-
PDF
- Split View
-
Views
-
Cite
Cite
Qi Yang, Katherine A Borkovich, Mutational Activation of a Gαi Causes Uncontrolled Proliferation of Aerial Hyphae and Increased Sensitivity to Heat and Oxidative Stress in Neurospora crassa, Genetics, Volume 151, Issue 1, 1 January 1999, Pages 107–117, https://doi.org/10.1093/genetics/151.1.107
Close - Share Icon Share
Abstract
Heterotrimeric G proteins, consisting of α, β, and γ subunits, transduce environmental signals through coupling to plasma membrane-localized receptors. We previously reported that the filamentous fungus Neurospora crassa possesses a Gα protein, GNA-1, that is a member of the Gαi superfamily. Deletion of gna-1 leads to defects in apical extension, differentiation of asexual spores, sensitivity to hyperosmotic media, and female fertility. In addition, Δgna-1 strains have lower intracellular cAMP levels under conditions that promote morphological abnormalities. To further define the function of GNA-1 in signal transduction in N. crassa, we examined properties of strains with mutationally activated gna-1 alleles (R178C or Q204L) as the only source of GNA-1 protein. These mutations are predicted to inhibit the GTPase activity of GNA-1 and lead to constitutive signaling. In the sexual cycle, gna-1R178C and gna-1Q204L strains are female-fertile, but produce fewer and larger perithecia than wild type. During asexual development, gna-1R178C and gna-1Q204L strains elaborate abundant, long aerial hyphae, produce less conidia, and possess lower levels of carotenoid pigments in comparison to wild-type controls. Furthermore, gna-1R178C and gna-1Q204L strains are more sensitive to heat shock and exposure to hydrogen peroxide than wild-type strains, while Δgna-1 mutants are more resistant. In contrast to Δgna-1 mutants, gna-1R178C and gna-1Q204L strains have higher steady-state levels of cAMP than wild type. The results suggest that GNA-1 possesses several Gβγ-independent functions in N. crassa. We propose that GNA-1 mediates signal transduction pathway(s) that regulate aerial hyphae development and sensitivity to heat and oxidative stresses, possibly through modulation of cAMP levels.
HETEROTRIMERIC G proteins (αβγ) are central components of signaling pathways in eukaryotic cells (Birnbaumer 1992). The heterotrimer is coupled to seven-helix plasma membrane receptors, which sense extracellular ligands. Ligand binding to the receptor causes GDP-GTP exchange on the Gα subunit, leading to dissociation of the heterotrimer into Gα and Gβγ subunits. Depending on the system, either Gα or Gβγ can function to regulate downstream effector proteins.
Hydrolysis of GTP to GDP by the Gα subunit leads to inactivation of signaling and reassociation of the Gα with Gβγ. Gα mutations resulting in defective GTPase activity have been identified that are dominant in trans and lead to constitutive signaling (Johnson et al. 1994). In mammals, mutation of either a conserved arginine (201 in Gαs or 178 in Gαi1) or a glutamine (227 in Gαs or 204 in Gαi1) leads to activation of Gα proteins (Freissmuth and Gilman 1989; Graziano and Gilman 1989; Coleman et al. 1994). Mutation of these residues in Gαs causes ∼100-fold lower GTPase activity in vitro (Freissmuth and Gilman 1989; Graziano and Gilman 1989). The slower rate of GTP hydrolysis results in constitutive activation of Gαs due to higher GTP occupancy (Freissmuth and Gilman 1989; Graziano and Gilman 1989). Comparison of crystal structures for wild-type and mutant forms of the Gαi1 protein demonstrates that the R178 and Q204 residues are critical for stabilization of the transition state during GTP hydrolysis (Coleman et al. 1994). Studies using mutant Gαi2 alleles demonstrated that Q205L had higher transformation potential than R179C; this result was predicted based on the greater stability of the Q205L protein (Hudson et al. 1981; Gupta et al. 1992).
Phenotypes observed in cells with GTPase-deficient Gα alleles can result from the action of the activated Gα or the free βγ dimer on effector pathways (Birnbaumer 1992). An example in which free βγ is the active unit is the mating/pheromone response pathway in the yeast Saccharomyces cerevisiae. In this system, the Ste4p/Ste18p βγ heterodimer positively regulates a mitogen-activated protein (MAP) kinase pathway, leading to cell-cycle arrest and mating (reviewed by Borkovich 1996). The function of the Gpa1p Gα is to tether βγ, rendering it inactive; thus, strains with null or GTPase-deficient gpa1 Gα alleles exhibit similar cell-cycle arrest phenotypes (reviewed by Kurjan 1992).
Neurospora crassa GNA-1 is a member of the Gαi superfamily, based on amino acid sequence identity and its ability to serve as a substrate for pertussis toxin (Turner and Borkovich 1993; Ivey et al. 1996). Δgna-1 strains are defective in several cellular processes, including apical extension on solid medium with or without hyperosmotic agents, aerial hyphae development, and female fertility (Ivey et al. 1996). Furthermore, Δgna-1 strains have reduced intracellular cAMP levels under conditions that promote morphological abnormalities (D. Ivey, Q. Yang and K. Borkovich, unpublished results).
cAMP is implicated in regulation of several processes in N. crassa. Carotenoid pigment accumulation is negatively correlated with the steady-state level of cAMP (Kritsky et al. 1982), exogenous cAMP reduces carotenoid synthesis (Harding 1973), and lower cAMP levels trigger derepression of carotenoid gene transcription (reviewed by Bramley and Mackenzie 1988). Mutation of the regulatory subunit of cAMP-dependent protein kinase (PKA) causes loss of growth polarity (Bruno et al. 1996), while hyphal elongation is regulated by a kinase exhibiting homology to the catalytic subunit of PKAs (Yarden et al. 1992). Furthermore, analysis of an adenylyl cyclase-deficient mutant (cr-1) has provided evidence that cAMP plays a positive regulatory role during basal hyphae growth and in aerial hyphae formation (Terenzi et al. 1974). Studies using Δgna-1 strains also support a positive role for cAMP in hyphal growth and aerial hyphae formation (D. Ivey, Q. Yang and K. Borkovich, unpublished results).
cAMP has been linked to responses to heat and oxidative stress in several fungal species. In S. cerevisiae and N. crassa, mutations in adenylyl cyclase lead to increased resistance to lethal heat treatment. This phenomenon results from a decrease in cAMP levels and subsequent lower PKA activity (Shin et al. 1987; Cruz et al. 1988). S. cerevisiae bcy1 mutants are deficient in the regulatory subunit of PKA and contain a constitutively active catalytic PKA subunit; these strains are more sensitive to heat (Shin et al. 1987). Available evidence suggests that cAMP control of heat sensitivity and heat shock protein synthesis is largely independent of heat shock promoter elements (HSEs) and the heat shock transcription factor Hsf1p (Mager and De Kruijff 1995). Recently, a cAMP-regulated stress responsive promoter element, or STRE, has been identified in S. cerevisiae (Marchler et al. 1993). This sequence is implicated in regulation of gene expression by a variety of environmental stresses, including heat and oxidants. However, regulation by STRE alone cannot explain the observed levels of expression of all stress-regulated genes. Hence, it has been postulated that both general control by STRE and specific control by other elements is required for appropriate regulation of genes in response to environmental stress (Mager and De Kruijff 1995).
