eye phenotype (Plough and Ives 1935) and was subsequently shown to be due to a truncation of the p150 subunit of dynactin caused by a transposon insertion in the Glued gene (Swaroop et al. 1985). The truncated Glued polypeptide is unable to assemble into a functional dynactin complex (McGrail et al. 1995); however, it retains the region identified as important for interaction with the dynein intermediate chain (Vaughan and Vallee 1995). Consequently the Glued1 truncation could act as a “poison” to dynein function, by uncoupling dynein from its cargo. The interaction between Glued1 and Dic depends on the dosage of Dic, suggesting a model where Glued1 acts by reducing the level of dynein intermediate chain available for cargo binding below a threshold required for normal eye development. Similar to a deficiency for the intermediate chain locus, the lethal dynein intermediate chain alleles identified in our screen and the viable allele sw all dominantly enhance the rough eye phenotype of Glued1. This result indicates that the intermediate chain alleles are all loss-of-function mutations that reduce the level of wild-type intermediate chain available to interact with the dynactin complex, causing an enhanced rough eye phenotype. Additional evidence for a dosage-sensitive interaction between the dynein intermediate chain and Glued1 comes from the observation that in males, the combination of the viable Dic allele, sw, with Glued1 is lethal. Moreover, this result shows that the interaction between the dynein intermediate chain and Glued is essential for viability and is not restricted to eye development.
Phenotypes associated with dynein intermediate chain and heavy chain mutations
| Genotype . | Phenotype . | Reference . |
|---|---|---|
| sw(Dic)/Y | Wings have defects in size and venation; eyes rough and elliptical | This study |
| Dic2/Y | Larval paralysis | This study |
| Dhc3-2/Dhc6-10 | Eyes small and rough; bristles short and thin | McGrail et al. (1995) |
| Gepner et al. (1996) | ||
| Dhc4-19/Dhc8-1 | Eyes darker in color and rounder in shape than those of wild type; bristles short and thin with bent tips | Gepner et al. (1996) |
| Dhc3-2/Dhc6-12 | Female sterile; male fertile | Gepner et al. (1996) |
| McGrail and Hays (1997) | ||
| Dhc6-6/Dhc6-10 | Female sterile | McGrail and Hays (1997) |
| Dhc6-10/Dhc6-12 | Female sterile; male sterile | Gepner et al. (1996) |
| Dhcx18/Df(3L)10H | Short bristles, female sterile | K. L. M. Boylan and T. S. Hays (unpublished observations) |
| Genotype . | Phenotype . | Reference . |
|---|---|---|
| sw(Dic)/Y | Wings have defects in size and venation; eyes rough and elliptical | This study |
| Dic2/Y | Larval paralysis | This study |
| Dhc3-2/Dhc6-10 | Eyes small and rough; bristles short and thin | McGrail et al. (1995) |
| Gepner et al. (1996) | ||
| Dhc4-19/Dhc8-1 | Eyes darker in color and rounder in shape than those of wild type; bristles short and thin with bent tips | Gepner et al. (1996) |
| Dhc3-2/Dhc6-12 | Female sterile; male fertile | Gepner et al. (1996) |
| McGrail and Hays (1997) | ||
| Dhc6-6/Dhc6-10 | Female sterile | McGrail and Hays (1997) |
| Dhc6-10/Dhc6-12 | Female sterile; male sterile | Gepner et al. (1996) |
| Dhcx18/Df(3L)10H | Short bristles, female sterile | K. L. M. Boylan and T. S. Hays (unpublished observations) |
Phenotypes associated with dynein intermediate chain and heavy chain mutations
| Genotype . | Phenotype . | Reference . |
|---|---|---|
| sw(Dic)/Y | Wings have defects in size and venation; eyes rough and elliptical | This study |
| Dic2/Y | Larval paralysis | This study |
| Dhc3-2/Dhc6-10 | Eyes small and rough; bristles short and thin | McGrail et al. (1995) |
| Gepner et al. (1996) | ||
| Dhc4-19/Dhc8-1 | Eyes darker in color and rounder in shape than those of wild type; bristles short and thin with bent tips | Gepner et al. (1996) |
| Dhc3-2/Dhc6-12 | Female sterile; male fertile | Gepner et al. (1996) |
| McGrail and Hays (1997) | ||
| Dhc6-6/Dhc6-10 | Female sterile | McGrail and Hays (1997) |
| Dhc6-10/Dhc6-12 | Female sterile; male sterile | Gepner et al. (1996) |
| Dhcx18/Df(3L)10H | Short bristles, female sterile | K. L. M. Boylan and T. S. Hays (unpublished observations) |
| Genotype . | Phenotype . | Reference . |
|---|---|---|
| sw(Dic)/Y | Wings have defects in size and venation; eyes rough and elliptical | This study |