In this study, we characterize the properties of N. crassa strains with null, wild-type, gna-1Q204L, or gna-1R178C GTPase-deficient alleles as the only source of GNA-1 protein. We analyze levels of G protein subunits and report phenotypes for the strains during both vegetative and sexual phases of the life cycle. We also quantitate intracellular cAMP and carotenoid levels, and measure sensitivity to heat and hydrogen peroxide in the various strains. We demonstrate a Gβγ-independent role for GNA-1 during several processes in N. crassa. We further show that in many cases the extent of the observed phenotypes can be correlated with the predicted GTP-occupancy of GNA-1.
MATERIALS AND METHODS
Growth of N. crassa and E. coli strains, plasmid constructs, and N. crassa electroporation: All media were supplemented with 10 μg/ml pyridoxine-HCl. Media for his-3 strains contained 100 μg/ml histidine. Eight-day-old conidia isolated from Vogel's minimal medium (VM; Vogel 1964) agar flasks were used to inoculate cultures for physiological studies (Davis and Deserres 1970). N. crassa strain genotypes are listed in Table 1. Escherichia coli strain DH5α was used to maintain all plasmids (Hanahan 1983).
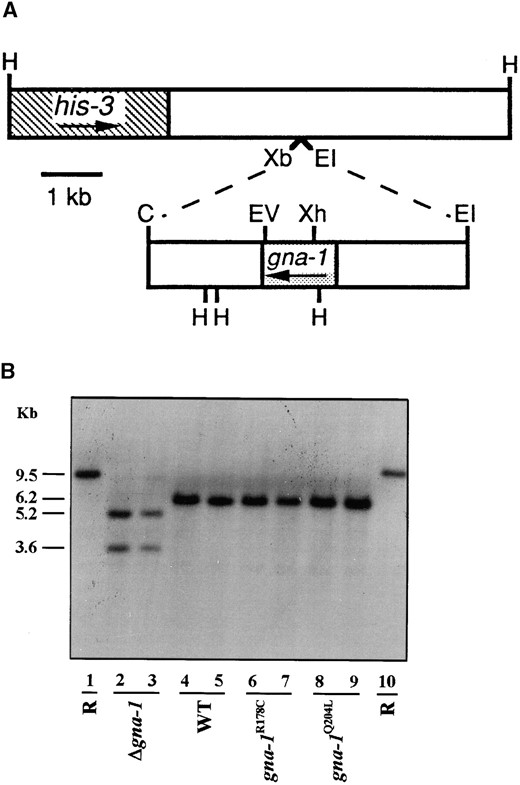
Two constitutively activating mutations, R178C and Q204L (Johnson et al. 1994), were made in gna-1 using site-directed mutagenesis essentially as previously described (Kunkel et al. 1987). The entire gna-1 gene region subcloned in pPNO5 (Ivey et al. 1996) is shown in Figure 1A; the template for mutagenesis (plasmid pPNO3) contained the 2.92-kb XhoI-ClaI fragment from this region. The presence of each mutation was verified by DNA sequencing (data not shown). To clone the Q204L mutation, a 2.67-kb EcoRI-XhoI fragment from pPNO2 (5′ portion of gna-1; Figure 1A), a 0.89-kb XhoI-EcoRV fragment of the pPNO3 mutagenized replicative form DNA, and a 2.04-kb EcoRV-ClaI fragment from pPNO5 (see Figure 1A) were inserted into pRAUW122 between the EcoRI and XbaI sites (XbaI made blunt with Klenow fragment). pRAUW122 is a vector that allows targeting to the his-3 locus of N. crassa (Aramayo 1996; Figure 1A). To clone the R178C mutation, a 2.67-kb EcoRI-XhoI fragment from pPNO2 and a 2.92-kb XhoI-ClaI fragment from mutagenized replicative form pPNO3 DNA were inserted into pRAUW122 in the manner described for the Q204L mutation. A plasmid containing the wild-type allele of gna-1 was constructed by insertion of a 5.58-kb EcoRI-ClaI fragment (ClaI blunted using Klenow) from pPNO5 into pRAUW122 as described for the Q204L plasmid. The plasmids containing the wild-type, Q204L, and R178C gna-1 alleles are designated as pQY17, pQY21, and pQY15, respectively.
Electroporation of N. crassa (Vann 1995; Ivey et al. 1996) and gene-directed integration at the his-3 locus were performed as described (Aramayo 1996), with selection on sorbose-containing minimal medium (Case et al. 1979). Δgna-1 strain 30-1 was the recipient (Table 1), and 2 μg pRAUW122, pQY17, pQY21, or PQY15 DNA was used for electroporation.
Southern and Western blot analysis: Genomic DNA was isolated from transformants and subjected to Southern analysis as described (Sambrook et al. 1989). The 8.8-kb HindIII fragment of pRAUW122 was used as a probe. To identify strains with gna-1 wild-type or activated alleles at the his-3 locus, genomic DNA was digested with HindIII. To identify strains containing the his-3 targeting vector alone at the his-3 locus, genomic DNA was digested with HindIII and XbaI.
Heterokaryotic transformants containing the correct-sized
N. crassa strains
| Strain . | Relevant genotype . | Comments . | Source . |
|---|---|---|---|
| 74A | Wild-type A | R. L. Weiss (UCLA) | |
| FGSCa 6103 | his-3 A | J. J. Loros (Dartmouth) | |
| FGSC 4564 | ab-3B cyh-1 am1 | FGSC | |
| 6103/4564 | his-3 A+ ad-3B cyh-1 am1 heterokaryon | This study | |
| 3-7#5 | Δ gna-1::mtr+ pdx-1 a | Ivey et al. (1996) | |
| 30-1 | Δ gna-1::mtr+ his-3 pdx-1 a | 6103 × 3-7#5 progenyb | This study |
| R-10-2 | Δ gna-1::mtr+ his-3+ pdx-1 a | Δ gna-1 | This study |
| R-5-7 | Δ gna-1::mtr+ his-3+ pdx-1 a | Δ gna-1 | This study |
| 17-7-7 | Δ gna-1::mtr+ gna-1+ ::his-3+ pdx-1 a | wild-type gna-1 | This study |
| 17-3 | Δ gna-1::mtr+ gna-1+ ::his-3+ pdx-1 a | wild-type gna-1 | This study |
| 15-9-2 | Δ gna-1::mtr+ gna-1 R178C ::his-3+ pdx-1 a | gna-1R178C allele | This study |
| 15-38 | Δ gna-1::mtr+ gna-1 R178C ::his-3+ pdx-1 a | gna-1R178C allele | This study |
| 21-5-1 | Δ gna-1::mtr+ gna-1Q 204L ::his-3+ pdx-1 a | gna-1Q204L allele | This study |
| 21-3-7 | Δ gna-1::mtr+ gna-1Q 204L ::his-3+ pdx-1 a | gna-1Q204L allele | This study |
| Strain . | Relevant genotype . | Comments . | Source . |
|---|---|---|---|
| 74A | Wild-type A | R. L. Weiss (UCLA) | |
| FGSCa 6103 | his-3 A | J. J. Loros (Dartmouth) | |
| FGSC 4564 | ab-3B cyh-1 am1 | FGSC | |
| 6103/4564 | his-3 A+ ad-3B cyh-1 am1 heterokaryon | This study | |
| 3-7#5 | Δ gna-1::mtr+ pdx-1 a | Ivey et al. (1996) | |
| 30-1 | Δ gna-1::mtr+ his-3 pdx-1 a | 6103 × 3-7#5 progenyb | This study |
| R-10-2 | Δ gna-1::mtr+ his-3+ pdx-1 a | Δ gna-1 | This study |
| R-5-7 | Δ gna-1::mtr+ his-3+ pdx-1 a | Δ gna-1 | This study |
| 17-7-7 | Δ gna-1::mtr+ gna-1+ ::his-3+ pdx-1 a | wild-type gna-1 | This study |
| 17-3 | Δ gna-1::mtr+ gna-1+ ::his-3+ pdx-1 a | wild-type gna-1 | This study |
| 15-9-2 | Δ gna-1::mtr+ gna-1 R178C ::his-3+ pdx-1 a | gna-1R178C allele | This study |
| 15-38 | Δ gna-1::mtr+ gna-1 R178C ::his-3+ pdx-1 a | gna-1R178C allele | This study |
| 21-5-1 | Δ gna-1::mtr+ gna-1Q 204L ::his-3+ pdx-1 a | gna-1Q204L allele | This study |
| 21-3-7 | Δ gna-1::mtr+ gna-1Q 204L ::his-3+ pdx-1 a | gna-1Q204L allele | This study |
FGSC: Fungal Genetics Stock Center, Kansas City.
The his-36103 strain was crossed as a heterokaryon with the am1 4564 helper strain to strain 3-7#5. Because the am1 nucleus is not passed during crosses, progeny carry genes from 6103 and 3-7#5 only.
N. crassa strains
| Strain . | Relevant genotype . | Comments . | Source . |
|---|---|---|---|
| 74A | Wild-type A | R. L. Weiss (UCLA) | |
| FGSCa 6103 | his-3 A | J. J. Loros (Dartmouth) | |
| FGSC 4564 | ab-3B cyh-1 am1 | FGSC | |
| 6103/4564 | his-3 A+ ad-3B cyh-1 am1 heterokaryon | This study | |
| 3-7#5 | Δ gna-1::mtr+ pdx-1 a | Ivey et al. (1996) | |
| 30-1 | Δ gna-1::mtr+ his-3 pdx-1 a | 6103 × 3-7#5 progenyb | This study |
| R-10-2 | Δ gna-1::mtr+ his-3+ pdx-1 a | Δ gna-1 | This study |
| R-5-7 | Δ gna-1::mtr+ his-3+ pdx-1 a | Δ gna-1 | This study |
| 17-7-7 | Δ gna-1::mtr+ gna-1+ ::his-3+ pdx-1 a | wild-type gna-1 | This study |
| 17-3 | Δ gna-1::mtr+ gna-1+ ::his-3+ pdx-1 a | wild-type gna-1 | This study |
| 15-9-2 | Δ gna-1::mtr+ gna-1 R178C ::his-3+ pdx-1 a | gna-1R178C allele | This study |
| 15-38 | Δ gna-1::mtr+ gna-1 R178C ::his-3+ pdx-1 a | gna-1R178C allele | This study |
| 21-5-1 | Δ gna-1::mtr+ gna-1Q 204L ::his-3+ pdx-1 a | gna-1Q204L allele | This study |
| 21-3-7 | Δ gna-1::mtr+ gna-1Q 204L ::his-3+ pdx-1 a | gna-1Q204L allele | This study |
| Strain . | Relevant genotype . | Comments . | Source . |
|---|---|---|---|
| 74A | Wild-type A | R. L. Weiss (UCLA) | |
| FGSCa 6103 | his-3 A | J. J. Loros (Dartmouth) | |
| FGSC 4564 | ab-3B cyh-1 am1 | FGSC | |
| 6103/4564 | his-3 A+ ad-3B cyh-1 am1 heterokaryon | This study | |
| 3-7#5 | Δ gna-1::mtr+ pdx-1 a | Ivey et al. (1996) | |
| 30-1 | Δ gna-1::mtr+ his-3 pdx-1 a | 6103 × 3-7#5 progenyb | This study |
| R-10-2 | Δ gna-1::mtr+ his-3+ pdx-1 a | Δ gna-1 | This study |
| R-5-7 | Δ gna-1::mtr+ his-3+ pdx-1 a | Δ gna-1 | This study |
| 17-7-7 | Δ gna-1::mtr+ gna-1+ ::his-3+ pdx-1 a | wild-type gna-1 | This study |
| 17-3 | Δ gna-1::mtr+ gna-1+ ::his-3+ pdx-1 a | wild-type gna-1 | This study |
| 15-9-2 | Δ gna-1::mtr+ gna-1 R178C ::his-3+ pdx-1 a | gna-1R178C allele | This study |
| 15-38 | Δ gna-1::mtr+ gna-1 R178C ::his-3+ pdx-1 a | gna-1R178C allele | This study |
| 21-5-1 | Δ gna-1::mtr+ gna-1Q 204L ::his-3+ pdx-1 a | gna-1Q204L allele | This study |
| 21-3-7 | Δ gna-1::mtr+ gna-1Q 204L ::his-3+ pdx-1 a | gna-1Q204L allele | This study |
FGSC: Fungal Genetics Stock Center, Kansas City.
The his-36103 strain was crossed as a heterokaryon with the am1 4564 helper strain to strain 3-7#5. Because the am1 nucleus is not passed during crosses, progeny carry genes from 6103 and 3-7#5 only.
hybridizing fragments were purified to homokaryons by repeated plating on sorbose-containing plates. Southern analysis was used to verify that in such homokaryons the endogenous his-3 fragment was replaced with the appropriate vector sequences.
For plasma membrane isolation, conidia were inoculated into liquid VM at a final concentration of 3.6 × 106 conidia/ml. Cultures were incubated in the dark at 30° with shaking at 200 rpm for 16 hr. Mycelia were collected by filtration and cells were broken using a Bead-Beater (Biospec Products, Bartlesville, OK). The plasma membrane fraction was isolated and protein concentration determined as described (Turner and Borkovich 1993). Western blot analysis of plasma membrane proteins was performed as described (Borkovich et al. 1989), using GNA-1, GNA-2, and GNB-1 antiserum at 1:1000, 1:300, and 1:5000 dilutions, respectively.
Phenotypic analysis: Apical extension rates at 30° on normal and hyperosmotic medium were determined as described (Ivey et al. 1996). For dry weight accumulation measurements on solid media, VM plates overlaid with cellophane were inoculated in the center with conidial suspensions. Plates were incubated at room temperature in constant light for 3 days. The colonies were then scraped from the plates onto filter paper and dried at 80° before weighing.
Sexual fertility assays were conducted on synthetic crossing medium (SCM; Westergaard and Mitchell 1947) at room temperature in constant light as described (Ivey et al. 1996). Strain 74A conidia were used to fertilize protoperithecia on plates after 10 days of growth.
cAMP and carotenoid pigment measurements: To measure the in vivo cAMP levels of wild-type and mutant N. crassa cells, VM plates overlaid with cellophane were inoculated with conidial suspensions. Plates were incubated at room temperature under constant light for 3 days. Mycelial pads were scraped from the plates, frozen in liquid nitrogen, and ground to a fine powder. Powdered mycelia were suspended in 6% ice-cold tricarboxylic acid, and incubated on ice for at least 20 min. A 1-ml aliquot of each sample was centrifuged at 16,200 × g for 25 min. The supernatant was chromatographed over a Dowex 50W (H+ form) column and concentrated to purify cAMP as described (Salomon et al. 1974). cAMP was quantitated using a competitive binding protein assay as described (Brown et al. 1971), with liquid scintillation counting in a Beckman LS 5801 counter (Beckman Instruments, Fullerton, CA).