| Dic2/Y | Larval paralysis | This study |
| Dhc3-2/Dhc6-10 | Eyes small and rough; bristles short and thin | McGrail et al. (1995) |
| Gepner et al. (1996) | ||
| Dhc4-19/Dhc8-1 | Eyes darker in color and rounder in shape than those of wild type; bristles short and thin with bent tips | Gepner et al. (1996) |
| Dhc3-2/Dhc6-12 | Female sterile; male fertile | Gepner et al. (1996) |
| McGrail and Hays (1997) | ||
| Dhc6-6/Dhc6-10 | Female sterile | McGrail and Hays (1997) |
| Dhc6-10/Dhc6-12 | Female sterile; male sterile | Gepner et al. (1996) |
| Dhcx18/Df(3L)10H | Short bristles, female sterile | K. L. M. Boylan and T. S. Hays (unpublished observations) |
The wing defects present in the sw Dic mutant identify a novel dynein phenotype. Our previous analysis of mutations in the dynein heavy chain (Dhc64C) has revealed heteroallelic combinations of alleles that complement for viability but have phenotypes in the eye and bristles and during oogenesis (Gepner et al. 1996; McGrail and Hays 1997), but no wing phenotypes have been observed. A major question raised by this observation is whether the wing phenotype reflects a tissue-specific function for the dynein intermediate chain and dynein transport. This seems unlikely since sw/sw females fail to exhibit a wing phenotype. An alternative explanation is that different tissues require different levels of dynein function during development. However, if this were the case one might expect the mutant phenotypes to “accumulate” on the basis of the level of dynein function provided by a particular mutant allele. For example, if the level of dynein function required for oogenesis is higher than that required for proper eye development, then all dynein mutants with a rough eye phenotype might also be expected to exhibit female sterility. As shown in Table 6, this is not the case, suggesting that the different dynein mutant alleles affect different aspects of dynein function. Consistent with the explanation that levels of dynein function account for different phenotypes in different tissues would be the prediction that noncomplementation may arise between Dhc and Dic mutations. However, so far we have failed to observe any such genetic interactions between Dic mutations and mutations in other dynein subunits. Further experiments will be necessary to establish whether, in addition to its essential functions, the dynein intermediate chain subunit serves tissue-restricted functions. The identified Dic alleles will provide new tools to identify interacting components that, similar to Glued (dynactin), play a role in regulating dynein function and its interaction with specific cargoes.
Acknowledgement
We thank Morgan Montgomery, Carl Erickson, and Pat Rosen for help with the screening and for preparation of fly food and Dr. Michael J. Simmons for critical reading of the manuscript. This work was completed by K.L.M.B. in partial fulfillment of the requirements for a Ph.D. (University of Minnesota) and was supported by grants to T.S.H. from the National Institutes of Health (GM-53956) and the American Heart Association. K.L.M.B. was supported in part by a research training grant from the National Science Foundation (DIR-91-11-44).
Footnotes
Communicating editor: R. S. Hawley
LITERATURE CITED



![—The rough eye phenotype caused by shortwing is rescued by the Dic transgene. (A) Scanning electron micrograph (SEM) showing the rough eye phenotype of sw/Y. (B) SEM of an sw eye in the presence of the Dic transgene [genotype sw/Y; P(Dic+)/+]. (C) The wing phenotype of shortwing is variable, including defects in wing size and venation and incised margins. Arrow, bifurcated wing vein; arrowheads, ectopic vein material. (D) The wing defects are rescued by the wild-type Dic transgene [genotype sw/Y; P(Dic+)/+].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/genetics/162/3/10.1093_genetics_162.3.1211/4/m_gen4796.f4.jpeg?Expires=1716500004&Signature=f3O9vptwbeCVFDvZPWs~U0BwySoP9CkHHZnBqNs34kVkBTO~4WR85GM-Jr3gCxQuRS-U8fySfwo8GOlFXzr0FJdagcofCdqaZE6m2aphZezraJGfFAsvJbIHzY~60UI0wyh1TkNJw6N8CpZUAccF6lE4tXtukzJkecZwFKHGlxbvM8kijlmjr~205XXzxd9mvlZ8xnoeDZaoWkd82OMIKdmaIbdMFvLPTK9lmxH0KpbPsT~8wW1FrD3PJLu3b9xVy699jDrgWs34ftfsTBSttw-FEmI57Zzc0wFoBISurMsEHQsmrbIur2cQNXfEeju41BJvosFB2ZJKNcAqTVKMPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)