Mycelial pads, isolated as described above for the cAMP measurements, were lyophilized, weighed, ground to a fine powder, and extracted in 6 ml methanol for 20 min at 60°. After filtration, the residue was reextracted with acetone for 20 min at 50° (Paietta and Sargent 1981; Linden et al. 1997). The filtrates were combined and the pigment content was estimated by optical density on a DS-2000 Spectrophotometer (SLM Instruments, Urbana, IL).
Assays for sensitivity to heat and hydrogen peroxide: Five-day-old conidia were inoculated into liquid VM medium at a concentration of 1 × 106 cells/ml and germinated for 2 hr with shaking at 200 rpm in the dark at 30°. For heat shock studies, 1-ml samples were placed in glass tubes and held at 30° (no induced thermotolerance) or given a 44° pretreatment for 30 min (to induce thermotolerance). Samples were then incubated at 52° for 20 min (heat treatment). A control tube was also prepared that contained germlings incubated at 30° that were never subjected to a heat treatment (30° control). Aliquots were diluted and spread on sorbose plates, incubated for 2 to 3 days at 30°, and colonies counted. Percent survival was obtained by dividing the number of viable colonies after heat treatment by the number on the 30° control plate, and multiplying by 100.
For hydrogen peroxide sensitivity assays, 1-ml aliquots of 2-hr germlings prepared as described above were brought up to 10 mm hydrogen peroxide (final concentration) and incubated for 2 hr at 30°. Samples were then diluted and plated on sorbose plates as described above. Percent survival was scored by dividing the number of colonies obtained after peroxide treatment by the number on a plate containing cells that were not exposed to hydrogen peroxide, and multiplying by 100.
RESULTS
Construction of isogenic strains and G protein analysis: Previous studies have demonstrated that GNA-1 regulates
—Construction of N. crassa strains with gna-1 alleles targeted to the his-3 locus. (A) Diagram of the his-3 region in the pRAUW122 plasmid, the gna-1 gene region in pPNO5, and targeting strategy. The top bar shows the genomic DNA insert of pRAUW122; the striped area is a portion of the his-3 gene. The bottom bar is the genomic DNA insert of pPNO5. The shaded area within the gna-1 gene region is the aminoacid coding sequence. The arrows below his-3 and gna-1 show the direction of translation. gna-1+, gna-1R178C, and gna-1Q204L alleles were inserted between the XbaI and EcoRI sites in the pRAUW122 polylinker as shown; the XbaI site of pRAUW122 and the ClaI site of gna-1 were made blunt with Klenow. The positions of various restriction enzyme sites are indicated. Enzyme abbreviations are as follows: H, HindIII; Xb, XbaI; EI, EcoRI; C, ClaI; EV, EcoRV; and Xh, XhoI. (B) Southern analysis of his-3 targeted homokaryotic strains. Genomic DNA was digested with HindIII and XbaI (lanes 1–3) or HindIII (lanes 4–10). The entire 8.8-kb insert from pRAUW122 depicted in A was used as a probe. Lanes 1 and 10 are the his-3 Δgna-1 recipient (R) strain 30-1; all other lanes are from his-3+ transformants. The relevant genotype of his-3+ strains is shown below the lane markings. Strains are lane 2, R-10-2; lane 3, R-5-7; lane 4, 17-7-7; lane 5, 17-3; lane 6, 15-9-2; lane 7, 15-38; lane 8, 21-5-1; and lane 9, 21-3-7. A 0.7-kb fragment was deleted from pRAUW122 during its construction (Aramayo 1996); thus, the HindIII fragment containing the endogenous his-3 locus in strain 30-1 is larger than that resulting from vector integration (9.5 vs. 8.8 kb). Integration of gna-1 wild-type or activated alleles yields two 6.2-kb hybridizing fragments due to the presence of three HindIII sites in gna-1. After HindIII-XbaI digestion, the chromosomally encoded his-3 fragment is 9.5 kb, as there are no XbaI sites in this region. Integration of the his-3 vector without insert (for Δgna-1 negative controls) yields 5.2-kb and 3.6-kb fragments, due to cleavage of the XbaI site in the plasmid polylinker.
several processes during growth and development in N. crassa (Ivey et al. 1996); however, an active role for GNA-1 could not be assigned based on the analysis of a Δgna-1 mutation alone. Therefore, to better characterize the function of GNA-1, we analyzed phenotypes in strains that contained null, wild-type, or activated (R178C or Q204L) gna-1 alleles, but were otherwise isogenic. For construction of these strains, we took advantage of an efficient gene targeting system for N. crassa that directs DNA sequences to the his-3 locus (Ebbole 1990; Aramayo 1996; Figure 1A). The constructs for wild-type or activated gna-1 alleles all contained a 5.6-kb fragment of gna-1 genomic DNA previously shown to support expression of the gene from ectopic sites (Ivey et al. 1996). Primary heterokaryotic transformants and purified homokaryotic strains were identified using Southern analysis (Figure 1B).
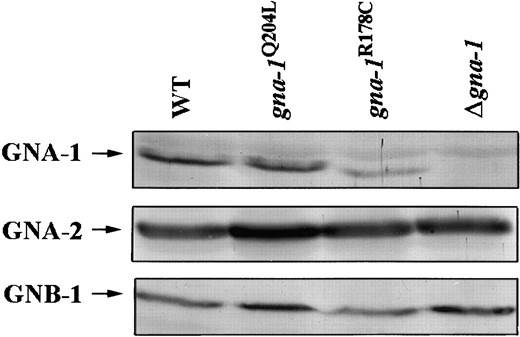
In mammalian cells, changes in expression of wild-type, inactivated, or activated Gα proteins can influence the expression of Gβ proteins (Hermouet et al. 1993). Altered expression of Gβ or Gα proteins due to mutation of gna-1 could influence the phenotypes observed in the N. crassa isogenic strains. Therefore, we determined whether activation of GNA-1 affected the expression of other known N. crassa G proteins. Levels of GNA-1, GNA-1R178C, GNA-1Q204L, GNA-2, and the only known N. crassa Gβ protein, GNB-1 (Q. Yang and K. Borkovich, unpublished results), were measured in the isogenic strains using Western analysis (Figure 2). GNA-1 and GNA-1Q204L are expressed to the same level in the respective strains, but the GNA-1R178C protein level is reduced ∼50% (Figure 2). The mobility of the GNA-1R178C protein is also slightly increased relative to wild-type GNA-1. Expression of GNA-2 and GNB-1 was similar in wild-type, Δgna-1, gna-1R178C, and gna-1Q204L strains (Figure 2), indicating that mutationally activated gna-1 alleles do not influence steady-state levels of other known G protein subunits in N. crassa.
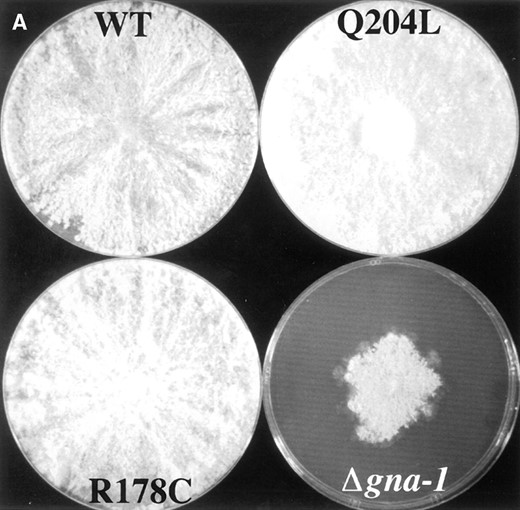
Strains containing GTPase-deficient gna-1 alleles produce abundant, long aerial hyphae: The colony morphology of Δgna-1, gna-1+, gna-1R178C, and gna-1Q204L strains was compared after growth on solid medium in light (Figure 3A). Under these conditions, mycelia from N. crassa wild-type strains produce aerial hyphae with conidiophores at their tips. These structures then give rise to asexual spores termed conidia (Springer 1993; Figure 3A). As noted previously, Δgna-1 strains have a reduced apical extension rate, producing smaller colonies than wild type (Figure 3A; Ivey et al. 1996). Furthermore,
—Levels of GNA-1, GNA-2, and GNB-1 proteins. Samples containing 30 μg plasma membrane proteins were subjected to Western analysis as described in materials and methods. Strains are 17-7-7 (gna-1+), 21-5-1 (gna-1Q204L), 15-9-2 (gna-1R178C), and R-10-2 (Δgna-1). The migration positions of GNA-1, GNA-2, and GNB-1 are shown on the left side of the figure. GNA-1, GNA-2, and GNB-1 antibodies were used at dilutions of 1:1000, 1:300, and 1:5000, respectively.
the Δgna-1 mutants produce fewer aerial hyphae per cross-sectional area than wild type (Figure 3A; data not shown). In contrast, the two strains with activating gna-1 mutations produce longer, more abundant aerial hyphae than wild type, with the greatest density seen in strains with the gna-1Q204L allele (Figure 3A). In spite of the presence of more aerial hyphae, the activated allele-containing
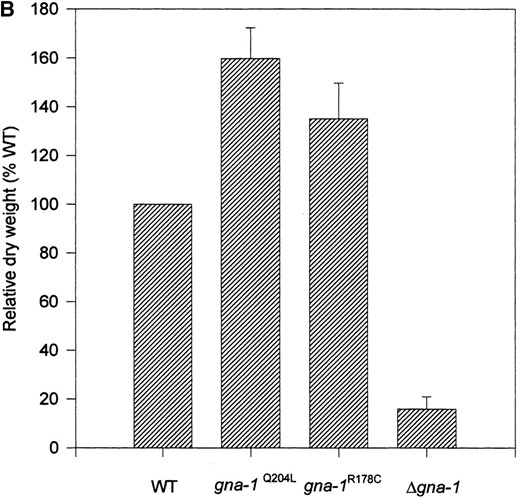
—Colony morphology and mass accumulation. (A) Colony morphology: Cellophane-overlaid VM plates were inoculated with conidia from the various strains and incubation at room temperature for 3 days in constant light. Strains are 17-7-7 (WT/wild type, gna-1+), 21-5-1 (gna-1Q204L), 15-9-2 (gna-1R178C), and R-10-2 (Δgna-1). In this photograph aerial hyphae are the white fluffy material on the plate surface. (B) Relative mass on cellophane-overlaid VM plates. Strains are the same as in A. Values are expressed as the mean of the relative mass of wild type ± SEM from four independent experiments. Wild-type dry weight is 312.5 ± 28.3 mg.
strains produce the same amount (gna-1R178C) or slightly fewer conidia than wild type (gna-1Q204L; data not shown). Conversely, although the Δgna-1 strains possess fewer aerial hyphae, they produce the same amount of conidia per cross-sectional area as wild-type controls (Ivey et al. 1995; data not shown). Thus, Δgna-1 mutants form more conidia per aerial hyphae than wild type, while strains containing GTPase-deficient gna-1 alleles produce fewer.
We previously reported that wild-type strains accumulate more mass than Δgna-1 mutants, with the greatest effect seen after growth in light (Ivey et al. 1996). On this basis, we predicted that the synthesis of more aerial hyphae would result in greater mass for the gna-1R178C and gna-1Q204L strains. Therefore, we measured colony dry weights for the four strains after incubation in light (Figure 3B). As noted previously, Δgna-1 strains have less mass than wild type (16.0% of wild type; Figure 3B). gna-1R178C and gna-1Q204L strains accumulate greater mass than wild-type or Δgna-1 mutant strains, with gna-1Q204L weights the highest (160% of wild type; Figure 3B). Thus, the dry weight results roughly correlate with the appearance of aerial hyphae on plates. However, these experiments do not rule out the possibility that differences in basal hyphae production among the various strains could also contribute to the observed mass differences.
These results indicate that the aerial hyphae and dry weight phenotypes of Δgna-1, gna-1+, and gna-1R178C and
Relative growth rates on solid medium
| . | Relevant genotype . | Growth rate (% wild type)a . | |||
|---|---|---|---|---|---|
| Strain . | VMb . | VM + sorbitolc . | VM + KClc . | VM + NaClc . | |
| 17-7-7 | Wild type | 100 | 100 | 100 | 100 |
| 21-5-1 | gna-1 Q204L | 77.4 ± 2.0 | 87.8 ± 4.6 | 76.8 ± 1.3 | 77.4 ± 1.5 |
| 15-9-2 | gna-1 R178C | 83.8 ± 2.1 | 93.2 ± 4.4 | 91.2 ± 2.3 | 92.3 ± 2.9 |
| R-10-2 | Δ gna-1 | 53.3 ± 0.5 | 42.3 ± 3.1 | 30.0 ± 3.9 | 32.0 ± 2.3 |
| . | Relevant genotype . | Growth rate (% wild type)a . | |||
|---|---|---|---|---|---|
| Strain . | VMb . | VM + sorbitolc . | VM + KClc . | VM + NaClc . | |
| 17-7-7 | Wild type | 100 | 100 | 100 | 100 |
| 21-5-1 | gna-1 Q204L | 77.4 ± 2.0 | 87.8 ± 4.6 | 76.8 ± 1.3 | 77.4 ± 1.5 |
| 15-9-2 | gna-1 R178C | 83.8 ± 2.1 | 93.2 ± 4.4 | 91.2 ± 2.3 | 92.3 ± 2.9 |
| R-10-2 | Δ gna-1 | 53.3 ± 0.5 | 42.3 ± 3.1 | 30.0 ± 3.9 | 32.0 ± 2.3 |
Apical extension rates were determined for the indicated strains by measuring colony diameters at various times. Wild-type growth rates (mm/hr) were 5.13 (VM), 2.08 (VM + Sorbitol, 2.92 (VM + KCl), and 2.70 (VM + NaCl).
Values are mean ± standard error of the mean (SEM) from two independent experiments.
Values are mean ± SEM from three independent experiments. The final concentrations of sorbitol, KCl, and NaCl were 1.5 m, 0.75 m, and 0.75 m, respectively.
Relative growth rates on solid medium
| . | Relevant genotype . | Growth rate (% wild type)a . | |||
|---|---|---|---|---|---|
| Strain . | VMb . | VM + sorbitolc . | VM + KClc . | VM + NaClc . | |
| 17-7-7 | Wild type | 100 | 100 | 100 | 100 |
| 21-5-1 | gna-1 Q204L | 77.4 ± 2.0 | 87.8 ± 4.6 | 76.8 ± 1.3 | 77.4 ± 1.5 |
| 15-9-2 | gna-1 R178C | 83.8 ± 2.1 | 93.2 ± 4.4 | 91.2 ± 2.3 | 92.3 ± 2.9 |
| R-10-2 | Δ gna-1 | 53.3 ± 0.5 | 42.3 ± 3.1 | 30.0 ± 3.9 | 32.0 ± 2.3 |
| . | Relevant genotype . | Growth rate (% wild type)a . | |||
|---|---|---|---|---|---|
| Strain . | VMb . | VM + sorbitolc . | VM + KClc . | VM + NaClc . | |
| 17-7-7 | Wild type | 100 | 100 | 100 | 100 |
| 21-5-1 | gna-1 Q204L | 77.4 ± 2.0 | 87.8 ± 4.6 | 76.8 ± 1.3 | 77.4 ± 1.5 |
| 15-9-2 | gna-1 R178C | 83.8 ± 2.1 | 93.2 ± 4.4 | 91.2 ± 2.3 | 92.3 ± 2.9 |
| R-10-2 | Δ gna-1 | 53.3 ± 0.5 | 42.3 ± 3.1 | 30.0 ± 3.9 | 32.0 ± 2.3 |
Apical extension rates were determined for the indicated strains by measuring colony diameters at various times. Wild-type growth rates (mm/hr) were 5.13 (VM), 2.08 (VM + Sorbitol, 2.92 (VM + KCl), and 2.70 (VM + NaCl).
Values are mean ± standard error of the mean (SEM) from two independent experiments.
Values are mean ± SEM from three independent experiments. The final concentrations of sorbitol, KCl, and NaCl were 1.5 m, 0.75 m, and 0.75 m, respectively.
gna-1Q204L strains differ. Since Gβγ is predicted to be free in both Δgna-1 and activated allele-containing strains, these results support a Gβγ-independent role for GNA-1 during signaling. Furthermore, the increased aerial hyphae formation and dry weight accumulation observed in strains with activating mutations suggest that GNA-1 is a positive regulator of these processes. In contrast, activation of GNA-1 correlates with decreased conidia formation per aerial hyphae in N. crassa.
Activation of gna-1 does not greatly impact apical extension rate, osmotic sensitivity, or sexual fertility: Previously we reported that Δgna-1 mutants are more sensitive to hyperosmotic medium than wild type (Ivey et al. 1996). Therefore, we compared the osmotic sensitivity of Δgna-1, gna-1+, gna-1R178C, and gna-1Q204L strains after growth on solid medium containing no additions, or 1.5 m sorbitol, 0.75 m KCl, or 0.75 m NaCl (Table 2). In accordance with previous results (Ivey et al. 1996), the Δgna-1 strain grew more slowly on both normal and hyperosmotic media than wild type (Table 2). In contrast, both activated alleles gave significant complementation of the Δgna-1 defect, with the gna-1R178C allele more effective than gna-1Q204L (Table 2). These results are consistent with a Gβγ-independent role for GNA-1 in regulation of apical extension rate on both normal and hyperosmotic media. Furthermore, the observation that the osmotic tolerance of gna-1R178C and gna-1Q204L strains is not greater than wild type implies that wild-type strains cannot increase their hyperosmotic tolerance through mutational activation of gna-1.
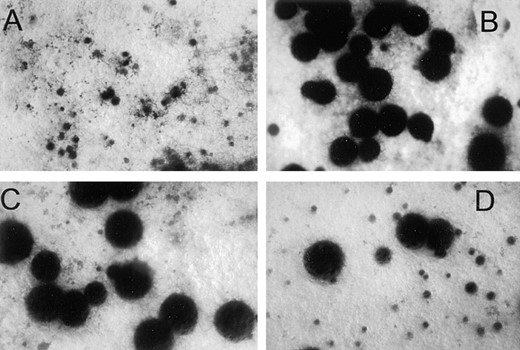
In earlier studies, it was demonstrated that Δgna-1 strains are male fertile, but female sterile (Ivey et al. 1996). To determine if GNA-1 plays a direct role in female fertility independent of Gβγ, we compared the ability of Δgna-1, wild-type, and activated gna-1 allele-containing strains to serve as female parents during sexual crosses. As seen previously (Ivey et al. 1996), Δgna-1 strains were female sterile, while wild-type strains were fertile and produced perithecia containing viable ascospores after fertilization (Figure 4, A and B; data not shown). gna-1R178C and gna-1Q204L strains were female fertile, although these strains produced fewer and, on average, slightly larger perithecia than wild type (Figure 4, C and D). Because the phenotypes of Δgna-1, wild-type, and activated-allele-containing strains differ, GNA-1 plays an active role in regulating female fertility in N. crassa. There was no effect on male fertility due to mutation of gna-1; all four strains could serve as male parents (data not shown).
gna-1R178C and gna-1Q204L strains have lower carotenoid, but higher cAMP levels than wild-type strains: In N. crassa, carotenoid synthesis is induced by blue light in mycelia, whereas synthesis in conidia is constitutive (Rau and Mitzka-Schnabel 1985; Bramley and Mackenzie 1988). Previous studies have demonstrated a negative correlation between cAMP levels and carotenoid
—Female fertility. SCM plates were inoculated with various strains, cultured to allow protoperithecial formation, and then fertilized using strain 74A conidia. Plates were photographed 7 days postfertilization. Strains are Δgna-1 R-10-2 (A); gna-1+ 17-7-7 (B); gna-1R178C 15-9-2 (C); and gna-1Q204L 21-3-7 (D). Examples of normal protoperithecia (small dark structures) and perithecia (larger, dark-colored spheres) can be seen in B for wild-type strain 17-7-7.
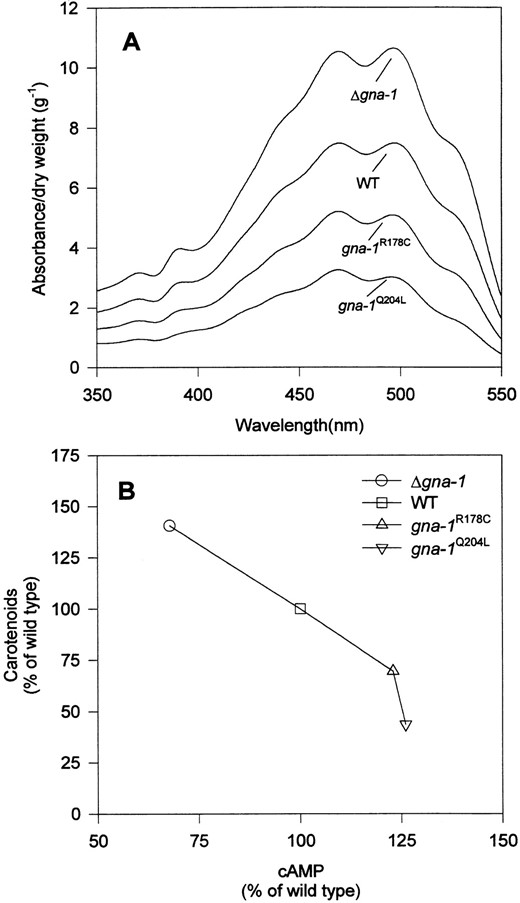
accumulation (Kritsky et al. 1982). In addition, Δgna-1 strains have lower cAMP levels and are more pigmented than wild type (Ivey et al. 1996; D. Ivey and K. Borkovich, unpublished results). In this study, we observed that carotenoid pigment production was visibly reduced in gna-1R178C and gna-1Q204L colonies in comparison to wild type (Figure 3A). The lighter appearance of the gna-1R178C and gna-1Q204L strains results not only from reduced pigmentation in aerial and basal hyphae, but also from the production of fewer, less-pigmented conidia (data not shown). Therefore, we extracted and quantitated carotenoids from Δgna-1, gna-1+, and gna-1R178C and gna-1Q204L strains grown in constant light. The spectrum of the extracted material is indicative of carotenoids, with peaks at ∼470 and 495 nm (Paietta and Sargent 1981; Armstrong and Hearst 1996; Figure 5A). The Δgna-1 strain has the highest carotenoid content, whereas the two strains containing activated gna-1 alleles have lower carotenoid levels than wild type (Figure 5A).
We have shown that deletion of gna-1 leads to lower adenylyl cyclase activity and intracellular cAMP levels in cells grown on solid medium (D. Ivey, Q. Yang and K. Borkovich, unpublished results). To ascertain whether activation of gna-1 would lead to higher cAMP levels, we measured steady-state cAMP levels in Δgna-1, gna-1+, and gna-1R178C and gna-1Q204L strains. Similar to previous results, the Δgna-1 strain has reduced levels of cAMP (67.8% of wild type; see also Figure 5B). There is an increase in cAMP levels in strains containing GTPase-deficient gna-1 alleles; gna-1R178C strains have 123% and gna-1Q204L strains have 126% wild-type levels of cAMP (see also Figure 5B). Thus, there is a positive correlation among cAMP levels, aerial hyphae formation, and GNA-1 activation state in N. crassa. In addition, the combined results from the carotenoid and cAMP assays demonstrate an inverse relationship between cAMP and carotenoid levels in the four strains (Figure 5B). Consistent with previous observations, our results support the hypothesis that aerial hyphae development is positively regulated by cAMP, but that lower cAMP levels promote production of conidia and carotenoid accumulation (Kritsky et al. 1982; Murayama et al. 1995).
Strains containing GTPase-deficient gna-1 alleles exhibit increased sensitivity to heat and hydrogen peroxide-induced oxidative stress: Carotenoids provide resistance to oxidative stress through quenching of free radicals (Krinsky 1992). In various fungal species, carotenoids are correlated with protection against oxidative stress (Moore et al. 1989; Schroeder and Johnson 1995). It also has been shown that exposure to oxidants can regulate synthesis of carotenoid pigments (Schroeder and Johnson 1995). As mentioned previously, resistance to oxidative stress is often linked to heat tolerance. Therefore, we measured the sensitivity of the four strains to heat and to oxidative stress induced by hydrogen peroxide and paraquat.
—Carotenoid spectra and relationship between carotenoid and cAMP levels. (A) Absorbance spectra of carotenoid pigments: The visible absorption spectra of the carotenoid extracts were recorded and normalized to the dry weight of the original mycelial pad. The spectra peak at 470 and 495 nm. Strains are Δgna-1 R-10-2, gna-1+ 17-7-7, gna-1R178C 15-9-2, and gna-1Q204L 21-5-1. (B) Inverse correlation between cAMP and carotenoid pigment level: Strains are the same as in A. Values for cAMP and carotenoids were converted to percentage wild type before plotting. The cAMP value for wild type is 4.88 ± 0.55 pmoles/mg protein. Carotenoid levels are taken from Figure 5A, using the value of 470 nm for each strain. The carotenoid level of wild type is 7.48 absorbance units/g dry weight.
Exposure of N. crassa cells to temperatures of 50° or above results in significant lethality (Kapoor 1983; Plesofsky-Vig and Brambl 1985; data not shown), while incubation at temperatures between 40° and 47° leads to heat shock protein synthesis and significant retention of viability (Kapoor 1983; Plesofsky-Vig and Brambl 1985). It has been demonstrated in N. crassa and other organisms that incubation of cells at sublethal heat shock temperatures yields increased resistance to subsequent exposure to a lethal temperature; this phenomenon is termed induced thermotolerance (Plesofsky-Vig and Brambl 1985; Lindquist and Craig 1988). Induced thermotolerance is due at least in part to synthesis of heat shock proteins, which protect the cell from the normally lethal temperature (reviewed by Mager and De Kruijff 1995).
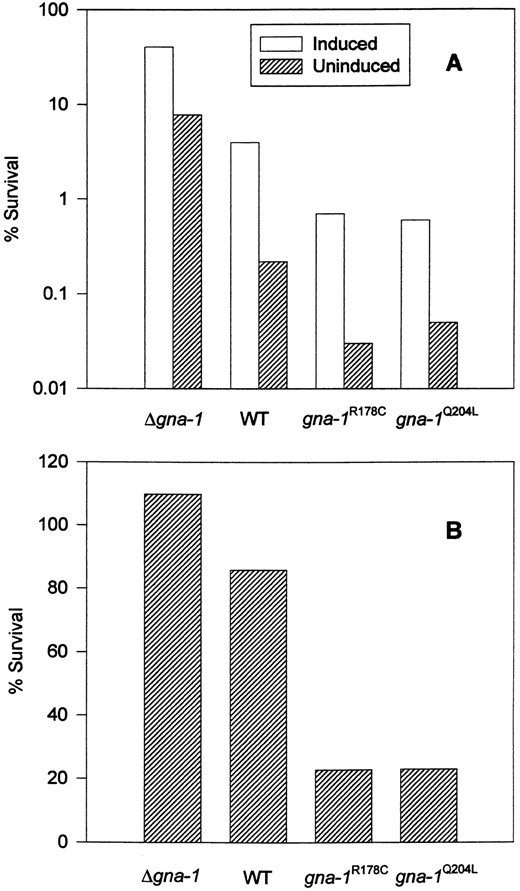
To assess thermotolerance in the N. crassa strains, we quantitated survival after exposure to a 52° lethal heat treatment with and without prior exposure to the nonlethal heat shock temperature of 44°. There was an inverse correlation between the extent of both uninduced and induced thermotolerance and the predicted GTP occupancy of the GNA-1 protein expressed in the four isogenic strains (Figure 6A). Δgna-1 strains exhibited the greatest survival after heat treatment (10- to 35-fold more resistant than wild type), while strains with GTPase-deficient alleles had the lowest survival (six times more sensitive than wild type). Thus, the activation state of GNA-1 in N. crassa negatively influences sensitivity to heat.
We next measured viability in the presence of hydrogen peroxide to evaluate the oxidative stress response and found an inverse relationship between sensitivity to this oxidant and activation of GNA-1 (Figure 6B). Δgna-1 mutants are most resistant to 10 mm hydrogen peroxide, with no killing observed under these conditions (104% viability). Wild-type strains exhibit 81.1% viability, while strains containing activating gna-1 alleles have 18.2–26.0% viability. In contrast to the above results, all four strains exhibited essentially the same sensitivity to paraquat, a superoxide-generating agent (data not shown). These findings suggest that GNA-1 negatively regulates the activity or expression of enzymes important in the defense against hydrogen peroxide but not superoxide.
DISCUSSION
Previous work from our laboratory demonstrated that loss of gna-1 leads to several phenotypes during the life cycle of N. crassa (Ivey et al. 1996). However, Δgna-1 strains are predicted to have increased free Gβγ levels, which can regulate downstream effectors (Simon et al. 1991). Hence, we could not conclude that the phenotypes of Δgna-1 strains directly resulted from loss of effector regulation by GNA-1. In the present study, we explored the relationship between GNA-1 and Gβγ in signal transduction by constructing a set of isogenic strains containing null, wild-type, or activated gna-1 alleles. Because Gβγ would be free to signal in strains with either null or mutationally activated gna-1 alleles, a comparison of strain phenotypes would indicate whether GNA-1 possesses a Gβγ-independent role in signaling. Our results demonstrate that Δgna-1, wild-type, and gna-1R178C or gna-1Q204L strains often have differing phenotypes, implying that GNA-1 has some functions
—Sensitivity to heat and hydrogen peroxide. (A) Measurement of induced and uninduced thermotolerance: For uninduced thermotolerance, 2-hr germlings were incubated at 52° for 20 min, while induced thermotolerance was assessed by introducing a 30-min exposure to 44° before the 52° heat treatment. See materials and methods for details. Strains are Δgna-1 R-10-2, gna-1+ 17-7-7, gna-1R178C 15-9-2, and gna-1Q204L 21-5-1. (B) Sensitivity to hydrogen peroxide: Twohour germlings were incubated in 10 mm hydrogen peroxide at 30° for 2 hr. See materials and methods for details. Strains are the same as in A.
independent of Gβγ in N. crassa. For example, apical extension rates on normal and hyperosmotic solid medium are similar for wild-type and activated gna-1 allele-containing strains, while Δgna-1 strains have substantially lower rates. Likewise, strains with wild-type, gna-1R178C, or gna-1Q204L genes have similar phenotypes during the sexual cycle, while Δgna-1 strains differ.
We also have identified several processes in which a more pronounced phenotype is observed depending on the activated state of GNA-1: aerial hyphae formation, conidia production, dry weight accumulation on solid medium, carotenogenesis, sensitivity to heat shock, and resistance to hydrogen peroxide-induced oxidative stress. For example, Δgna-1 mutants elaborate fewer aerial hyphae, but are more resistant to heat and hydrogen peroxide treatment than wild-type strains. In contrast, strains with mutationally activated gna-1 alleles are superior to wild type in the first trait, but are more sensitive to heat and hydrogen peroxide. These results suggest that the role of GNA-1 is dominant to Gβγ in these processes, with a greater concentration of GTP-bound GNA-1 leading to a stronger phenotype.
Our results demonstrate that GNA-1 has a profound effect on the survival of N. crassa during exposure to lethal temperatures. One possible model to explain this observation is that GNA-1 is a negative regulator of a heat-shock protein necessary for thermotolerance. At present, there is evidence for differential expression of various heat shock proteins during developmental stages in N. crassa, including aerial hyphae development (Fracella et al. 1997; Hafker et al. 1998). Thus, it is plausible that the regulatory roles of GNA-1 during aerial hyphae development and the heat stress response are related.
Oxidative metabolism is essential for the viability of obligate aerobes such as N. crassa. However, this metabolic state produces superoxide, peroxide, and hydroxyl radicals, which are deleterious to nucleic acids, proteins, and lipids (reviewed by Mager and De Kruijff 1995). Superoxide dismutases, catalases, and carotenoid pigments are key players in conferring resistance to these oxidants in microorganisms (Moore et al. 1989; Mager and De Kruijff 1995). In N. crassa, a CuZn superoxide dismutase gene, dos-1, has been cloned, and properties of a null mutant have been characterized (Chary et al. 1994). sod-1 null mutants are more sensitive to paraquat than wild-type strains, but have normal viability after a lethal heat treatment. Three forms of catalase have been identified in N. crassa, with differential regulation by developmental stage, incubation at lethal temperatures, or paraquat exposure (Chary and Natvig 1989). Conidia from N. crassa albino strains (lacking carotenoids) are more sensitive to singlet oxygen (Shimizu et al. 1979). Our results suggest that GNA-1 may regulate catalase and carotenoid biosynthetic enzyme activity or expression in N. crassa, while superoxide dismutase regulation is GNA-1 independent.
Increased production of reactive oxygen species is associated with all steps of conidiation in N. crassa: hyphal adhesion, aerial hyphae differentiation, and conidia formation (Hansberg et al. 1993). It has been suggested that organisms that are unable to reduce reactive oxygen species use mechanisms to avoid oxygen that result in differentiation; in the absence of these mechanisms they will die (Hansberg and Aguirre 1990). Thus, the involvement of GNA-1 in aerial hyphae development and resistance to hydrogen peroxideinduced oxidative stress may be linked; the oxidative stress response pathway is inhibited in strains with GTPase-deficient gna-1 mutations, causing elevated sensitivity to hydrogen peroxide and hyperactivation of aerial hyphae formation. In this regard, it is of interest that Aspergillus nidulans strains containing a GTPase-deficient allele of the gna-1 homologue fadA (fadAG42R) do not produce aerial hyphae or conidia; instead they exhibit colony lysis (Yu et al. 1996).
We have shown that deletion of gna-1 negatively influences adenylyl cyclase activity and cAMP levels in N. crassa (D. Ivey, Q. Yang and K. Borkovich, unpublished results). In this study, we again demonstrate that the Δgna-1 strain has lower cAMP levels than wild type. We also show that the activated gna-1 allele-containing strains have elevated levels of cAMP. It should be noted that steady-state, and not ligand-stimulated levels of cAMP, were measured in these experiments; the modest changes observed between the various strains may reflect compensatory mechanisms that maintain cAMP levels at relatively constant levels (reviewed by Beltman et al. 1993; Burns et al. 1996). Thus, the magnitude of fluctuations in cAMP level induced by environmental signals may be much greater than the steady-state values suggest. In addition, the proposed involvement of GNA-1 in regulation of cellular processes may involve both cAMP-dependent and -independent functions. For example, GNA-1 might regulate MAP kinase pathway(s) that respond to external stresses. In mammals, stress-activated MAP kinase cascades have been identified (reviewed by Paul et al. 1997), and the osmostress response in S. cerevisiae is mediated by the Hog1 MAP kinase pathway (Brewster et al. 1993).
The formation of a multicellular organism and subsequent differentiation of specialized structures involves many genes and their products. The cellular machinery required to withstand environmental stresses such as heat and oxidants is also regulated by numerous genetic loci. Our results are consistent with GNA-1 as a key mediator of these interrelated processes in N. crassa. Future efforts will be directed toward elucidating the signal transduction pathways regulated by GNA-1 in performing these essential functions.
Footnotes
Communicating editor: R. H. Davis
Acknowledgement
We thank D. Ebbole and R. Aramayo for plasmids; J. Spudich and X.-C. Yu for preparation of figures; J. Spudich and E. Spudich for use of equipment; and X.-N. Zhang and J. Sasaki for technical assistance. We acknowledge J. Bieszke, D. Ivey, A. Kays, E. Spudich, and G. Turner for their critical comments on the manuscript. This work was supported by National Institutes of Health grant GM-48626 (to K.A.B.) and by an American Cancer Society Junior Faculty Research Award JFRA-495 (to K.A.B.).
LITERATURE CITED